Sodium Houttuyfonate Prevents Seizures and Neuronal Cell Loss by Maintaining Glutamatergic System Stability in Male Rats with Kainic Acid-Induced Seizures
Abstract
:1. Introduction
2. Materials and Methods
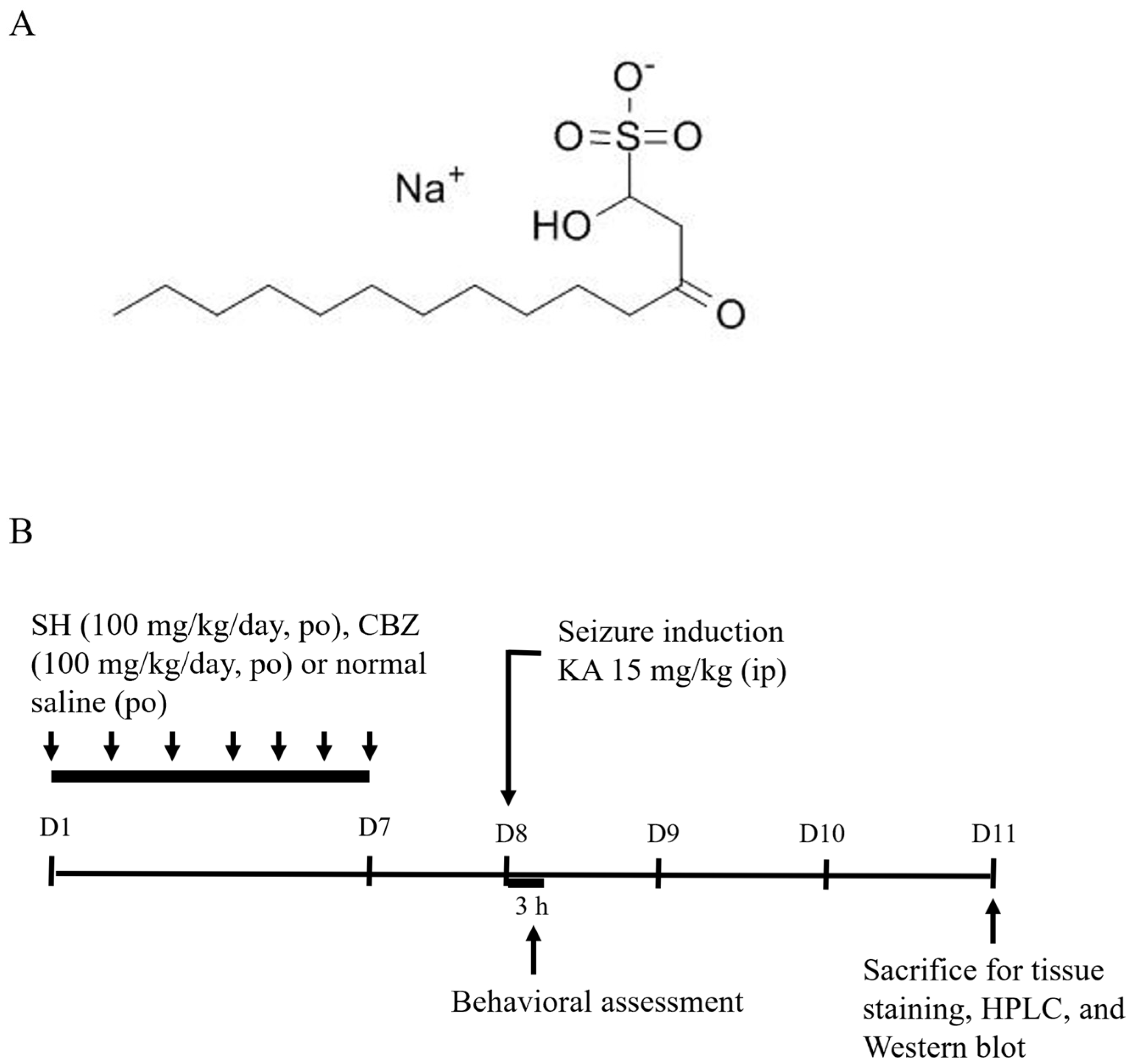
2.1. Drugs and Chemicals
2.2. Animals
2.3. Seizure Model and Drug Treatment
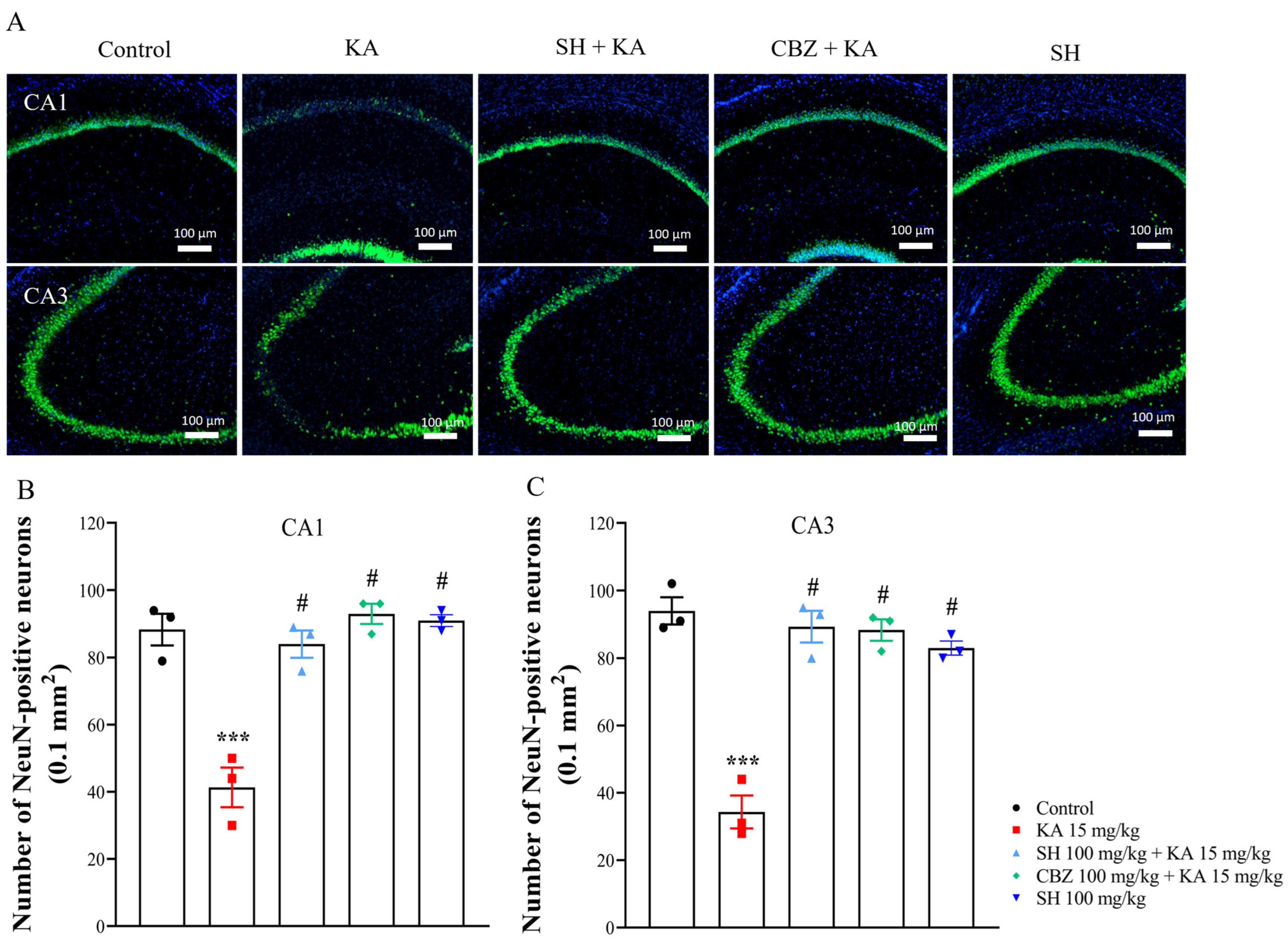
2.4. Nissl Staining and NeuN Immunofluorescence
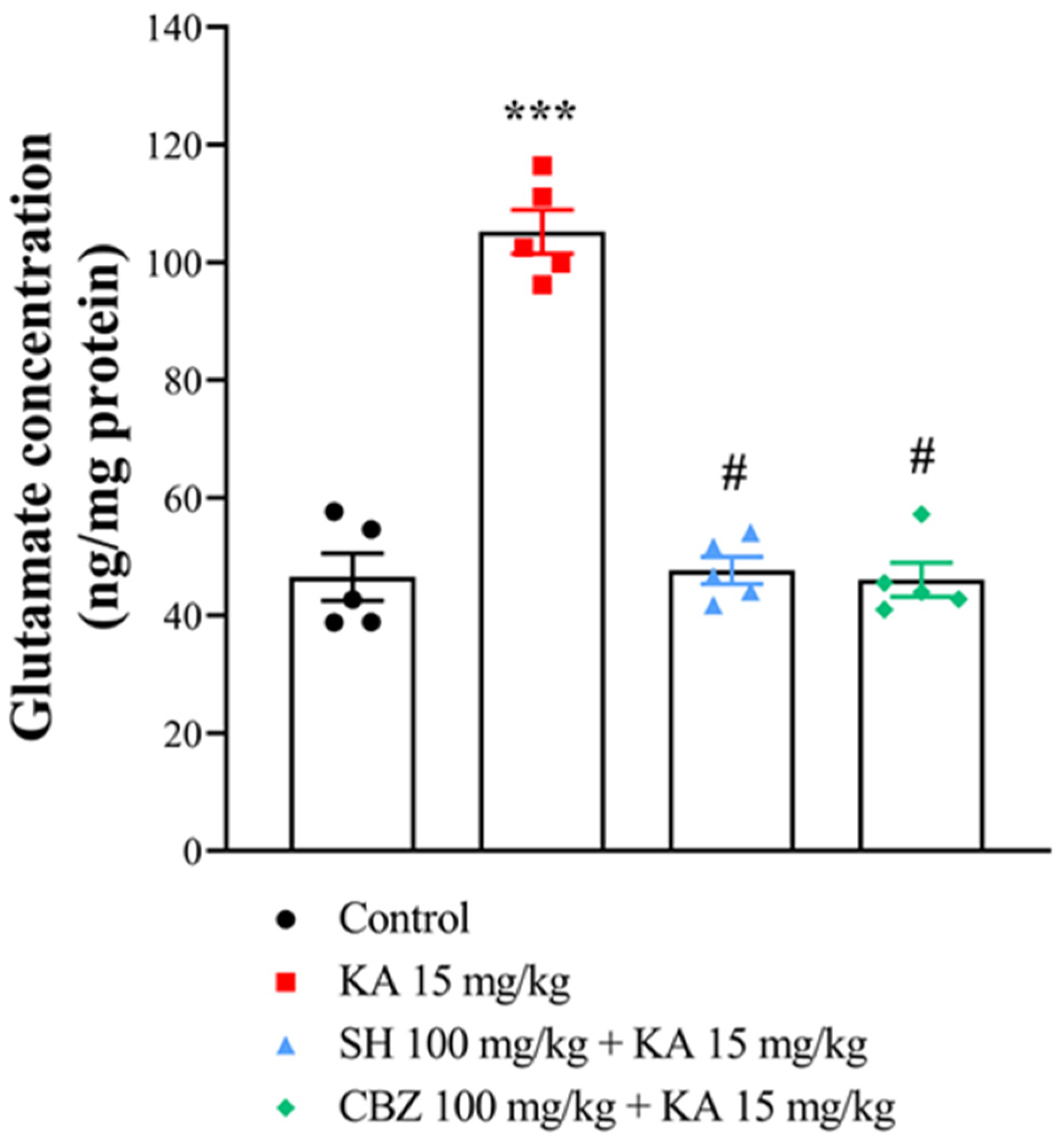
2.5. Glutamate Level
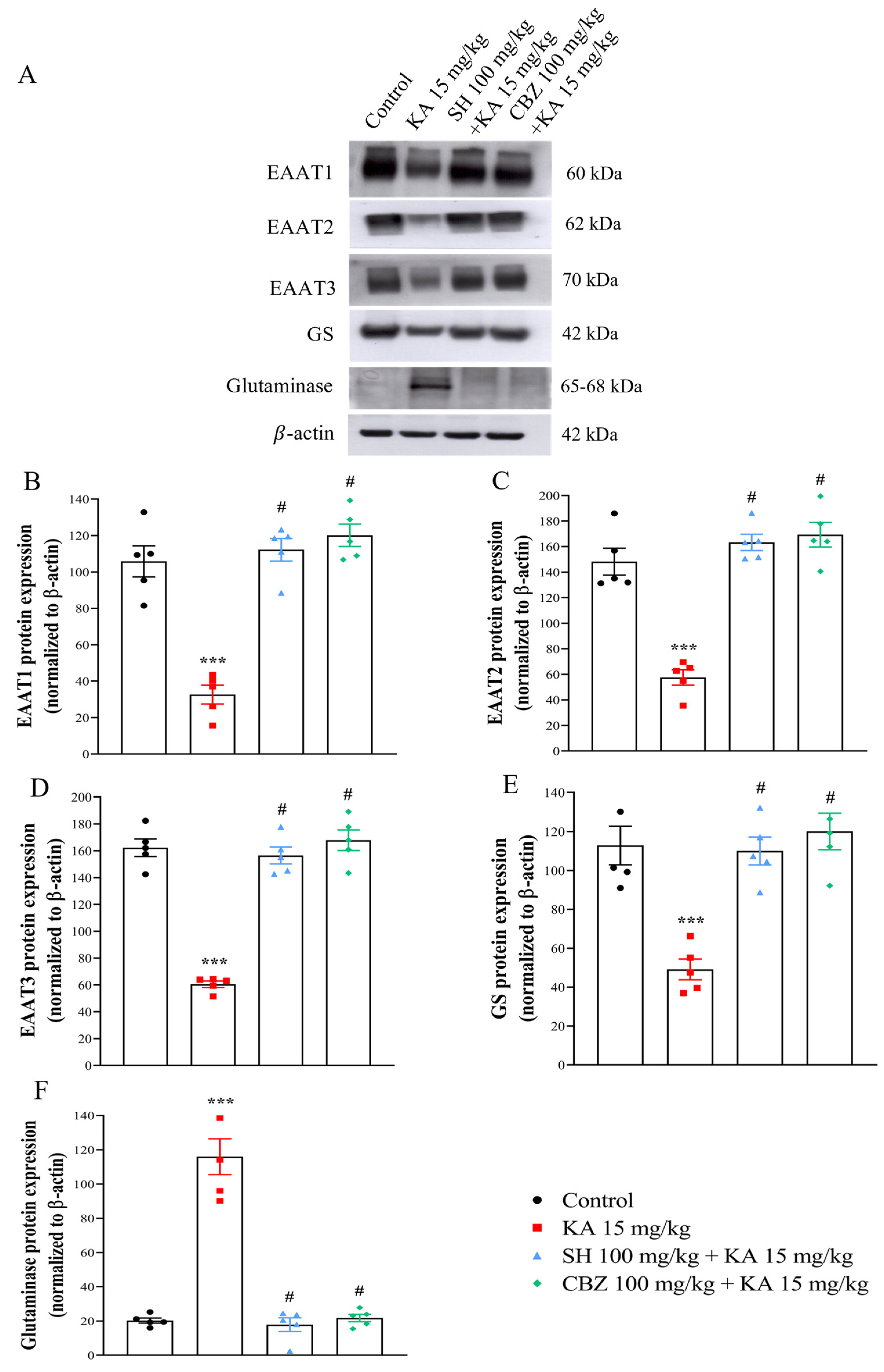
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Seizure Onset, Seizure Score, and Percentage of Animals That Developed Seizures
3.2. Histological Examination
3.3. Glutamate Level
3.4. EAAT1, EAAT2, EAAT3, GS, and Glutaminase Levels
3.5. GluA1, GluA2, GluNR1, GluN2A, and GluN2B Levels
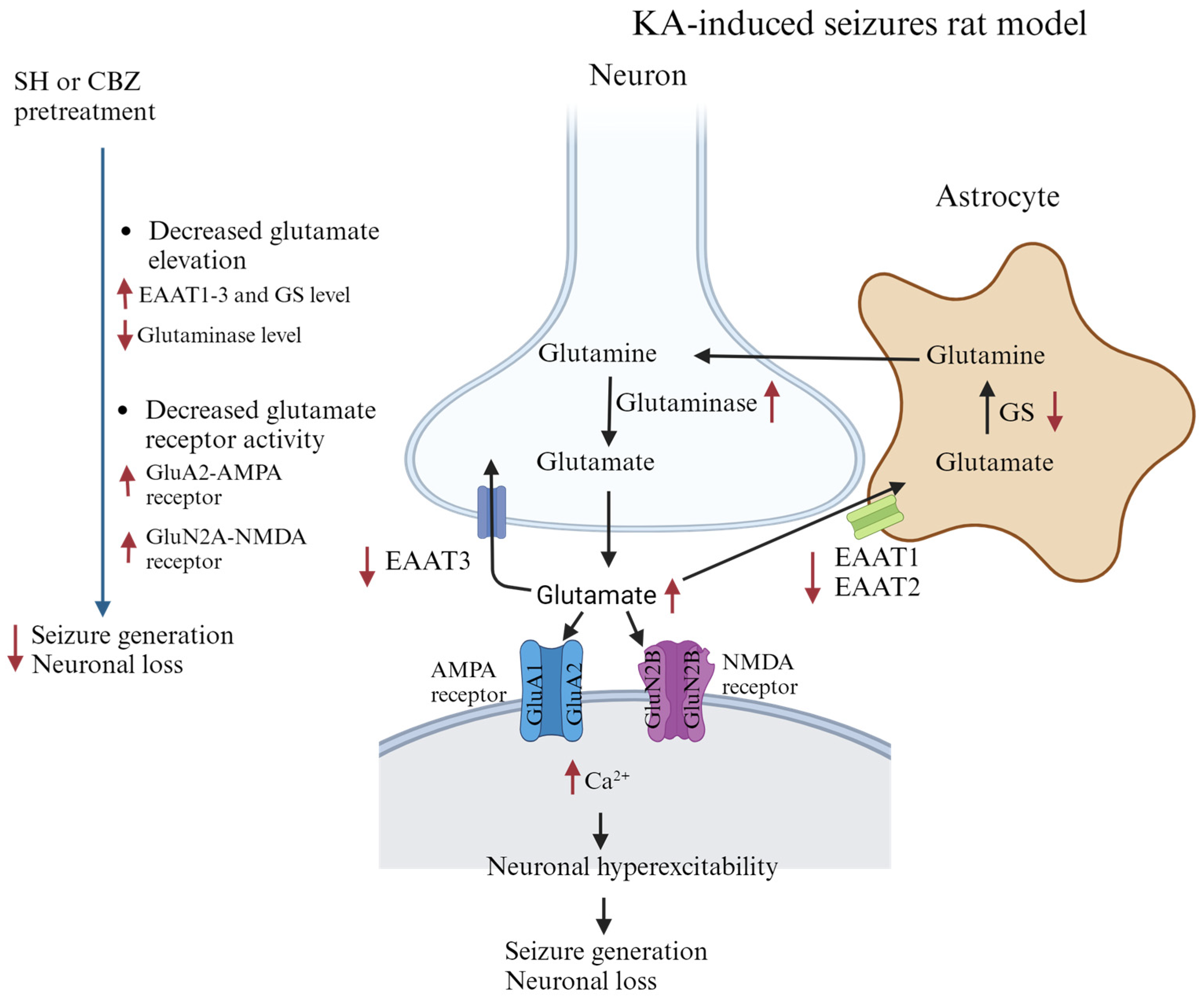
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Hu, M.B.; Li, R.L.; Zhao, R.; Fan, L.H.; He, L.; Lu, F.; Ye, X.; Huang, Y.L.; Wu, C.J. Natural medicines for the treatment of epilepsy: Bioactive components, pharmacology, and mechanism. Front. Pharmacol. 2021, 12, 604–640. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.M.; Liang, Y.Z.; Yi, L.Z.; Wu, X.J. Anti-inflammatory effect of Houttuynia cordata injection. J. Ethnopharmacol. 2006, 104, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharm. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.; Xu, W.T.; Zhong, X. Sodium houttuyfonate plays a protective role in the asthmatic airway by alleviating the NLRP3-related pyroptosis and Th1/Th2 immune imbalance. Mol. Immunol. 2023, 160, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhong, L.; Xie, J.; Sui, Y.; Li, G.; Ma, Z.; Yang, L. Sodium houttuyfonate: A review of its antimicrobial, anti-inflammatory and cardiovascular protective effects. Eur. J. Pharmacol. 2021, 902, 174110. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, H.; Wang, C.; Wu, D.; Zhu, X.; Zhu, L.; Sun, Z. Sodium houttuyfonate, a potential phytoanticipin derivative of antibacterial agent, inhibits bacterial attachment and pyocyanine secretion of Pseudomonas aeruginosa by attenuating flagella-mediated swimming motility. World J. Microbiol. Biotechnol. 2013, 29, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, Y.; Feng, T. Sodium Houttuyfonate Ameliorates β-amyloid1-42-Induced Memory Impairment and Neuroinflammation through Inhibiting the NLRP3/GSDMD Pathway in Alzheimer’s Disease. Mediat. Inflamm. 2021, 2021, 8817698. [Google Scholar] [CrossRef]
- Yao, X.; Wang, S.; Chen, Y.; Sheng, L.; Li, H.; You, H.; Ye, J.; Zhang, Q.; Li, J. Sodium houttuyfonate attenuates neurological defects after traumatic brain injury in mice via inhibiting NLRP3 inflammasomes. J. Biochem. Mol. Toxicol. 2021, 35, e22850. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, L.; Yan, Y.; Xia, Y. Sodium houttuyfonate exerts its neuroprotection effect by inhibiting the M1 microglia polarization in a TLR4/NF-κB signal pathway. Brain Res. 2023, 1809, 148358. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, J.; Sinn, D.I.; Kim, J.M.; Park, D.K.; Lee, J.J.; Kim, S.U.; Kim, M. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpineinduced status epilepticus. Neurobiol. Dis. 2006, 23, 237–246. [Google Scholar] [CrossRef]
- Erdogan, A.; Erdogan, M.A.; Gurgul, S.; Erbas, O. Effects of diclofenac sodium on seizure activity in rats with pentylenetetrazole-induced convulsions. Neurochem. Res. 2023, 48, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, E.F.M.; de Lima Rosa, G.; Domingues, A.M.; Padilha, R.B.; Coitinho, A.S. Reduction of seizures and inflammatory markers by betamethasone in a kindling seizure model. Steroids 2023, 193, 109202. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Luteolin protects the hippocampus against neuron impairments induced by kainic acid in rats. Neurotoxicology 2016, 55, 48–57. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Pan, T.L.; Wang, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Asiatic acid prevents cognitive deficits by inhibiting calpain activation and preserving synaptic and mitochondrial function in rats with kainic acid-induced seizure. Biomedicines 2021, 9, 284. [Google Scholar] [CrossRef]
- Chang, A.; Chang, Y.; Wang, S.J. Rutin prevents seizures in kainic acid-treated rats: Evidence of glutamate levels, inflammation and neuronal loss modulation. Food Funct. 2022, 13, 10401–10414. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Chiu, K.M.; Lee, M.Y.; Huang, J.H.; Wang, S.J. Silymarin Inhibits Glutamate Release and Prevents against Kainic Acid-Induced Excitotoxic Injury in Rats. Biomedicines 2020, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- During, M.J.; Spencer, D.D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 1993, 341, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Egbenya, D.L.; Hussain, S.; Lai, Y.C.; Xia, J.; Anderson, A.E.; Davanger, S. Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol. Cell Neurosci. 2018, 92, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zubareva, O.E.; Kovalenko, A.A.; Kalemenev, S.V.; Schwarz, A.P.; Karyakin, V.B.; Zaitsev, A.V. Alterations in mRNA expression of glutamate receptor subunits and excitatory amino acid transporters following pilocarpine-induced seizures in rats. Neurosci. Lett. 2018, 686, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rusina, E.; Bernard, C.; Williamson, A. The kainic acid models of temporal lobe epilepsy. eNeuro 2021, 8, ENEURO.0337. [Google Scholar] [CrossRef]
- Friedman, L.K.; Pellegrini-Giampietro, D.E.; Sperber, E.F.; Bennett, M.V.; Moshé, S.L.; Zukin, R.S. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: An in situ hybridization study. J. Neurosci. 1994, 14, 2697–2707. [Google Scholar] [CrossRef]
- Spigolon, G.; Veronesi, C.; Bonny, C.; Vercelli, A. c-Jun n-terminal kinase signaling pathway in excitotoxic cell death following kainic acid-induced status epilepticus. Eur. J. Neurosci. 2010, 31, 1261–1272. [Google Scholar] [CrossRef]
- Friedman, L.K. Selective reduction of GluR2 protein in adult hippocampal CA3 neurons following status epilepticus but prior to cell loss. Hippocampus 1998, 8, 511–525. [Google Scholar] [CrossRef]
- Pai, M.; Wang, K.C.; Yeh, K.C.; Wang, S.J. Stabilization of mitochondrial function by chlorogenic acid protects against kainic acid-induced seizures and neuronal cell death in rats. Eur. J. Pharmacol. 2023, 961, 176197. [Google Scholar] [CrossRef] [PubMed]
- Soukupova, M.; Binaschi, A.; Falcicchia, C.; Palma, E.; Roncon, P.; Zucchini, S.; Simonato, M. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscience 2015, 301, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 2017, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.; Wan Roslina, W.Y.; Sirajudeen, K.N.S.; Zulkarnain, M.; Chandran, G. Decreased glutamine synthetase, increased citrulline-nitric oxide cycle activities and oxidative stress in different regions of brain in epilepsy rat model. J. Physiol. Biochem. 2011, 67, 105–113. [Google Scholar] [CrossRef]
- Eid, T.; Behar, K.; Bumanglag, A.V.; Lee, T.S. Role of glutamine synthetase inhibition in epilepsy. Neurochem. Res. 2012, 37, 2339–2350. [Google Scholar] [CrossRef]
- Lu, C.W.; Wu, C.C.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. Inhibition of synaptic glutamate exocytosis and prevention of glutamate neurotoxicity by eupatilin from Artemisia argyi in the rat cortex. Int. J. Mol. Sci. 2022, 23, 13406. [Google Scholar] [CrossRef]
- Jean, W.H.; Huang, C.T.; Hsu, J.H.; Chiu, K.M.; Lee, M.Y.; Shieh, J.S.; Lin, T.Y.; Wang, S.J. Anticonvulsive and neuroprotective effects of eupafolin in rats are associated with the inhibition of glutamate overexcitation and upregulation of the Wnt/β-catenin signaling pathway. ACS Chem. Neurosci. 2022, 13, 1594–1603. [Google Scholar] [CrossRef]
- Liu, S.J.; Zukin, R.S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007, 30, 126–134. [Google Scholar] [CrossRef]
- Paul, S.; Connor, J.A. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J. Neurochem. 2010, 114, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.M.; Geddes-Klein, D.M.; Hockenberry, A.; Scarsella, D.; Mesfin, M.N.; Singh, P.; Patel, T.P.; Meaney, D.F. NR2A and NR2B subunits differentially mediate MAP kinase signaling and mitochondrial morphology following excitotoxic insult. Neurochem. Int. 2012, 60, 506–516. [Google Scholar] [CrossRef]
- Grooms, S.Y.; Opitz, T.; Bennett, M.V.; Zukin, R.S. Status epilepticus decreases glutamate receptor 2 mRNA and protein expression in hippocampal pyramidal cells before neuronal death. Proc. Natl. Acad. Sci. USA 2000, 97, 3631–3636. [Google Scholar] [CrossRef]
- Sitges, M.; Chiu, L.M.; Guarneros, A.; Nekrassov, V. Effects of carbamazepine, phenytoin, lamotrigine, oxcarbazepine, topiramate and vinpocetine on Na+ channel-mediated release of [3H] glutamate in hippocampal nerve endings. Neuropharmacology 2007, 52, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Sitges, M.; Chiu, L.M.; Reed, R.C. Effects of levetiracetam, carbamazepine, phenytoin, valproate, lamotrigine, oxcarbazepine, topiramate, vinpocetine and sertraline on presynaptic hippocampal Na+ and Ca2+ channels permeability. Neurochem. Res. 2016, 41, 758–769. [Google Scholar] [CrossRef]
- Wang, W.; Hu, X.; Shen, P.; Zhang, N.; Fu, Y. Sodium houttuyfonate inhibits LPS-induced inflammatory response via suppressing TLR4/NF-κB signaling pathway in bovine mammary epithelial cells. Microb. Pathog. 2017, 107, 12–16. [Google Scholar] [CrossRef]
- Pereno, G.L.; Balaszczuk, V.; Beltramino, C.A. Effect of sex differences and gonadal hormones on kainic acid-induced neurodegeneration in the bed nucleus of the stria terminalis of the rat. Exp. Toxicol. Pathol. 2012, 64, 283–289. [Google Scholar] [CrossRef]
- Scharfman, H.E.; MacLusky, N.J. Sex differences in the neurobiology of epilepsy: A preclinical perspective. Neurobiol. Dis. 2014, 72, 180–192. [Google Scholar] [CrossRef]

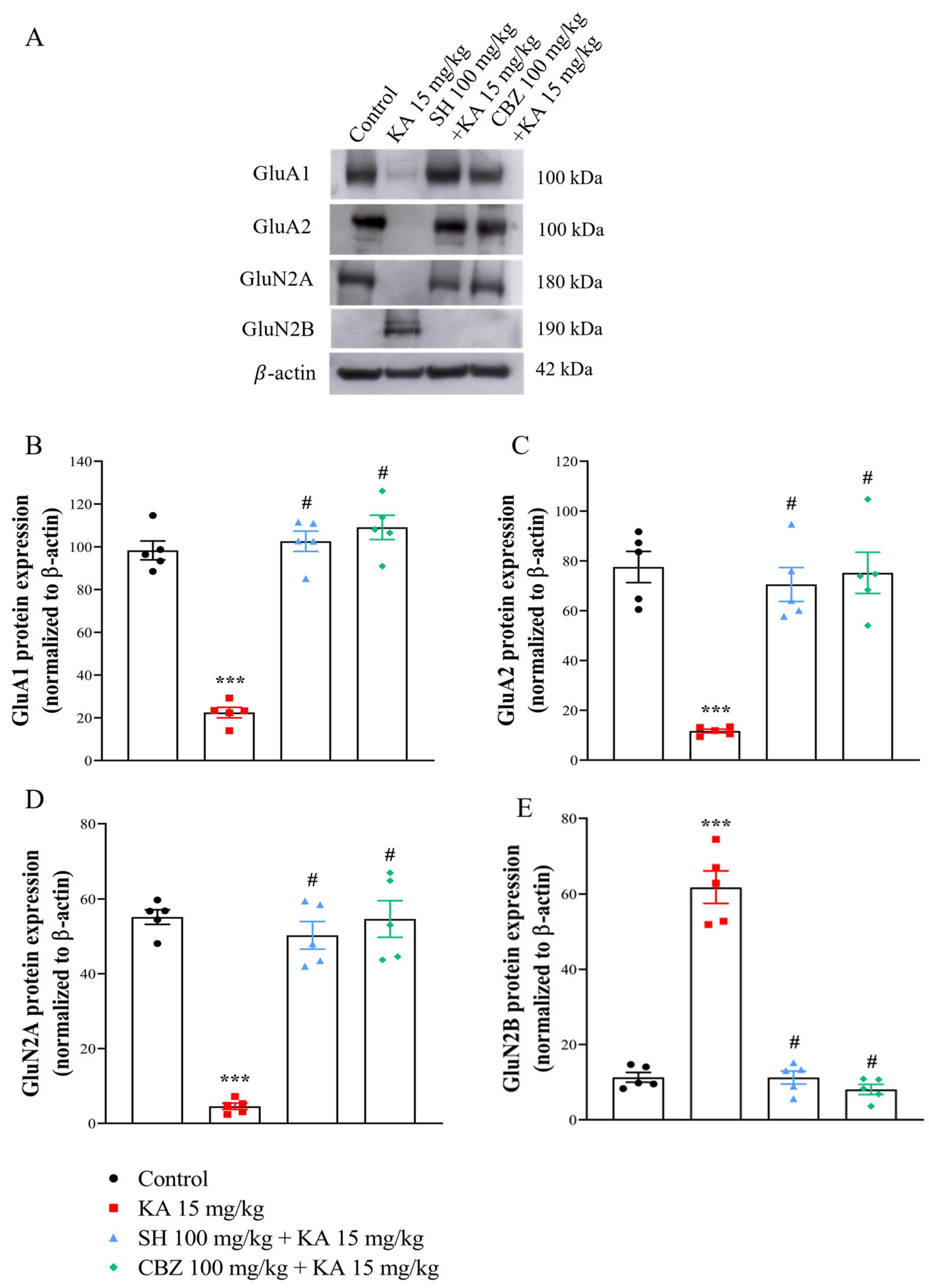
| Group | Seizure Onset (min) | Seizure Score (Racine Scale) | % Seizure |
|---|---|---|---|
| KA 15 mg/kg | 65.2 ± 3.9 | 4.5 ± 0.2 | 100% (11/11) |
| SH 50 mg/kg + KA 15 mg/kg | 101.3 ± 15.8 ** | 3.9 ± 0.4 | 55% (5/9) |
| SH 100 mg/kg + KA 15 mg/kg | 167 | 0.6 ± 0.5 *** | 9% (1/11) |
| CBZ 100 mg/kg + KA 15 mg/kg | - | 0.3 ± 0.2 *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chen, Y.-J.; Wang, S.-J. Sodium Houttuyfonate Prevents Seizures and Neuronal Cell Loss by Maintaining Glutamatergic System Stability in Male Rats with Kainic Acid-Induced Seizures. Biomedicines 2024, 12, 1312. https://doi.org/10.3390/biomedicines12061312
Chang Y, Chen Y-J, Wang S-J. Sodium Houttuyfonate Prevents Seizures and Neuronal Cell Loss by Maintaining Glutamatergic System Stability in Male Rats with Kainic Acid-Induced Seizures. Biomedicines. 2024; 12(6):1312. https://doi.org/10.3390/biomedicines12061312
Chicago/Turabian StyleChang, Yi, Yi-Jun Chen, and Su-Jane Wang. 2024. "Sodium Houttuyfonate Prevents Seizures and Neuronal Cell Loss by Maintaining Glutamatergic System Stability in Male Rats with Kainic Acid-Induced Seizures" Biomedicines 12, no. 6: 1312. https://doi.org/10.3390/biomedicines12061312





