ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PMN Leukocyte Isolation
2.3. Incubations for 5-LOX Product Synthesis in Cells
2.4. Calcium Flux Assay
2.5. PMNLs Adhesion Assessment
2.6. Microscopy
2.7. Statistics
3. Results
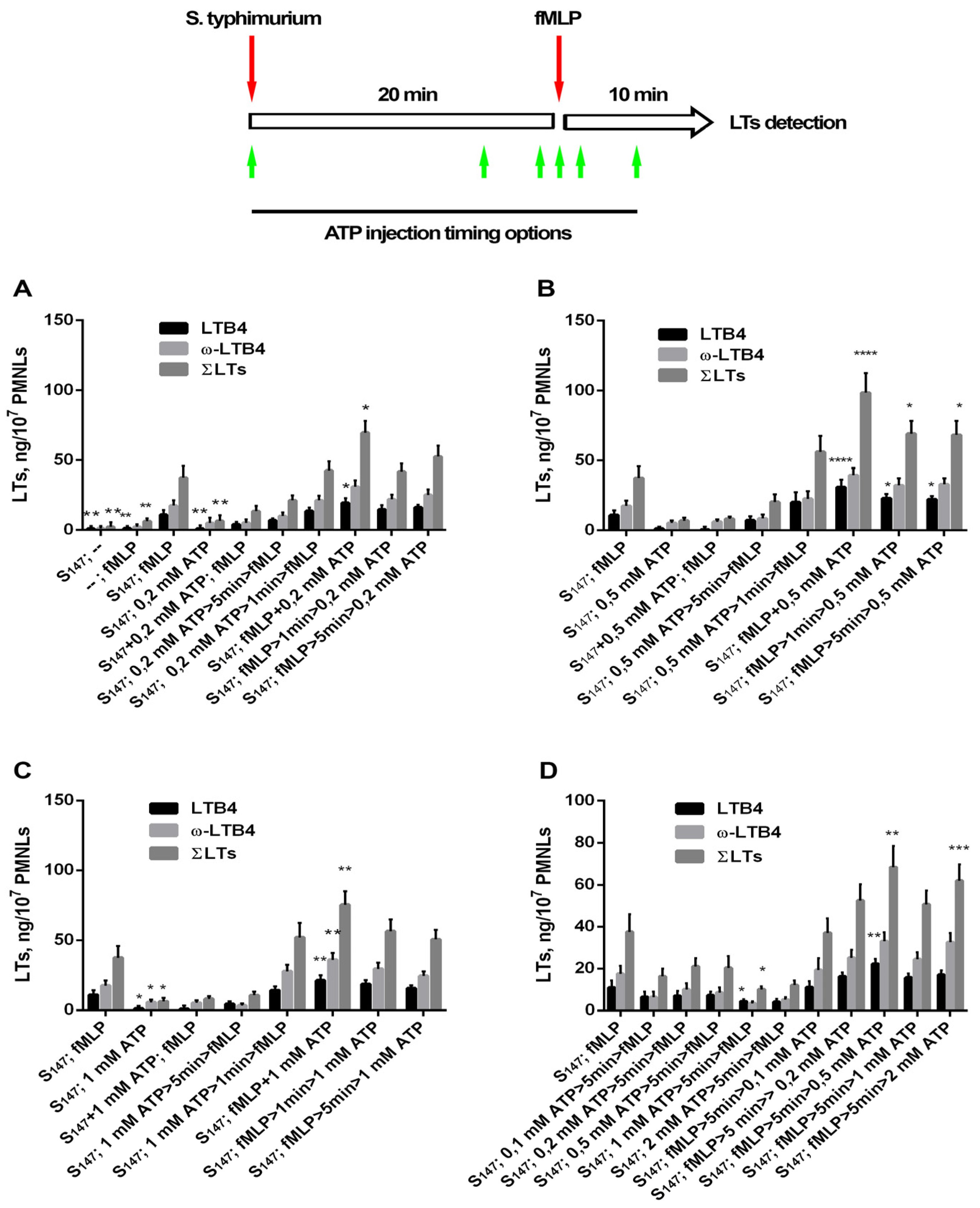
3.1. Extracellular ATP (eATP) Facilitated Formyl Peptide-Induced LT Synthesis
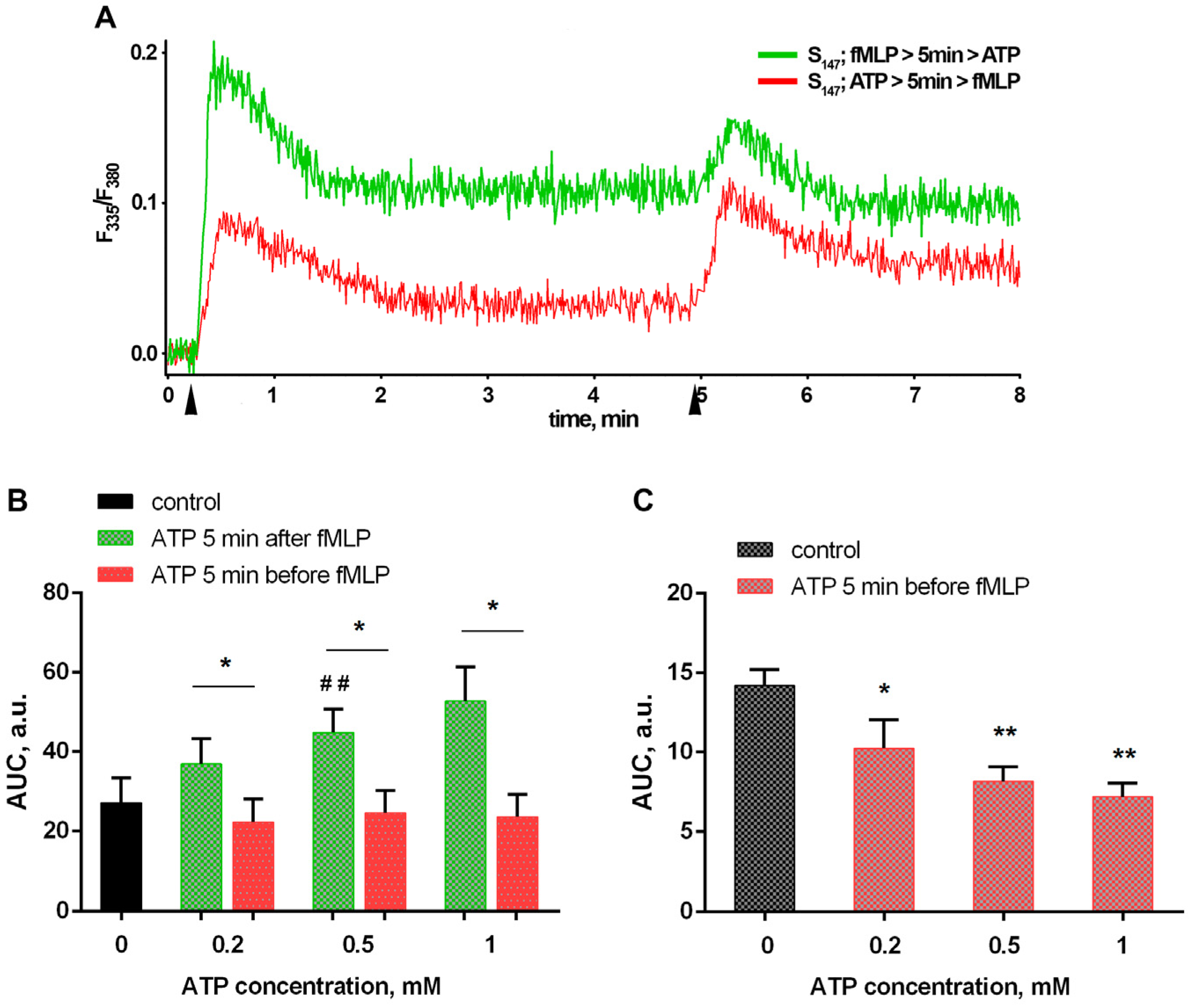
3.2. Bacteria and eATP Modulate Ca2+ Fluxes in Neutrophils Exposed to the Bacterium Salmonella typhimurium
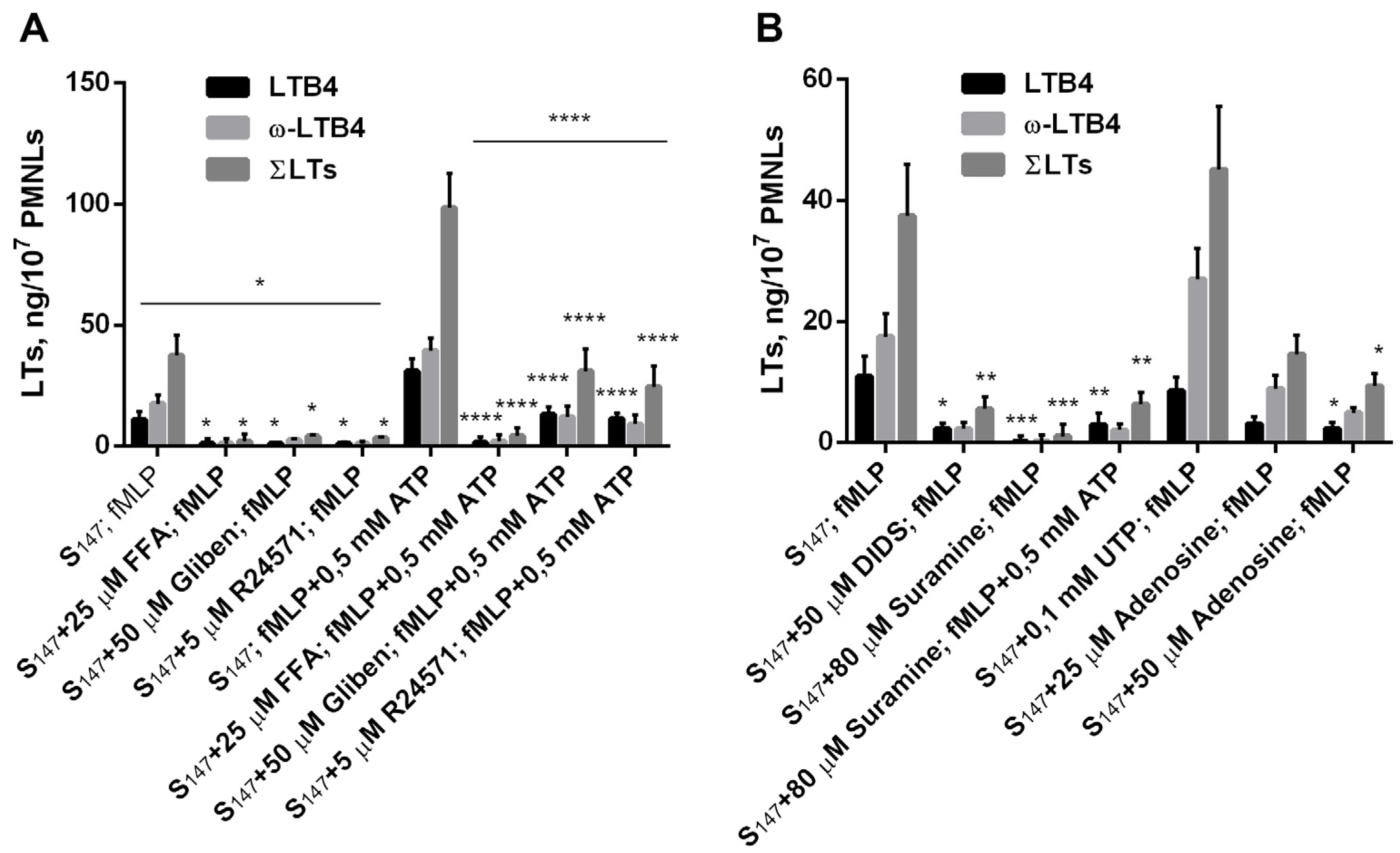
3.3. Purinergic ATP Signaling Influences fMLP-Induced LT Synthesis in Neutrophils Exposed to the Bacterium Salmonella typhimurium
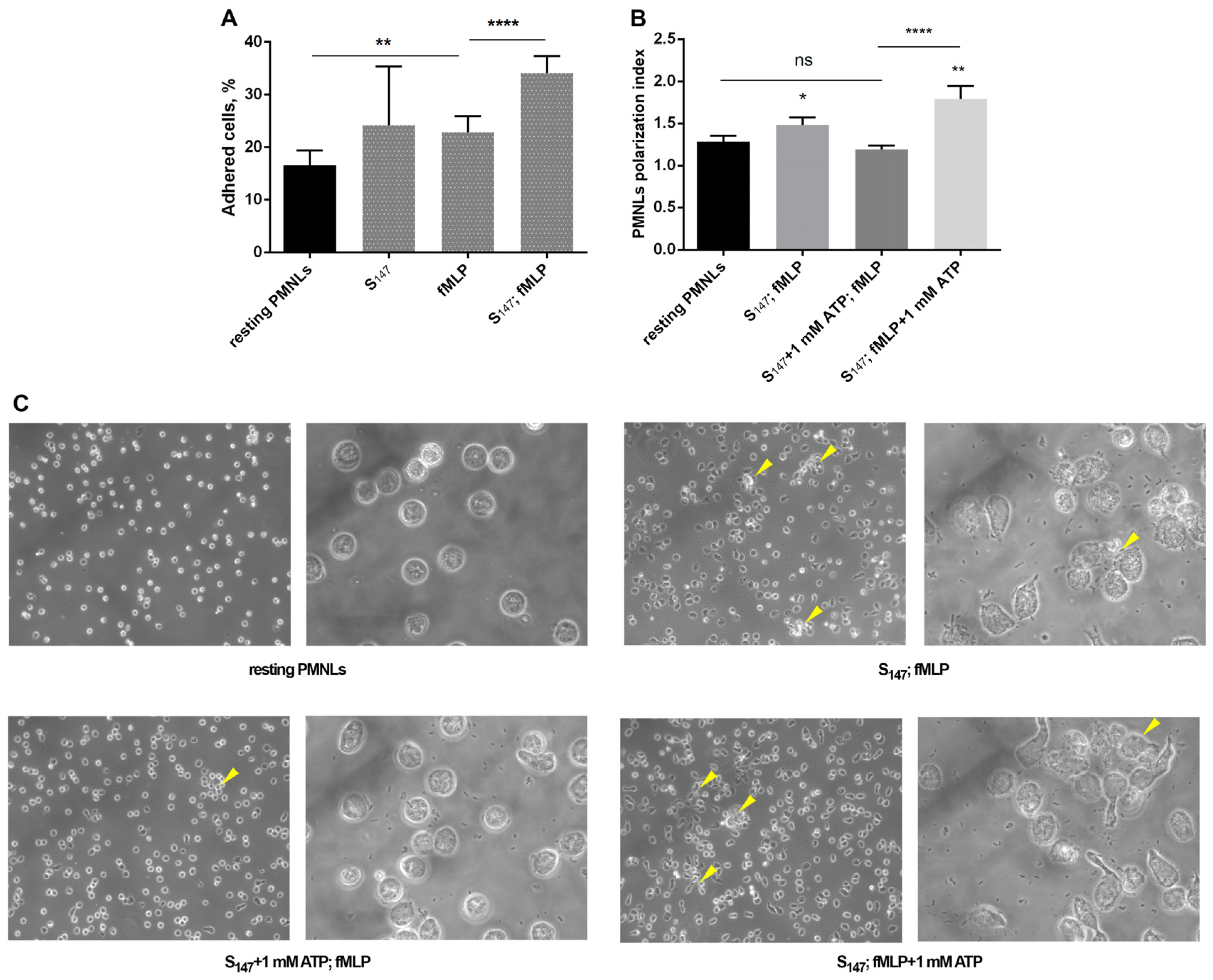
3.4. eATP and Adhesive Properties of Neutrophils
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metschnikoff, E. Lecture on Phagocytosis and Immunity. Br. Med. J. 1891, 1, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Metzemaekers, M.; Malengier-Devlies, B.; Gouwy, M.; De Somer, L.; Cunha, F.Q.; Opdenakker, G.; Proost, P. Fast and furious: The neutrophil and its armamentarium in health and disease. Med. Res. Rev. 2023, 43, 1537–1606. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Ye, R.D. The N-formyl peptide receptor: A model for the study of chemoattractant receptor structure and function. Pharmacol. Ther. 1997, 74, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Bhattacharya, S.; Clemens, R.A.; Dinauer, M.C. Molecular regulation of neutrophil swarming in health and disease: Lessons from the phagocyte oxidase. iScience 2023, 26, 108034. [Google Scholar] [CrossRef] [PubMed]
- Showell, H.J.; Freer, R.J.; Zigmond, S.H.; Schiffmann, E.; Aswanikumar, S.; Corcoran, B.; Becker, E.L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J. Exp. Med. 1976, 143, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Johri, S.; Chakraborty, K. Immunomodulatory role of mitochondrial DAMPs: A missing link in pathology? FEBS J. 2023, 290, 4395–4418. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, D. Purinergic Regulation of Neutrophil Function. Front. Immunol. 2018, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef]
- Spari, D.; Beldi, G. Extracellular ATP as an Inter-Kingdom Signaling Molecule: Release Mechanisms by Bacteria and Its Implication on the Host. Int. J. Mol. Sci. 2020, 21, 5590. [Google Scholar] [CrossRef]
- Dixit, A.; Cheema, H.; George, J.; Iyer, S.; Dudeja, V.; Dawra, R.; Saluja, A.K. Extracellular release of ATP promotes systemic inflammation during acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G463–G475. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2017, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rubenich, D.S.; de Souza, P.O.; Omizzollo, N.; Lenz, G.S.; Sevigny, J.; Braganhol, E. Neutrophils: Fast and furious—The nucleotide pathway. Purinergic Signal. 2021, 17, 371–383. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Y.; Sumi, Y.; Li, A.; To, U.K.; Elkhal, A.; Inoue, Y.; Woehrle, T.; Zhang, Q.; Hauser, C.; et al. Purinergic signaling: A fundamental mechanism in neutrophil activation. Sci. Signal. 2010, 3, ra45. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Ectonucleoside triphosphate diphosphohydrolases and ecto-5′-nucleotidase in purinergic signaling: How the field developed and where we are now. Purinergic Signal. 2021, 17, 117–125. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Lammas, D.A.; Stober, C.; Harvey, C.J.; Kendrick, N.; Panchalingam, S.; Kumararatne, D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 1997, 7, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lei, L.; Collins, J.W.M.; Briones, M.; Ma, L.; Sturdevant, G.L.; Su, H.; Kashyap, A.K.; Dorward, D.; Bock, K.W.; et al. Chlamydia evasion of neutrophil host defense results in NLRP3 dependent myeloid-mediated sterile inflammation through the purinergic P2X7 receptor. Nat. Commun. 2021, 12, 5454. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Laskay, T. Purinergic Enhancement of Anti-Leishmanial Effector Functions of Neutrophil Granulocytes. Front. Immunol. 2021, 12, 747049. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Schlatterer, K.; Beck, C.; Peschel, A.; Kretschmer, D. Formyl-Peptide Receptor Activation Enhances Phagocytosis of Community-Acquired Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 2020, 221, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Poplimont, H.; Georgantzoglou, A.; Boulch, M.; Walker, H.A.; Coombs, C.; Papaleonidopoulou, F.; Sarris, M. Neutrophil Swarming in Damaged Tissue Is Orchestrated by Connexins and Cooperative Calcium Alarm Signals. Curr. Biol. 2020, 30, 2761–2776.e2767. [Google Scholar] [CrossRef] [PubMed]
- Reategui, E.; Jalali, F.; Khankhel, A.H.; Wong, E.; Cho, H.; Lee, J.; Serhan, C.N.; Dalli, J.; Elliott, H.; Irimia, D. Microscale arrays for the profiling of start and stop signals coordinating human-neutrophil swarming. Nat. Biomed. Eng. 2017, 1, 94. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Kienle, K.; Glaser, K.M.; Eickhoff, S.; Mihlan, M.; Knopper, K.; Reategui, E.; Epple, M.W.; Gunzer, M.; Baumeister, R.; Tarrant, T.K.; et al. Neutrophils self-limit swarming to contain bacterial growth in vivo. Science 2021, 372, 1303. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Human neutrophils not equal murine neutrophils: Does it matter? Immunol. Rev. 2023, 314, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, M.; Kubes, P. Neutrophils in host defense, healing, and hypersensitivity: Dynamic cells within a dynamic host. J. Allergy Clin. Immunol. 2023, 151, 634–655. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, D.A.; Zagryagskaya, A.N.; Pushkareva, M.A.; Bachschmid, M.; Peters-Golden, M.; Werz, O.; Steinhilber, D.; Sud’ina, G.F. Cholesterol and its anionic derivatives inhibit 5-lipoxygenase activation in polymorphonuclear leukocytes and MonoMac6 cells. FEBS J. 2006, 273, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Golenkina, E.A.; Galkina, S.I.; Pletjushkina, O.; Chernyak, B.; Gaponova, T.V.; Romanova, Y.M.; Sud’ina, G.F. Gram-Negative Bacteria Salmonella typhimurium Boost Leukotriene Synthesis Induced by Chemoattractant fMLP to Stimulate Neutrophil Swarming. Front. Pharmacol. 2021, 12, 814113. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.F.; Campbell, D.E.; Douglas, S.D. O-phenylenediamine oxidation by phorbol myristate acetate-stimulated human polymorphonuclear leukocytes: Characterization of two distinct oxidative mechanisms. Clin. Immunol. Immunopathol. 1987, 42, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gijon, M.A.; Leslie, C.C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 1999, 65, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Radmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Luo, M.; Jones, S.M.; Peters-Golden, M.; Brock, T.G. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc. Natl. Acad. Sci. USA 2003, 100, 12165–12170. [Google Scholar] [CrossRef]
- Axtell, R.A.; Sandborg, R.R.; Smolen, J.E.; Ward, P.A.; Boxer, L.A. Exposure of human neutrophils to exogenous nucleotides causes elevation in intracellular calcium, transmembrane calcium fluxes, and an alteration of a cytosolic factor resulting in enhanced superoxide production in response to FMLP and arachidonic acid. Blood 1990, 75, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Fodor, S.; Mocsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Da Silva-Santos, J.E.; Santos-Silva, M.C.; Cunha Fde, Q.; Assreuy, J. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J. Pharmacol. Exp. Ther. 2002, 300, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T.; Mager, A.; Kuper, N.; Karcher, C.; Weissmuller, T.; Boengler, K.; Schulz, R.; Robson, S.C.; Colgan, S.P. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 2006, 99, 1100–1108. [Google Scholar] [CrossRef]
- Dahl, G.; Qiu, F.; Wang, J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 2013, 75, 583–593. [Google Scholar] [CrossRef]
- Sandoval, A.J.; Riquelme, J.P.; Carretta, M.D.; Hancke, J.L.; Hidalgo, M.A.; Burgos, R.A. Store-operated calcium entry mediates intracellular alkalinization, ERK1/2, and Akt/PKB phosphorylation in bovine neutrophils. J. Leukoc. Biol. 2007, 82, 1266–1277. [Google Scholar] [CrossRef]
- Barr, T.P.; Albrecht, P.J.; Hou, Q.; Mongin, A.A.; Strichartz, G.R.; Rice, F.L. Air-stimulated ATP release from keratinocytes occurs through connexin hemichannels. PLoS ONE 2013, 8, e56744. [Google Scholar] [CrossRef]
- Capuozzo, E.; Verginelli, D.; Crifo, C.; Salerno, C. Effects of calmodulin antagonists on calcium pump and cytosolic calcium level in human neutrophils. Biochim. Biophys. Acta 1997, 1357, 123–127. [Google Scholar] [CrossRef]
- Nelson, G.A.; Andrews, M.L.; Karnovsky, M.J. Control of erythrocyte shape by calmodulin. J. Cell Biol. 1983, 96, 730–735. [Google Scholar] [CrossRef]
- Ma, W.; Hui, H.; Pelegrin, P.; Surprenant, A. Pharmacological Characterization of Pannexin-1 Currents Expressed in Mammalian Cells. J. Pharmacol. Exp. Ther. 2009, 328, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Hasko, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Qin, J.S.; Roediger, B.; Wang, Y.; Jain, R.; Cavanagh, L.L.; Smith, A.L.; Jones, C.A.; de Veer, M.; Grimbaldeston, M.A.; et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Investig. Dermatol. 2011, 131, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.O.; de Souza, P.O.; Haas, C.B.; da Silveira, F.; Mohr, K.R.; Bertoni, A.P.S.; Soares, M.S.; Azambuja, J.H.; Pra, M.D.; da Cruz, L.L.P.; et al. ATPergic signaling disruption in human sepsis as a potential source of biomarkers for clinical use. Clin. Exp. Med. 2023, 23, 3651–3662. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Woehrle, T.; Chen, Y.; Bao, Y.; Li, X.; Yao, Y.; Inoue, Y.; Tanaka, H.; Junger, W.G. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock 2014, 42, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 2014, 5, e1102. [Google Scholar] [CrossRef]
- Ledderose, C.; Valsami, E.A.; Newhams, M.; Elevado, M.J.; Novak, T.; Randolph, A.G.; Junger, W.G. ATP breakdown in plasma of children limits the antimicrobial effectiveness of their neutrophils. Purinergic Signal. 2023, 19, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Csoka, B.; Nemeth, Z.H.; Toro, G.; Idzko, M.; Zech, A.; Koscso, B.; Spolarics, Z.; Antonioli, L.; Cseri, K.; Erdelyi, K.; et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015, 29, 3626–3637. [Google Scholar] [CrossRef]
- Ren, H.; Teng, Y.; Tan, B.; Zhang, X.; Jiang, W.; Liu, M.; Jiang, W.; Du, B.; Qian, M. Toll-like receptor-triggered calcium mobilization protects mice against bacterial infection through extracellular ATP release. Infect. Immun. 2014, 82, 5076–5085. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kudo, F.; Nishiguchi, N.; Mizuike, R.; Sato, H.; Ito, K.; Nakano, M.; Ito, K. Neutrophil phagocytosis is down-regulated by nucleotides until encounter with pathogens. Immunol. Lett. 2012, 144, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ledderose, C.; Seier, T.; Graf, A.F.; Brix, B.; Chong, E.; Junger, W.G. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J. Biol. Chem. 2014, 289, 26794–26803. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ledderose, C.; Slubowski, C.J.; Fakhari, M.; Sumi, Y.; Sueyoshi, K.; Bezler, A.K.; Aytan, D.; Arbab, M.; Junger, W.G. Frontline Science: Escherichia coli use LPS as decoy to impair neutrophil chemotaxis and defeat antimicrobial host defense. J. Leukoc. Biol. 2019, 106, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Petit-Jentreau, L.; Jouvion, G.; Charles, P.; Majlessi, L.; Gicquel, B.; Tailleux, L. Ecto-5′-Nucleotidase (CD73) Deficiency in Mycobacterium tuberculosis-Infected Mice Enhances Neutrophil Recruitment. Infect. Immun. 2015, 83, 3666–3674. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Campbell, G.; Satchell, D.; Smythe, A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970, 40, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S172–S181. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Munoz-Planillo, R.; Liu, Q.; Nunez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef]
- Carminita, E.; Crescence, L.; Brouilly, N.; Altie, A.; Panicot-Dubois, L.; Dubois, C. DNAse-dependent, NET-independent pathway of thrombus formation in vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2100561118. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, T.; Provost, P.; Persson, B.; Radmark, O. The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J. Biol. Chem. 2000, 275, 38787–38793. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Klemm, J.; Samuelsson, B.; Radmark, O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. USA 2000, 97, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Zarini, S.; Gijon, M.A.; Folco, G.; Murphy, R.C. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J. Biol. Chem. 2006, 281, 10134–10142. [Google Scholar] [CrossRef]
- Glaser, K.M.; Mihlan, M.; Lammermann, T. Positive feedback amplification in swarming immune cell populations. Curr. Opin. Cell Biol. 2021, 72, 156–162. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golenkina, E.A.; Viryasova, G.M.; Galkina, S.I.; Iakushkina, I.V.; Gaponova, T.V.; Romanova, Y.M.; Sud’ina, G.F. ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion. Biomedicines 2024, 12, 1184. https://doi.org/10.3390/biomedicines12061184
Golenkina EA, Viryasova GM, Galkina SI, Iakushkina IV, Gaponova TV, Romanova YM, Sud’ina GF. ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion. Biomedicines. 2024; 12(6):1184. https://doi.org/10.3390/biomedicines12061184
Chicago/Turabian StyleGolenkina, Ekaterina A., Galina M. Viryasova, Svetlana I. Galkina, Iuliia V. Iakushkina, Tatjana V. Gaponova, Yulia M. Romanova, and Galina F. Sud’ina. 2024. "ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion" Biomedicines 12, no. 6: 1184. https://doi.org/10.3390/biomedicines12061184
APA StyleGolenkina, E. A., Viryasova, G. M., Galkina, S. I., Iakushkina, I. V., Gaponova, T. V., Romanova, Y. M., & Sud’ina, G. F. (2024). ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion. Biomedicines, 12(6), 1184. https://doi.org/10.3390/biomedicines12061184






