Expression of IMP3 and LIN28A RNA-Binding Proteins in Placentas of Patients with Pre-Eclampsia with and without Severe Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Immunohistochemistry
2.3. Double Immunofluorescence Staining
2.4. qPCR
2.5. Statistical Analysis
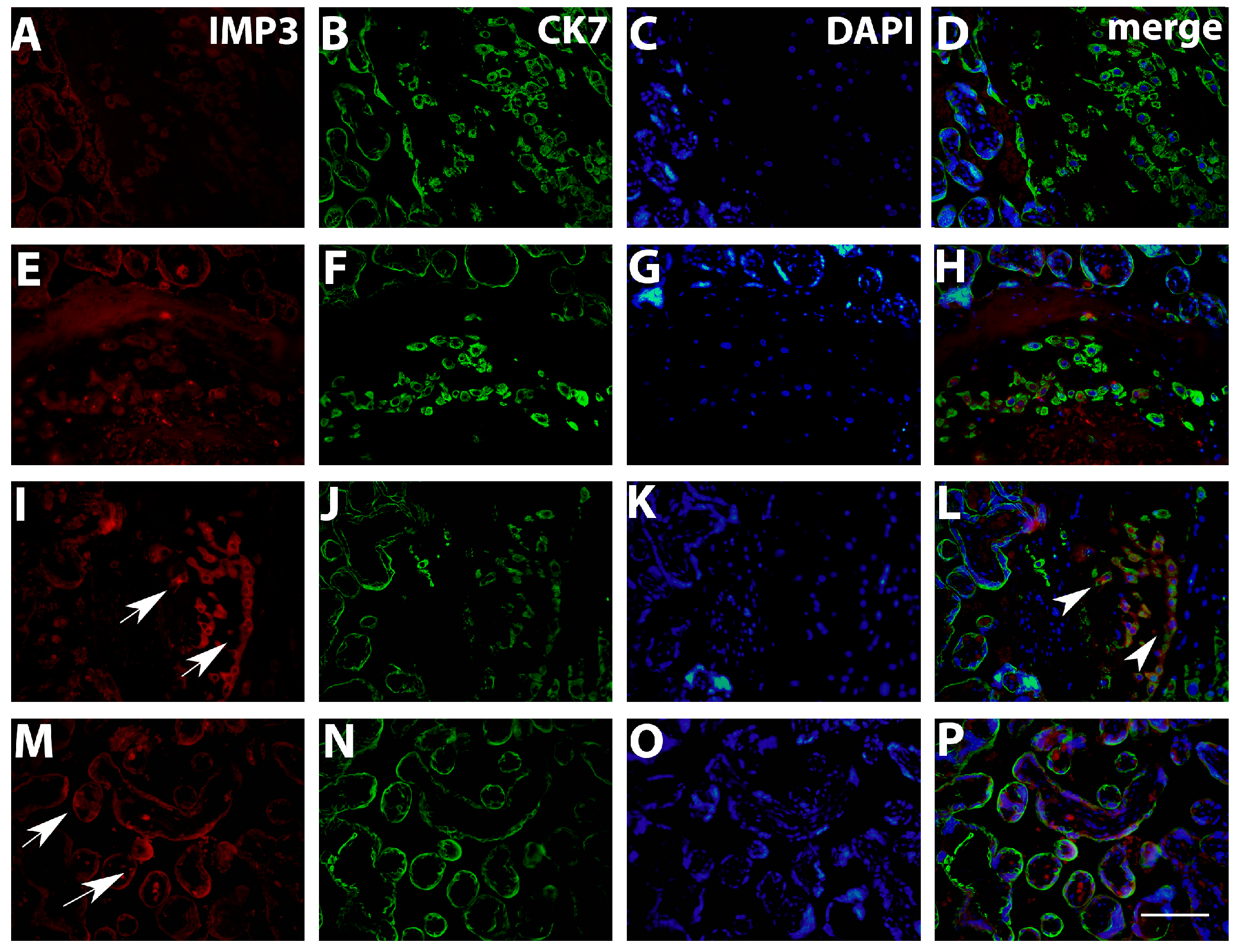
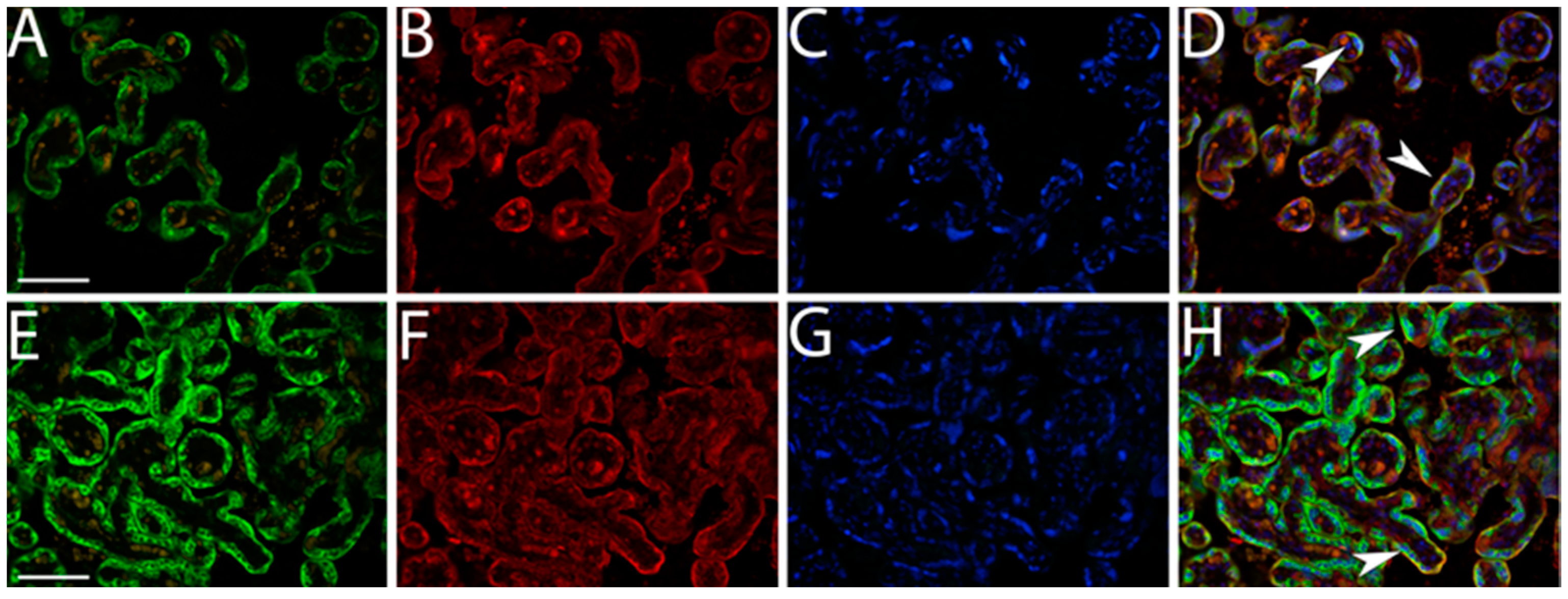
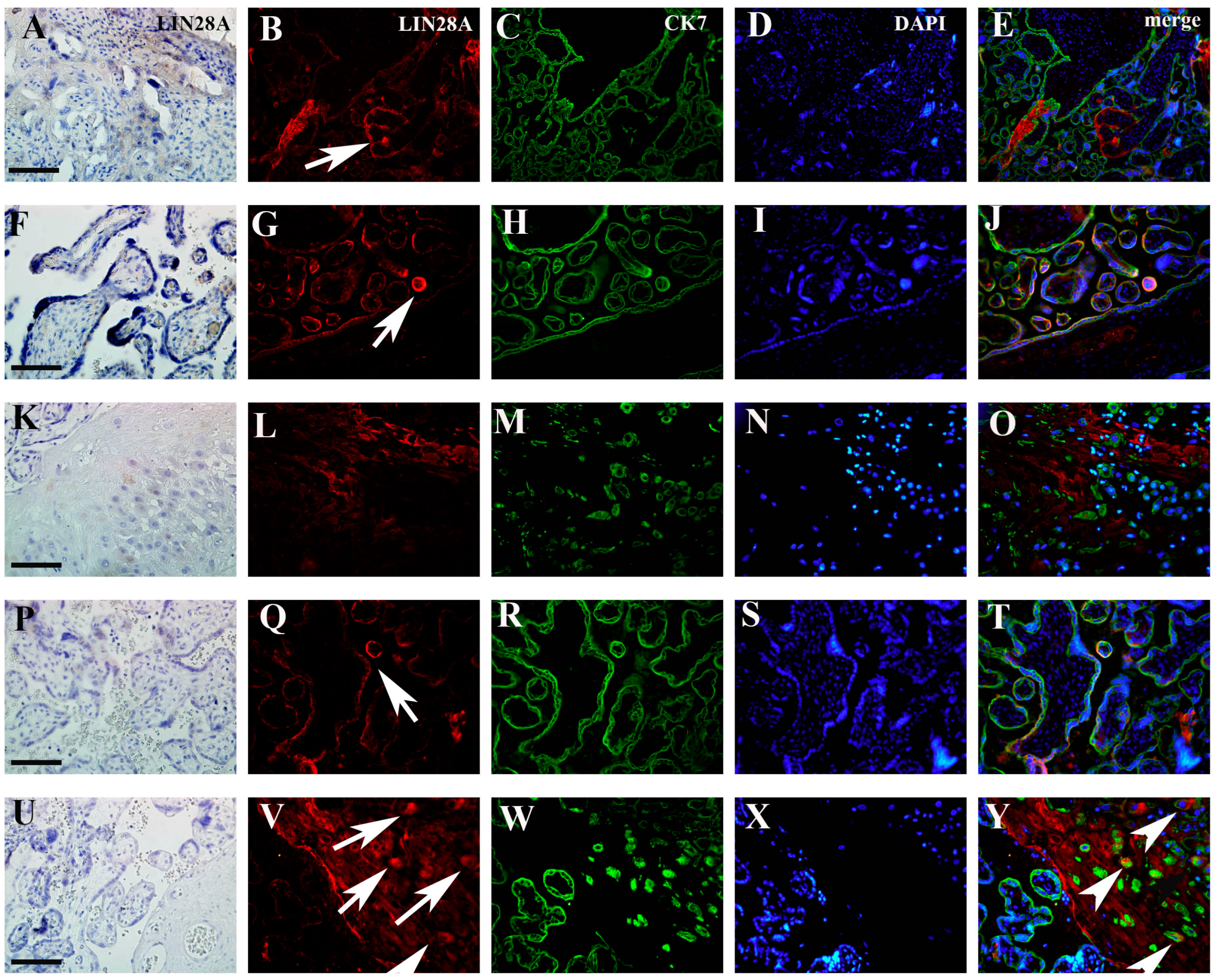
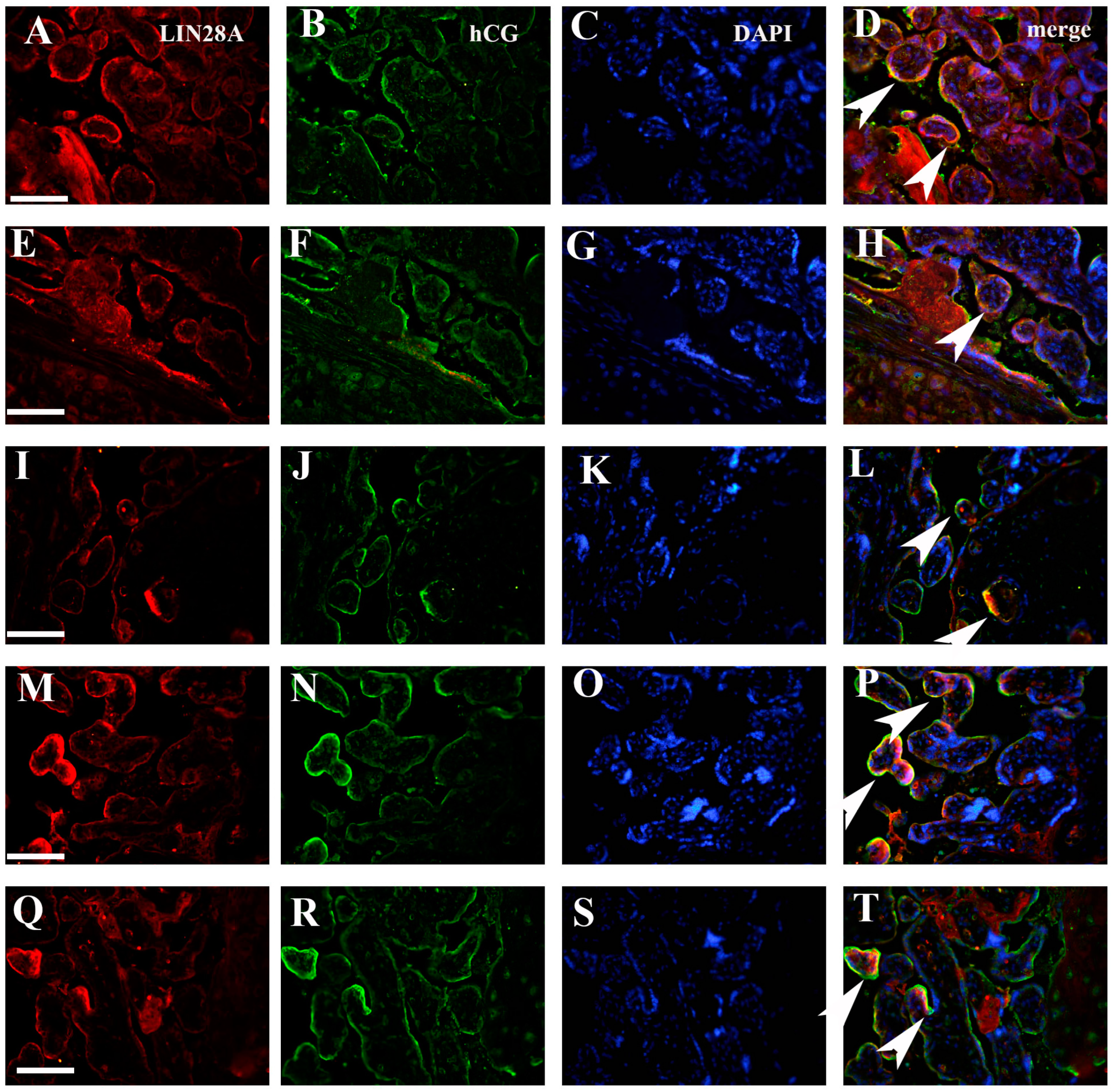
3. Results
3.1. Quantification IMP3 and LIN28A Expression in the EVT and VT of Placentas with PE with and without Severe Features Compared to That of Normal Healthy Pregnancies
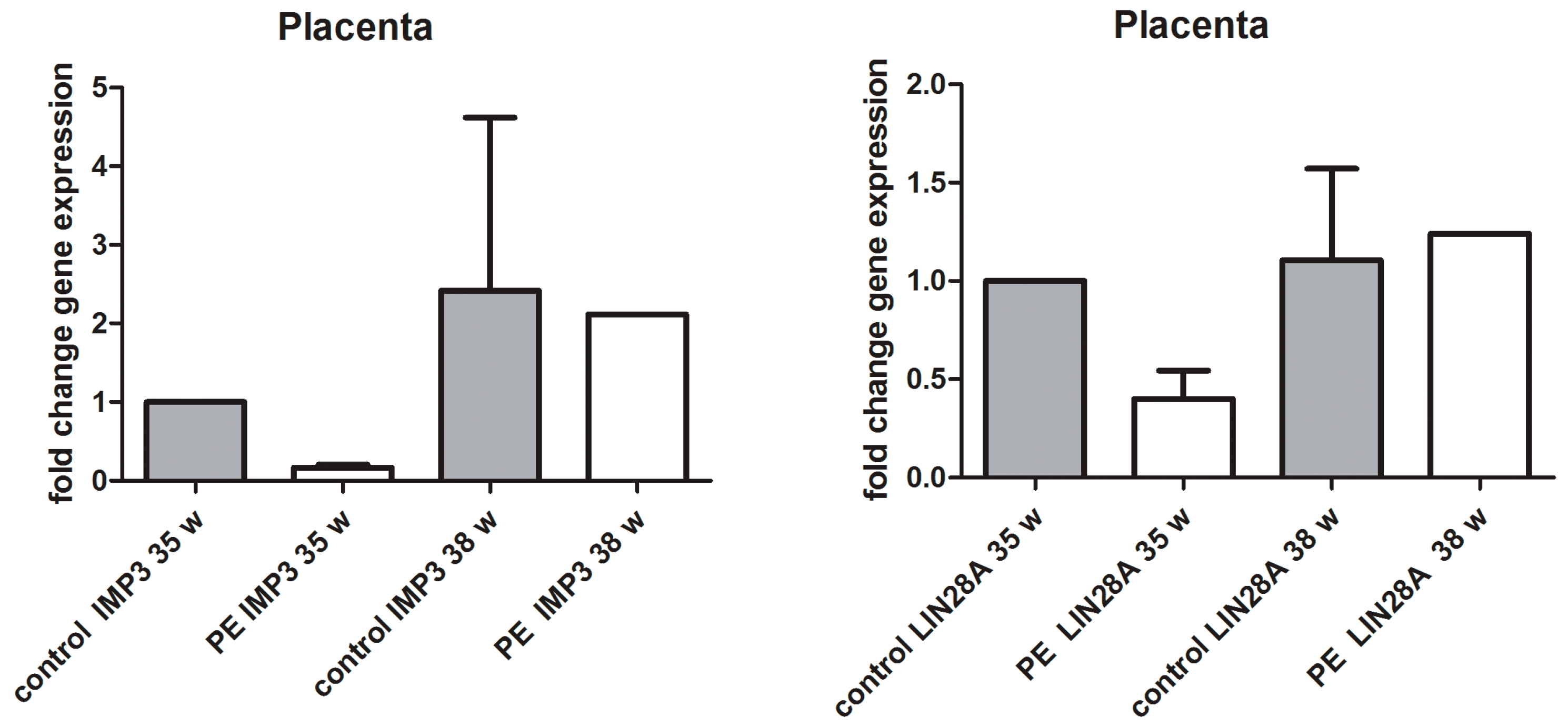
3.2. qPCR Analysis of IMP3 and LIN28A in Whole Placental Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baines, K.J.; Renaud, S.J. Transcription Factors That Regulate Trophoblast Development and Function. Prog. Mol. Biol. Transl. Sci. 2017, 145, 39–88. [Google Scholar] [CrossRef]
- Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2019, 21, 289. [Google Scholar] [CrossRef]
- Mendes, S.; Timoteo-Ferreira, F.; Almeida, H.; Silva, E. New Insights into the Process of Placentation and the Role of Oxidative Uterine Microenvironment. Oxidative Med. Cell. Longev. 2019, 2019, 9174521. [Google Scholar] [CrossRef]
- Salomon, C.; Yee, S.W.; Mitchell, M.D.; Rice, G.E. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed. Res. Int. 2014, 2014, 693157. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef]
- Burke, S.D.; Karumanchi, S.A. Spiral artery remodeling in preeclampsia revisited. Hypertension 2013, 62, 1013–1014. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Ellery, S.J.; Murthi, P.; Gatta, P.A.D.; May, A.K.; Davies-Tuck, M.L.; Kowalski, G.M.; Callahan, D.L.; Bruce, C.R.; Wallace, E.M.; Walker, D.W.; et al. The Effects of Early-Onset Pre-Eclampsia on Placental Creatine Metabolism in the Third Trimester. Int. J. Mol. Sci. 2020, 21, 806. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Kohan-Ghadr, H.R.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef]
- Doridot, L.; Miralles, F.; Barbaux, S.; Vaiman, D. Trophoblasts, invasion, and microRNA. Front. Genet. 2013, 4, 248. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Orendi, K.; Huppertz, B. Factors involved in regulating trophoblast fusion: Potential role in the development of preeclampsia. Placenta 2009, 30 (Suppl. A), S49–S54. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Q.; Lu, J.; Yang, G.; Tian, N.; Wang, X.; Tan, Y.; Tan, D. MSX2 Induces Trophoblast Invasion in Human Placenta. PLoS ONE 2016, 11, e0153656. [Google Scholar] [CrossRef]
- Ali, A.; Anthony, R.V.; Bouma, G.J.; Winger, Q.A. LIN28-let-7 axis regulates genes in immortalized human trophoblast cells by targeting the ARID3B-complex. FASEB J. 2019, 33, 12348–12363. [Google Scholar] [CrossRef]
- Gong, Y.; Woda, B.A.; Jiang, Z. Oncofetal protein IMP3, a new cancer biomarker. Adv. Anat. Pathol. 2014, 21, 191–200. [Google Scholar] [CrossRef]
- Szarvas, T.; vom Dorp, F.; Niedworok, C.; Melchior-Becker, A.; Fischer, J.W.; Singer, B.B.; Reis, H.; Bankfalvi, A.; Schmid, K.W.; Romics, I.; et al. High insulin-like growth factor mRNA-binding protein 3 (IMP3) protein expression is associated with poor survival in muscle-invasive bladder cancer. BJU Int. 2012, 110, E308–E317. [Google Scholar] [CrossRef]
- Hart, J.; Parab, M.; Mandich, D.; Cartun, R.W.; Ligato, S. IMP3 immunocytochemical staining increases sensitivity in the routine cytologic evaluation of biliary brush specimens. Diagn. Cytopathol. 2012, 40, 321–326. [Google Scholar] [CrossRef]
- Beljan Perak, R.; Durdov, M.G.; Capkun, V.; Ivcevic, V.; Pavlovic, A.; Soljic, V.; Peric, M. IMP3 can predict aggressive behaviour of lung adenocarcinoma. Diagn. Pathol. 2012, 7, 165. [Google Scholar] [CrossRef]
- Qin, R.; Zhou, J.; Chen, C.; Xu, T.; Yan, Y.; Ma, Y.; Zheng, Z.; Shen, Y.; Lu, Y.; Fu, D.; et al. LIN28 is involved in glioma carcinogenesis and predicts outcomes of glioblastoma multiforme patients. PLoS ONE 2014, 9, e86446. [Google Scholar] [CrossRef]
- Enriquez, V.A.; Cleys, E.R.; Da Silveira, J.C.; Spillman, M.A.; Winger, Q.A.; Bouma, G.J. High LIN28A Expressing Ovarian Cancer Cells Secrete Exosomes That Induce Invasion and Migration in HEK293 Cells. Biomed. Res. Int. 2015, 2015, 701390. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Chang, W.; Lu, X.; Wang, Y.L.; Wang, H.; Zhu, C.; Lin, H.Y.; Zhang, Y.; Zhou, J.; et al. Role of IGF2BP3 in trophoblast cell invasion and migration. Cell Death Dis. 2014, 5, e1025. [Google Scholar] [CrossRef]
- Seabrook, J.L.; Cantlon, J.D.; Cooney, A.J.; McWhorter, E.E.; Fromme, B.A.; Bouma, G.J.; Anthony, R.V.; Winger, Q.A. Role of LIN28A in mouse and human trophoblast cell differentiation. Biol. Reprod. 2013, 89, 95. [Google Scholar] [CrossRef]
- Chan, H.W.; Lappas, M.; Yee, S.W.; Vaswani, K.; Mitchell, M.D.; Rice, G.E. The expression of the let-7 miRNAs and Lin28 signalling pathway in human term gestational tissues. Placenta 2013, 34, 443–448. [Google Scholar] [CrossRef]
- Canfield, J.; Arlier, S.; Mong, E.F.; Lockhart, J.; VanWye, J.; Guzeloglu-Kayisli, O.; Schatz, F.; Magness, R.R.; Lockwood, C.J.; Tsibris, J.C.M.; et al. Decreased LIN28B in preeclampsia impairs human trophoblast differentiation and migration. FASEB J. 2019, 33, 2759–2769. [Google Scholar] [CrossRef]
- West, R.C.; McWhorter, E.S.; Ali, A.; Goetzman, L.N.; Russ, J.E.; Gonzalez-Berrios, C.L.; Anthony, R.V.; Bouma, G.J.; Winger, Q.A. HMGA2 is regulated by LIN28 and BRCA1 in human placental cells. Biol. Reprod. 2019, 100, 227–238. [Google Scholar] [CrossRef]
- Kosovic, I.; Prusac, I.K.; Berkovic, A.; Marusic, J.; Mimica, M.; Tomas, S.Z. Expression of EGF, EGFR, and proliferation in placentas from pregnancies complicated with preeclampsia. Hypertens. Pregnancy 2017, 36, 16–20. [Google Scholar] [CrossRef]
- Orlovic, M.; Tomic, V.; Vukojevic, K.; Hudic, I.; Mandic, V.; Azinovic, I.; Soldo, D.; Kajic, M.; Soljic, V. Decreased expression of MMP-9 in CD8(+) cells in placenta with severe preeclampsia. Biotech. Histochem. 2017, 92, 288–296. [Google Scholar] [CrossRef]
- Soljic, V.; Barbaric, M.; Vukoja, M.; Curlin, M.; Orlovic Vlaho, M.; Cerni Obrdalj, E.; Lasic Arapovic, L.; Bevanda Glibo, D.; Vukojevic, K. Decreased Expression of Cytotoxic Proteins in Decidual CD8(+) T Cells in Preeclampsia. Biology 2021, 10, 1037. [Google Scholar] [CrossRef]
- Moser, G.; Huppertz, B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta 2017, 56, 19–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Long, Y.; Zhang, Y.; Chen, R.; Shi, J.; Chen, J. circRNA N6-methyladenosine methylation in preeclampsia and the potential role of N6-methyladenosine-modified circPAPPA2 in trophoblast invasion. Sci. Rep. 2021, 11, 24357. [Google Scholar] [CrossRef]
- Chen, L.L.; Li, Y.Q.; Kang, Z.H.; Zhang, X.; Gu, S.Y.; Wang, N.; Shen, X.Y. Blocking the interaction between circTNRC18 and LIN28A promotes trophoblast epithelial-mesenchymal transformation and alleviates preeclampsia. Mol. Cell. Endocrinol. 2024, 579, 112073. [Google Scholar] [CrossRef]
- Pan, X.; Noguchi, S.; Ando, M.; Nishimura, T.; Tomi, M. MicroRNA-126 suppresses the invasion of trophoblast-model JEG-3 cells by targeting LIN28A. Biochem. Biophys. Res. Commun. 2021, 545, 132–137. [Google Scholar] [CrossRef]
- Wheeler, S.M.; Myers, S.O.; Swamy, G.K.; Myers, E.R. Estimated Prevalence of Risk Factors for Preeclampsia Among Individuals Giving Birth in the US in 2019. JAMA Netw. Open 2022, 5, e2142343. [Google Scholar] [CrossRef]
- Bustan-Nahumson, M.; Bornstein, S.; Feldstein, O.; Levy, M.; Schreiber, L.; Bar, J.; Kovo, M.; Weiner, E. Preeclampsia in Different Maternal Age Groups-Is There an Association with Pregnancy Outcomes and Placental Pathology? Reprod. Sci. 2020, 27, 1879–1887. [Google Scholar] [CrossRef]
- Mohamedain, A.; Rayis, D.A.; AlHabardi, N.; Adam, I. Association between previous spontaneous abortion and preeclampsia: A case-control study. BMC Pregnancy Childbirth 2022, 22, 715. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Strauss, R.A.; Westreich, D.J.; Thorp, J.M.; Stamilio, D.M.; Grantz, K.L. Adverse maternal and neonatal outcomes among women with preeclampsia with severe features < 34 weeks gestation with versus without comorbidity. Pregnancy Hypertens. 2020, 20, 75–82. [Google Scholar] [CrossRef]
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef]
- Coppage, K.H.; Polzin, W.J. Severe preeclampsia and delivery outcomes: Is immediate cesarean delivery beneficial? Am. J. Obstet. Gynecol. 2002, 186, 921–923. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Guida, J.P.; Simoes, M.; Marangoni-Junior, M.; Cralcev, C.; Santos, J.C.; Dias, T.Z.; Luz, A.G.; Costa, M.L. Can pre-eclampsia explain higher cesarean rates in the different groups of Robson’s classification? Int. J. Gynaecol. Obstet. 2021, 152, 339–344. [Google Scholar] [CrossRef]


| Control 1 (n = 10) | PE with Severe Features (n = 10) | Control 2 (n = 10) | PE without Severe Fetures (n = 10) | p Value | |
|---|---|---|---|---|---|
| Maternal age (years) mean ± SD | 28.06 ± 5.89 | 26.50 ± 3.51 | 27.52 ± 5.19 | 31.30 ± 5.73 | 0.390 |
| Gestational age (weeks) median (IqR) | 35 (34–38) | 34 (33–38) | 39 (38–41) | 38 (34–40) | <0.001 |
| Systolic RR (mmHg) mean ± SD | 115 ± 5.13 | 178 ± 19.05 | 119 ± 5.82 | 148 ± 3.45 | <0.001 |
| Diastolic RR (mmHg) median (IqR) | 73 (60–85) | 123 (110–140) | 77 (65–80) | 88 (86–100) | <0.001 |
| Birth weight (grams) median (IqR) | 2327 (2132–3218) | 1818 (1251–2205) | 3320 (2350–4515) | 3320 (1815–3960) | <0.001 |
| Cesarean deliveries (%) | 2 (20) | 10 (100) | 1 (10) | 2 (20) | <0.001 |
| Body mass index (BMI) mean ± SD | 24.93 ± 4.78 | 23.57 ± 3.42 | 25.64 ± 4.36 | 23.42 ± 2.36 | 0.06 |
| Intrauterin growth restriction (IUGR) (%) | 1 (10) | 7 (70) | 0 (0) | 1 (10) | <0.001 |
| Postpartal complications (%) | 0 (0) | 7(70) | 0 (0) | 1 (10) | <0.001 |
| Antibody | Dilution | Host | Cellular Localization | Developer |
|---|---|---|---|---|
| IMP3 | 1:200 | Mouse | Cytoplasm | Dako, Glostrup, Denmark M362629-2 |
| IMP3 | 1:250 | Rabbit | Cytoplasm | Abcam, Cambridge, UK ab179807 |
| LIN28A | 1:800 | Mouse | Cytoplasm | Cell Signaling Technology Inc, Danvers, MA, USA CT-5930S |
| LIN28A | 1:800 | Rabbit | Cytoplasm | Cell Signaling Technology Inc, Danvers, MA, USA CT-3978S |
| Cytokeratin 7 | 1:300 | Mouse | Cytoplasm | Dako, Glostrup, Denmark M7108 |
| Cytokeratin 7 | 1.100 | Rabbit | Cytoplasm | Ventana Medical Systems Inc., Tucson, AZ, USA SP52 |
| Anti-chorionic gonadotropin | 1:50 | Rabbit | Cytoplasm | Sigma Aldrich, Sant Louis, MI, USA C8534 |
| Intensity of staining | IMP3 | p | LIN28A | p | ||
| VT | VT | |||||
| PE | CG | PE | CG | |||
| 1.27 | 2.33 | 0.002 ** | 1 | 1 | 0.42 | |
| Percentage of positive cells | IMP3 | p | LIN28A | p | ||
| EVT | EVT | |||||
| PE | CG | PE | CG | |||
| 26.10 | 63.35 | 0.0002 *** | 45.2 | 51.1 | 0.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaric, M.; Vukojevic, K.; Kolobaric, A.; Orlovic Vlaho, M.; Kresic, T.; Soljic, V. Expression of IMP3 and LIN28A RNA-Binding Proteins in Placentas of Patients with Pre-Eclampsia with and without Severe Features. Biomedicines 2024, 12, 879. https://doi.org/10.3390/biomedicines12040879
Barbaric M, Vukojevic K, Kolobaric A, Orlovic Vlaho M, Kresic T, Soljic V. Expression of IMP3 and LIN28A RNA-Binding Proteins in Placentas of Patients with Pre-Eclampsia with and without Severe Features. Biomedicines. 2024; 12(4):879. https://doi.org/10.3390/biomedicines12040879
Chicago/Turabian StyleBarbaric, Maja, Katarina Vukojevic, Anita Kolobaric, Martina Orlovic Vlaho, Tanja Kresic, and Violeta Soljic. 2024. "Expression of IMP3 and LIN28A RNA-Binding Proteins in Placentas of Patients with Pre-Eclampsia with and without Severe Features" Biomedicines 12, no. 4: 879. https://doi.org/10.3390/biomedicines12040879
APA StyleBarbaric, M., Vukojevic, K., Kolobaric, A., Orlovic Vlaho, M., Kresic, T., & Soljic, V. (2024). Expression of IMP3 and LIN28A RNA-Binding Proteins in Placentas of Patients with Pre-Eclampsia with and without Severe Features. Biomedicines, 12(4), 879. https://doi.org/10.3390/biomedicines12040879







