Unveiling the Significance of HLA and KIR Diversity in Underrepresented Populations
Abstract
:1. Introduction
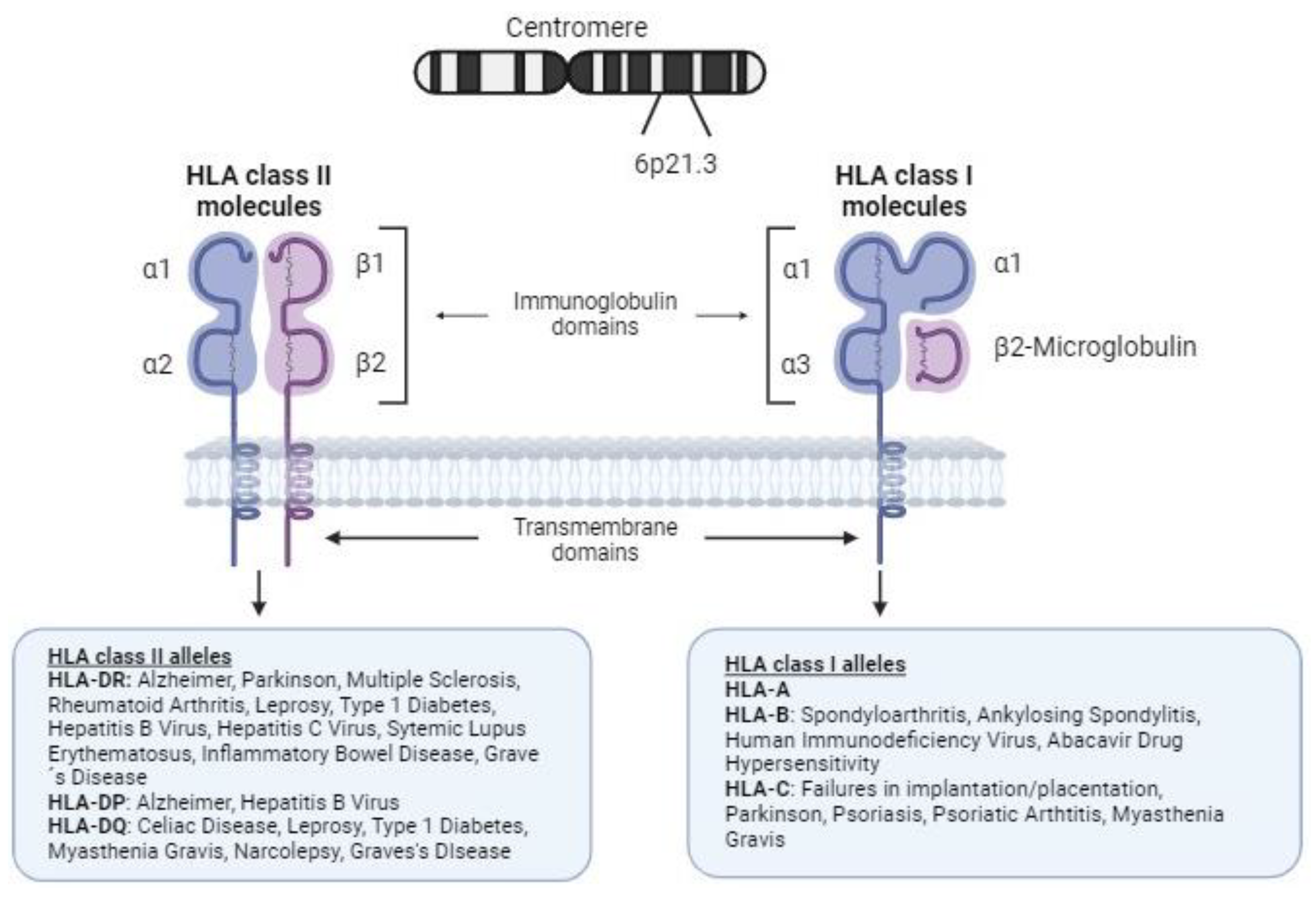
2. Major Histocompatibility Complex
2.1. Structure and Function of HLA Molecules
2.2. HLA Diversity and Disease Susceptibility
2.3. Genome-Wide Association Studies and Single-Nucleotide Polymorphisms
3. Killer Cell Immunoglobulin-like Receptors
3.1. KIR Structure
3.2. Haplotypic and Allelic Diversity
3.3. KIR Ligands
4. HLA/KIR Interaction and Association with Disease
4.1. HLA/KIR and Reproduction
4.2. HLA/KIR and the Response to Infection
4.3. HLA/KIR and Rejection Status
4.4. HLA/KIR and Autoimmunity
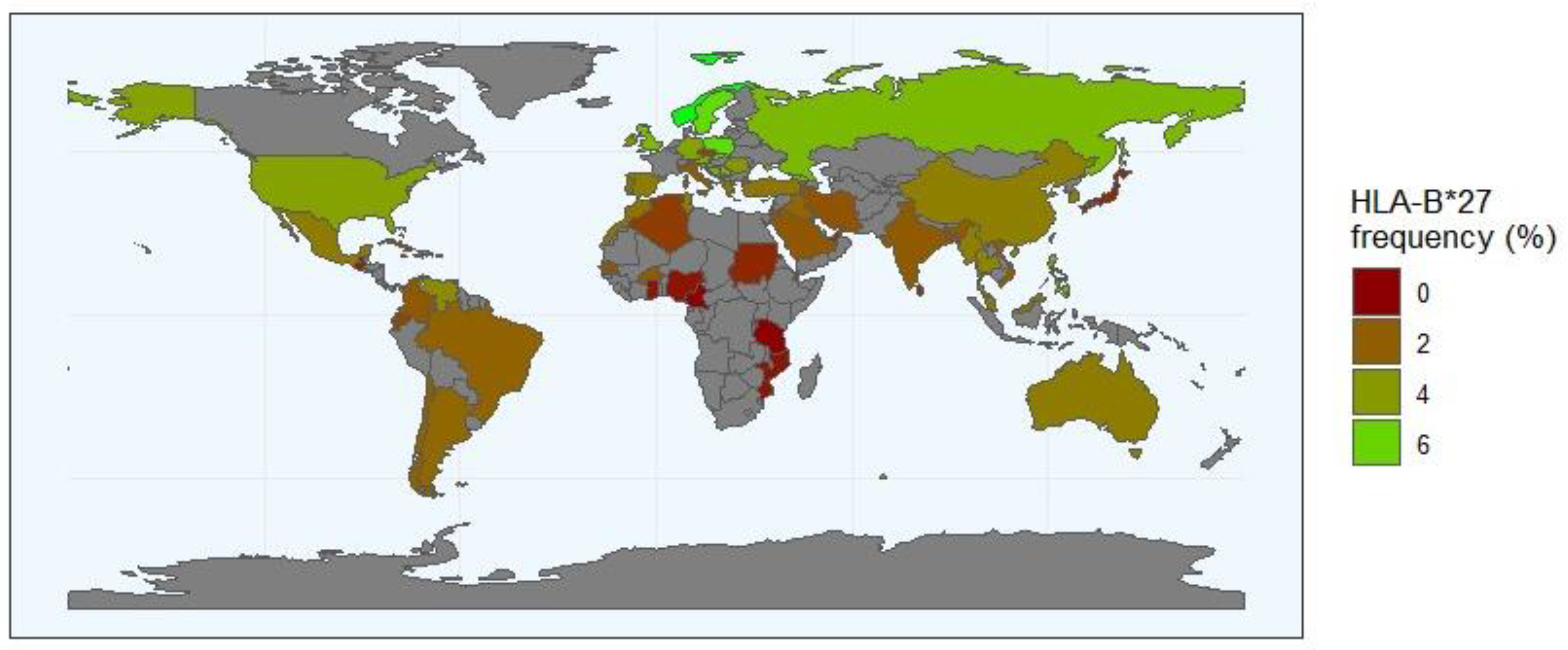
4.5. HLA/KIR Diversity and Distribution Worldwide
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Weijer, M.L.; Luteijn, R.D.; Wiertz, E.J.H.J. Viral Immune Evasion: Lessons in MHC Class I Antigen Presentation. Semin. Immunol. 2015, 27, 125–137. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK Cells and Cancer: You Can Teach Innate Cells New Tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Trowsdale, J.; Knight, J.C. Major Histocompatibility Complex Genomics and Human Disease. Annu. Rev. Genom. Hum. Genet. 2013, 14, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Matzaraki, V.; Kumar, V.; Wijmenga, C.; Zhernakova, A. The MHC Locus and Genetic Susceptibility to Autoimmune and Infectious Diseases. Genome Biol. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Schuster, H.; Backert, L.; Ghosh, M.; Rammensee, H.-G.; Stevanović, S. Unveiling the Peptide Motifs of HLA-C and HLA-G from Naturally Presented Peptides and Generation of Binding Prediction Matrices. J. Immunol. 2017, 199, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.J.W.; Stotz, Z.; Lu, J.; Brennan, P.; Long, E.O.; Sun, P.D. T Cells Discriminate between Groups C1 and C2 HLA-C. Elife 2022, 11, e75670. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; López-Vázquez, A.; López-Larrea, C. Old and New HLA Associations with Ankylosing Spondylitis. Tissue Antigens 2012, 80, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Okada, Y. The Current Landscape of Psoriasis Genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.-S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five Amino Acids in Three HLA Proteins Explain Most of the Association between MHC and Seropositive Rheumatoid Arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef]
- Dedmon, L.E. The Genetics of Rheumatoid Arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef]
- Ortíz-Fernández, L.; Martín, J.; Alarcón-Riquelme, M.E. A Summary on the Genetics of Systemic Lupus Erythematosus, Rheumatoid Arthritis, Systemic Sclerosis, and Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2023, 64, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Yates, R.L.; Pansieri, J.; Li, Q.; Bell, J.S.; Yee, S.A.; Palace, J.; Esiri, M.M.; DeLuca, G.C. The Influence of HLA-DRB1*15 on the Relationship between Microglia and Neurons in Multiple Sclerosis Normal Appearing Cortical Grey Matter. Brain Pathol. 2022, 32, e13041. [Google Scholar] [CrossRef] [PubMed]
- Megiorni, F.; Pizzuti, A. HLA-DQA1 and HLA-DQB1 in Celiac Disease Predisposition: Practical Implications of the HLA Molecular Typing. J. Biomed. Sci. 2012, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Kachooei-Mohaghegh-Yaghoobi, L.; Rezaei-Rad, F.; Sadeghniiat-Haghighi, K.; Zamani, M. The Impact of the HLA DQB1 Gene and Amino Acids on the Development of Narcolepsy. Int. J. Neurosci. 2022, 132, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Dallmann-Sauer, M.; Fava, V.M.; Gzara, C.; Orlova, M.; Van Thuc, N.; Thai, V.H.; Alcaïs, A.; Abel, L.; Cobat, A.; Schurr, E. The Complex Pattern of Genetic Associations of Leprosy with HLA Class I and Class II Alleles Can Be Reduced to Four Amino Acid Positions. PLoS Pathog. 2020, 16, e1008818. [Google Scholar] [CrossRef]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA Region in the Prediction of Type 1 Diabetes. Curr. Diab Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Vejrazkova, D.; Vcelak, J.; Vaclavikova, E.; Vankova, M.; Zajickova, K.; Duskova, M.; Vrbikova, J.; Bendlova, B. Genetic Predictors of the Development and Recurrence of Graves’ Disease. Physiol. Res. 2018, 67, S431–S439. [Google Scholar] [CrossRef] [PubMed]
- Salvado, M.; Caro, J.L.; Garcia, C.; Rudilla, F.; Zalba-Jadraque, L.; Lopez, E.; Sanjuan, E.; Gamez, J.; Vidal-Taboada, J.M. HLA-DQB1*05:02, *05:03, and *03:01 Alleles as Risk Factors for Myasthenia Gravis in a Spanish Cohort. Neurol. Sci. 2022, 43, 5057–5065. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker, K.L.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic Variations in HLA-B Region and Hypersensitivity Reactions to Abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef]
- Evans, D.M.; Spencer, C.C.A.; Pointon, J.J.; Su, Z.; Harvey, D.; Kochan, G.; Oppermann, U.; Opperman, U.; Dilthey, A.; Pirinen, M.; et al. Interaction between ERAP1 and HLA-B27 in Ankylosing Spondylitis Implicates Peptide Handling in the Mechanism for HLA-B27 in Disease Susceptibility. Nat. Genet. 2011, 43, 761–767. [Google Scholar] [CrossRef]
- Reveille, J.D.; Hirsch, R.; Dillon, C.F.; Carroll, M.D.; Weisman, M.H. The Prevalence of HLA-B27 in the US: Data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 2012, 64, 1407–1411. [Google Scholar] [CrossRef]
- Khan, M.A. HLA-B*27 and Ankylosing Spondylitis: 50 Years of Insights and Discoveries. Curr. Rheumatol. Rep. 2023, 25, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gelaz, M.A.; López-Vázquez, A.; García-Fernández, S.; Martínez-Borra, J.; González, S.; López-Larrea, C. Genetic Variability, Molecular Evolution, and Geographic Diversity of HLA-B27. Hum. Immunol. 2001, 62, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; Blanco-Gelaz, M.A.; Njobvu, P.; López-Vazquez, A.; Suárez-Alvarez, B.; López-Larrea, C. Influence of HLA-B*5703 and HLA-B*1403 on Susceptibility to Spondyloarthropathies in the Zambian Population. J. Rheumatol. 2008, 35, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; Ouédraogo, D.D.; López-Vázquez, A.; Sawadogo, S.A.; López-Larrea, C. Ankylosing Spondylitis in Three Sub-Saharan Populations: HLA-B*27 and HLA-B*14 Contribution. Tissue Antigens 2012, 80, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mazas, A. A Review of HLA Allele and SNP Associations with Highly Prevalent Infectious Diseases in Human Populations. Swiss Med. Wkly. 2020, 150, w20214. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rios, S.; Carlson, J.M.; John, M.; Mallal, S.; Brumme, Z.L. Clinical and Evolutionary Consequences of HIV Adaptation to HLA: Implications for Vaccine and Cure. Curr. Opin. HIV AIDS 2019, 14, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Thio, C.L.; Apps, R.; Qi, Y.; Gao, X.; Marti, D.; Stein, J.L.; Soderberg, K.A.; Moody, M.A.; Goedert, J.J.; et al. A Novel Variant Marking HLA-DP Expression Levels Predicts Recovery from Hepatitis B Virus Infection. J. Virol. 2012, 86, 6979–6985. [Google Scholar] [CrossRef] [PubMed]
- Tangamornsuksan, W.; Chaiyakunapruk, N.; Somkrua, R.; Lohitnavy, M.; Tassaneeyakul, W. Relationship between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2013, 149, 1025–1032. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Gutierrez-Arcelus, M.; Rich, S.S.; Raychaudhuri, S. Autoimmune Diseases - Connecting Risk Alleles with Molecular Traits of the Immune System. Nat. Rev. Genet. 2016, 17, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Zhang, Y.; Lin, Z.; Zhang, H.; Wang, T.-Y.; Cao, Y.; Morris, D.L.; Sheng, Y.; Yin, X.; Zhong, S.-L.; et al. Identification of 38 Novel Loci for Systemic Lupus Erythematosus and Genetic Heterogeneity between Ancestral Groups. Nat. Commun. 2021, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Sawcer, S.; Franklin, R.J.M.; Ban, M. Multiple Sclerosis Genetics. Lancet Neurol. 2014, 13, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, K.; Sakaue, S.; Terao, C.; Luo, Y.; Sonehara, K.; Yamaguchi, K.; Amariuta, T.; Too, C.L.; Laufer, V.A.; Scott, I.C.; et al. Multi-Ancestry Genome-Wide Association Analyses Identify Novel Genetic Mechanisms in Rheumatoid Arthritis. Nat. Genet. 2022, 54, 1640–1651. [Google Scholar] [CrossRef]
- Silva, N.S.B.; Bourguiba-Hachemi, S.; Douillard, V.; Koskela, S.; Degenhardt, F.; Clancy, J.; Limou, S.; Meyer, D.; Masotti, C.; Knorst, S.; et al. 18th International HLA and Immunogenetics Workshop: Report on the SNP-HLA Reference Consortium (SHLARC) Component. HLA 2024, 103, e15293. [Google Scholar] [CrossRef]
- Loos, R.J.F. 15 Years of Genome-Wide Association Studies and No Signs of Slowing Down. Nat. Commun. 2020, 11, 5900. [Google Scholar] [CrossRef]
- Mills, M.C.; Rahal, C. The GWAS Diversity Monitor Tracks Diversity by Disease in Real Time. Nat. Genet. 2020, 52, 242–243. [Google Scholar] [CrossRef]
- Douillard, V.; Dos Santos Brito Silva, N.; Bourguiba-Hachemi, S.; Naslavsky, M.S.; Scliar, M.O.; Duarte, Y.A.O.; Zatz, M.; Passos-Bueno, M.R.; Limou, S.; Gourraud, P.-A.; et al. Optimal Population-Specific HLA Imputation with Dimension Reduction. HLA 2024, 103, e15282. [Google Scholar] [CrossRef]
- Downing, J.; D’Orsogna, L. High-Resolution Human KIR Genotyping. Immunogenetics 2022, 74, 369–379. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Middleton, D.; Gonzelez, F. The Extensive Polymorphism of KIR Genes. Immunology 2010, 129, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.; Vierra-Green, C.; Pyo, C.-W.; Eng, K.; Hall, R.; Kuang, R.; Spellman, S.; Ranade, S.; Geraghty, D.E.; Maiers, M. Revealing Complete Complex KIR Haplotypes Phased by Long-Read Sequencing Technology. Genes Immun. 2017, 18, 127–134. [Google Scholar] [CrossRef]
- Gendzekhadze, K.; Norman, P.J.; Abi-Rached, L.; Graef, T.; Moesta, A.K.; Layrisse, Z.; Parham, P. Co-Evolution of KIR2DL3 with HLA-C in a Human Population Retaining Minimal Essential Diversity of KIR and HLA Class I Ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 18692–18697. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Martin, M.P.; Carrington, M. The Yin and Yang of HLA and KIR in Human Disease. Semin. Immunol. 2008, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, D.M.; Marangon, A.V.; Guimarães, F.; Marques, S.; Lieber, S.; Delamain, M.; Aranha, F.; Visentainer, J.E.L.; Souza, C.A. de Killer Cell Immunoglobulin-like Receptor (KIR) Genes and Their HLA Ligands in a Brazilian Population. Innate Immun. 2023, 29, 71–82. [Google Scholar] [CrossRef]
- Vierra-Green, C.; Roe, D.; Hou, L.; Hurley, C.K.; Rajalingam, R.; Reed, E.; Lebedeva, T.; Yu, N.; Stewart, M.; Noreen, H.; et al. Allele-Level Haplotype Frequencies and Pairwise Linkage Disequilibrium for 14 KIR Loci in 506 European-American Individuals. PLoS ONE 2012, 7, e47491. [Google Scholar] [CrossRef] [PubMed]
- Ligeiro, D.; Buhler, S.; Abecasis, M.; Abade, O.; Sanchez-Mazas, A.; da Silva, M.G.; Trindade, H. KIR Genotypic Diversity in Portuguese and Analysis of KIR Gene Allocation after Allogeneic Hematopoietic Stem Cell Transplantation. HLA 2016, 87, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Santin, I.; de Nanclares, G.P.; Calvo, B.; Gaafar, A.; Castaño, L.; GEPV-N Group; Bilbao, J.R. Killer Cell Immunoglobulin-like Receptor (KIR) Genes in the Basque Population: Association Study of KIR Gene Contents with Type 1 Diabetes Mellitus. Hum. Immunol. 2006, 67, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.J.; Hollenbach, J.A.; Nemat-Gorgani, N.; Guethlein, L.A.; Hilton, H.G.; Pando, M.J.; Koram, K.A.; Riley, E.M.; Abi-Rached, L.; Parham, P. Co-Evolution of Human Leukocyte Antigen (HLA) Class I Ligands with Killer-Cell Immunoglobulin-like Receptors (KIR) in a Genetically Diverse Population of Sub-Saharan Africans. PLoS Genet. 2013, 9, e1003938. [Google Scholar] [CrossRef]
- Pettifor, A.E.; Kleinschmidt, I.; Levin, J.; Rees, H.V.; MacPhail, C.; Madikizela-Hlongwa, L.; Vermaak, K.; Napier, G.; Stevens, W.; Padian, N.S. A Community-Based Study to Examine the Effect of a Youth HIV Prevention Intervention on Young People Aged 15-24 in South Africa: Results of the Baseline Survey. Trop. Med. Int. Health 2005, 10, 971–980. [Google Scholar] [CrossRef]
- Kevin-Tey, W.F.; Wen, W.X.; Bee, P.C.; Eng, H.S.; Ho, K.W.; Tan, S.M.; Anuar, N.A.; Pung, Y.F.; Zain, S.M. KIR Genotype and Haplotype Frequencies in the Multi-Ethnic Population of Malaysia. Hum. Immunol. 2023, 84, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Ameen, R.; Titus, R.; Geo, J.A.; Al Shemmari, S.; Geraghty, D.E.; Pyo, C.-W.; Askar, M. KIR Genotype and Haplotype Repertoire in Kuwaiti Healthy Donors, Hematopoietic Cell Transplant Recipients and Healthy Family Members. HLA 2023, 102, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Augusto, D.G.; Hollenbach, J.A.; Petzl-Erler, M.L. A Deep Look at KIR-HLA in Amerindians: Comprehensive Meta-Analysis Reveals Limited Diversity of KIR Haplotypes. Hum. Immunol. 2015, 76, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Jobim, M.; Salim, P.H.; Portela, P.; Wilson, T.J.; Fraportti, J.; Baronio, D.; Gil, B.; Penna, L.S.; Roesler, R.; Jobim, L.F.; et al. Killer Cell Immunoglobulin-like Receptor Gene Diversity in a Caucasian Population of Southern Brazil. Int. J. Immunogenet. 2010, 37, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, M.; Marin, N.D.; Karduss-Urueta, A.J.; Velasquez, S.Y.; Alvarez, C.M. Killer-Cell Immunoglobulin-like Receptor Diversity in an Admixed South American Population. Cells 2022, 11, 2776. [Google Scholar] [CrossRef] [PubMed]
- Single, R.M.; Martin, M.P.; Gao, X.; Meyer, D.; Yeager, M.; Kidd, J.R.; Kidd, K.K.; Carrington, M. Global Diversity and Evidence for Coevolution of KIR and HLA. Nat. Genet. 2007, 39, 1114–1119. [Google Scholar] [CrossRef]
- Bastidas-Legarda, L.Y.; Khakoo, S.I. Conserved and Variable Natural Killer Cell Receptors: Diverse Approaches to Viral Infections. Immunology 2019, 156, 319–328. [Google Scholar] [CrossRef]
- Deng, Z.; Zhen, J.; Harrison, G.F.; Zhang, G.; Chen, R.; Sun, G.; Yu, Q.; Nemat-Gorgani, N.; Guethlein, L.A.; He, L.; et al. Adaptive Admixture of HLA Class I Allotypes Enhanced Genetically Determined Strength of Natural Killer Cells in East Asians. Mol. Biol. Evol. 2021, 38, 2582–2596. [Google Scholar] [CrossRef]
- Yu, J.; Heller, G.; Chewning, J.; Kim, S.; Yokoyama, W.M.; Hsu, K.C. Hierarchy of the Human Natural Killer Cell Response Is Determined by Class and Quantity of Inhibitory Receptors for Self-HLA-B and HLA-C Ligands. J. Immunol. 2007, 179, 5977–5989. [Google Scholar] [CrossRef]
- Gumperz, J.E.; Litwin, V.; Phillips, J.H.; Lanier, L.L.; Parham, P. The Bw4 Public Epitope of HLA-B Molecules Confers Reactivity with Natural Killer Cell Clones That Express NKB1, a Putative HLA Receptor. J. Exp. Med. 1995, 181, 1133–1144. [Google Scholar] [CrossRef]
- Shaw, J.; Kollnberger, S. New Perspectives on the Ligands and Function of the Killer Cell Immunoglobulin-like Receptor KIR3DL2 in Health and Disease. Front. Immunol. 2012, 3, 339. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lozano, N.; de Pablo, R.; Puente, S.; Vilches, C. Recognition of HLA-G by the NK Cell Receptor KIR2DL4 Is Not Essential for Human Reproduction. Eur. J. Immunol. 2003, 33, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Walker, J.J.; O’shaughnessy, K.M.; Redman, C.W.G.; Carrington, M.; Trowsdale, J.; Moffett, A. Combinations of Maternal KIR and Fetal HLA-C Genes Influence the Risk of Preeclampsia and Reproductive Success. J. Exp. Med. 2004, 200, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; de Los Santos, M.J.; Lucia, A.; Castro-Santos, P. Understanding the Role of Killer Cell Immunoglobulin-like Receptors in Pregnancy Complications. J. Assist. Reprod. Genet. 2019, 36, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Apps, R.; Sharkey, A.M.; Farrell, L.E.; Gardner, L.; Mulder, A.; Claas, F.H.; Walker, J.J.; Redman, C.W.; Morgan, L.; et al. Maternal Activating KIRs Protect against Human Reproductive Failure Mediated by Fetal HLA-C2. J. Clin. Investig. 2010, 120, 4102–4110. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, J.H.; Hilton, H.G.; Guethlein, L.A.; Norman, P.J.; Nemat-Gorgani, N.; Nakimuli, A.; Chazara, O.; Moffett, A.; Parham, P. KIR2DS5 Allotypes That Recognize the C2 Epitope of HLA-C Are Common among Africans and Absent from Europeans. Immun. Inflamm. Dis. 2017, 5, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Radwan, P.; Tarnowska, A.; Wiśniewski, A.; Radwan, M.; Wilczyński, J.R.; Malinowski, A.; Nowak, I. ERAP, KIR, and HLA-C Profile in Recurrent Implantation Failure. Front. Immunol. 2021, 12, 755624. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, K.; Wiśniewski, A.; Malinowski, A.; Barcz, E.; Wilczyński, J.R.; Kuśnierczyk, P.; Nowak, I. ERAP, KIR and HLA-C Gene Interaction in Susceptibility to Recurrent Spontaneous Abortion in the Polish Population. Hum. Immunol. 2019, 80, 344–348. [Google Scholar] [CrossRef]
- Alecsandru, D.; Garrido, N.; Vicario, J.L.; Barrio, A.; Aparicio, P.; Requena, A.; García-Velasco, J.A. Maternal KIR Haplotype Influences Live Birth Rate after Double Embryo Transfer in IVF Cycles in Patients with Recurrent Miscarriages and Implantation Failure. Hum. Reprod. 2014, 29, 2637–2643. [Google Scholar] [CrossRef]
- Stefańska, K.; Tomaszewicz, M.; Dębska-Zielkowska, J.; Zamkowska, D.; Piekarska, K.; Sakowska, J.; Studziński, M.; Tymoniuk, B.; Adamski, P.; Jassem-Bobowicz, J.; et al. KIR- Ligand Interactions in Hypertensive Disorders in Pregnancy. Front. Immunol. 2022, 13, 868175. [Google Scholar] [CrossRef]
- Male, V.; Sharkey, A.; Masters, L.; Kennedy, P.R.; Farrell, L.E.; Moffett, A. The Effect of Pregnancy on the Uterine NK Cell KIR Repertoire. Eur. J. Immunol. 2011, 41, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Faridi, R.M.; Das, V.; Tripthi, G.; Talwar, S.; Parveen, F.; Agrawal, S. Influence of Activating and Inhibitory Killer Immunoglobulin-like Receptors on Predisposition to Recurrent Miscarriages. Hum. Reprod. 2009, 24, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Faridi, R.M.; Agrawal, S. Killer Immunoglobulin-like Receptors (KIRs) and HLA-C Allorecognition Patterns Implicative of Dominant Activation of Natural Killer Cells Contribute to Recurrent Miscarriages. Hum. Reprod. 2011, 26, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wang, H.; Zhang, B.; Kang, Y.; Guo, Q.; Xiao, H.; Yang, H.; Liao, S. Maternal Natural Killer Cell Immunoglobulin Receptor Genes and Human Leukocyte Antigen-C Ligands Influence Recurrent Spontaneous Abortion in the Han Chinese Population. Exp. Ther. Med. 2018, 15, 327–337. [Google Scholar] [CrossRef]
- Kelemu, T.; Erlandsson, L.; Seifu, D.; Hansson, E.; Abebe, M.; Teklu, S.; Girma, S.; Traherne, J.A.; Moffett, A.; Hansson, S.R. Polymorphism in Killer Cell Immunoglobulin-like Receptors and Human Leukocyte Antigen-c and Predisposition to Preeclampsia in Ethiopian Pregnant Women Population. J. Reprod. Immunol. 2020, 141, 103169. [Google Scholar] [CrossRef]
- Vidal-Castiñeira, J.R.; López-Vázquez, A.; Díaz-Peña, R.; Alonso-Arias, R.; Martínez-Borra, J.; Pérez, R.; Fernández-Suárez, J.; Melón, S.; Prieto, J.; Rodrigo, L.; et al. Effect of Killer Immunoglobulin-like Receptors in the Response to Combined Treatment in Patients with Chronic Hepatitis C Virus Infection. J. Virol. 2010, 84, 475–481. [Google Scholar] [CrossRef]
- Ruibal, P.; Franken, K.L.M.C.; van Meijgaarden, K.E.; van Wolfswinkel, M.; Derksen, I.; Scheeren, F.A.; Janssen, G.M.C.; van Veelen, P.A.; Sarfas, C.; White, A.D.; et al. Identification of HLA-E Binding Mycobacterium Tuberculosis-Derived Epitopes through Improved Prediction Models. J. Immunol. 2022, 209, 1555–1565. [Google Scholar] [CrossRef]
- Omraninava, M.; Mehranfar, S.; Khosrojerdi, A.; Jamalzehi, S.; Karami, J.; Motallebnezhad, M.; Javan, M.R.; Aslani, S.; Mohammadi, H.; Kousha, A. Systematic Review and Meta-Analytic Findings on the Association between Killer-Cell Immunoglobulin-like Receptor Genes and Susceptibility to Pulmonary Tuberculosis. Pathog. Glob. Health 2021, 115, 61–69. [Google Scholar] [CrossRef]
- Mousavi, T.; Shahsavar, F.; Farnia, P.; Tajik, N.; Soofi, M. Study of KIR Expression and HLA Ligands in CD56+ Lymphocytes of Drug Resistant Tuberculosis Patients. Iran. J. Allergy Asthma Immunol. 2011, 10, 189–194. [Google Scholar]
- Boulet, S.; Kleyman, M.; Kim, J.Y.; Kamya, P.; Sharafi, S.; Simic, N.; Bruneau, J.; Routy, J.-P.; Tsoukas, C.M.; Bernard, N.F. A Combined Genotype of KIR3DL1 High Expressing Alleles and HLA-B*57 Is Associated with a Reduced Risk of HIV Infection. AIDS 2008, 22, 1487–1491. [Google Scholar] [CrossRef]
- Martin, M.P.; Qi, Y.; Gao, X.; Yamada, E.; Martin, J.N.; Pereyra, F.; Colombo, S.; Brown, E.E.; Shupert, W.L.; Phair, J.; et al. Innate Partnership of HLA-B and KIR3DL1 Subtypes against HIV-1. Nat. Genet. 2007, 39, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hölzemer, A.; Thobakgale, C.F.; Jimenez Cruz, C.A.; Garcia-Beltran, W.F.; Carlson, J.M.; van Teijlingen, N.H.; Mann, J.K.; Jaggernath, M.; Kang, S.; Körner, C.; et al. Selection of an HLA-C*03:04-Restricted HIV-1 P24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa. PLoS Med. 2015, 12, e1001900. [Google Scholar] [CrossRef] [PubMed]
- de Sá, N.B.R.; Ribeiro-Alves, M.; da Silva, T.P.; Pilotto, J.H.; Rolla, V.C.; Giacoia-Gripp, C.B.W.; Scott-Algara, D.; Morgado, M.G.; Teixeira, S.L.M. Clinical and Genetic Markers Associated with Tuberculosis, HIV-1 Infection, and TB/HIV-Immune Reconstitution Inflammatory Syndrome Outcomes. BMC Infect. Dis. 2020, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, J.M.; Pittari, G.; Gooley, T.A.; Chewning, J.H.; Spellman, S.; Haagenson, M.; Gallagher, M.M.; Malkki, M.; Petersdorf, E.; Dupont, B.; et al. HLA-C-Dependent Prevention of Leukemia Relapse by Donor Activating KIR2DS1. N. Engl. J. Med. 2012, 367, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; Trachtenberg, E.; Bergemann, T.L.; Saeteurn, K.; Klein, J.; Le, C.T.; Marsh, S.G.E.; Guethlein, L.A.; Parham, P.; Miller, J.S.; et al. Donors with Group B KIR Haplotypes Improve Relapse-Free Survival after Unrelated Hematopoietic Cell Transplantation for Acute Myelogenous Leukemia. Blood 2009, 113, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.E.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor Selection for Natural Killer Cell Receptor Genes Leads to Superior Survival after Unrelated Transplantation for Acute Myelogenous Leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Weisdorf, D.J.; Wang, T.; Marsh, S.G.E.; Cereb, N.; Haagenson, M.D.; Spellman, S.R.; Lee, S.J.; Guethlein, L.A.; Parham, P.; et al. Donor Killer Cell Immunoglobulin-Like Receptor Genotype Does Not Improve Graft-versus-Leukemia Responses in Chronic Lymphocytic Leukemia after Unrelated Donor Transplant: A Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2019, 25, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Sablik, K.A.; Litjens, N.H.R.; Klepper, M.; Betjes, M.G.H. Increased CD16 Expression on NK Cells Is Indicative of Antibody-Dependent Cell-Mediated Cytotoxicity in Chronic-Active Antibody-Mediated Rejection. Transpl. Immunol. 2019, 54, 52–58. [Google Scholar] [CrossRef]
- Kildey, K.; Francis, R.S.; Hultin, S.; Harfield, M.; Giuliani, K.; Law, B.M.P.; Wang, X.; See, E.J.; John, G.; Ungerer, J.; et al. Specialized Roles of Human Natural Killer Cell Subsets in Kidney Transplant Rejection. Front. Immunol. 2019, 10, 1877. [Google Scholar] [CrossRef]
- Littera, R.; Piredda, G.; Argiolas, D.; Lai, S.; Congeddu, E.; Ragatzu, P.; Melis, M.; Carta, E.; Michittu, M.B.; Valentini, D.; et al. KIR and Their HLA Class I Ligands: Two More Pieces towards Completing the Puzzle of Chronic Rejection and Graft Loss in Kidney Transplantation. PLoS ONE 2017, 12, e0180831. [Google Scholar] [CrossRef]
- van Bergen, J.; Thompson, A.; Haasnoot, G.W.; Roodnat, J.I.; de Fijter, J.W.; Claas, F.H.J.; Koning, F.; Doxiadis, I.I.N. KIR-Ligand Mismatches Are Associated with Reduced Long-Term Graft Survival in HLA-Compatible Kidney Transplantation. Am. J. Transplant. 2011, 11, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK Cells in Autoimmune Diseases: Linking Innate and Adaptive Immune Responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Natural Killer Cells: Potential Biomarkers and Therapeutic Target in Autoimmune Diseases? Front. Immunol. 2021, 12, 616853. [Google Scholar] [CrossRef] [PubMed]
- Littera, R.; Chessa, L.; Onali, S.; Figorilli, F.; Lai, S.; Secci, L.; La Nasa, G.; Caocci, G.; Arras, M.; Melis, M.; et al. Exploring the Role of Killer Cell Immunoglobulin-Like Receptors and Their HLA Class I Ligands in Autoimmune Hepatitis. PLoS ONE 2016, 11, e0146086. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; Castro-Santos, P.; Durán, J.; Santiago, C.; Lucia, A. The Genetics of Spondyloarthritis. J. Pers. Med. 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D.; Zhou, X.; Lee, M.; Weisman, M.H.; Yi, L.; Gensler, L.S.; Zou, H.; Ward, M.M.; Ishimori, M.L.; Learch, T.J.; et al. HLA Class I and II Alleles in Susceptibility to Ankylosing Spondylitis. Ann. Rheum. Dis. 2019, 78, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Jepson, A.; Young, A.; Whittle, H.C.; Greenwood, B.M.; Wordsworth, B.P. Ankylosing Spondylitis in West Africans--Evidence for a Non-HLA-B27 Protective Effect. Ann. Rheum. Dis. 1997, 56, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; Vidal-Castiñeira, J.R.; Alonso-Arias, R.; Suarez-Alvarez, B.; Vicario, J.L.; Solana, R.; Collantes, E.; López-Vázquez, A.; Martínez-Borra, J.; López-Larrea, C. Association of the KIR3DS1*013 and KIR3DL1*004 Alleles with Susceptibility to Ankylosing Spondylitis. Arthritis Rheum. 2010, 62, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.L.; International Genetics of Ankylosing Spondylitis Consortium; Vukcevic, D.; Leslie, S.; Harris, J.; Lê Cao, K.-A.; Kenna, T.J.; Brown, M.A. Epistatic Interactions between Killer Immunoglobulin-like Receptors and Human Leukocyte Antigen Ligands Are Associated with Ankylosing Spondylitis. PLoS Genet. 2020, 16, e1008906. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Jamshidi, A.R.; Karami, J.; Mohseni, A.; Amirzargar, A.A.; Farhadi, E.; Ahmadzadeh, N.; Nicknam, M.H. Analysis of Killer Cell Immunoglobulin-like Receptor Genes and Their HLA Ligands in Iranian Patients with Ankylosing Spondylitis. Iran. J. Allergy Asthma Immunol. 2016, 15, 27–38. [Google Scholar]
- Chandran, V.; Bull, S.B.; Pellett, F.J.; Ayearst, R.; Pollock, R.A.; Gladman, D.D. Killer-Cell Immunoglobulin-like Receptor Gene Polymorphisms and Susceptibility to Psoriatic Arthritis. Rheumatology 2014, 53, 233–239. [Google Scholar] [CrossRef]
- Augusto, D.G.; Petzl-Erler, M.L. KIR and HLA under Pressure: Evidences of Coevolution across Worldwide Populations. Hum. Genet. 2015, 134, 929–940. [Google Scholar] [CrossRef] [PubMed]
- de Brito Vargas, L.; Beltrame, M.H.; Ho, B.; Marin, W.M.; Dandekar, R.; Montero-Martín, G.; Fernández-Viña, M.A.; Hurtado, A.M.; Hill, K.R.; Tsuneto, L.T.; et al. Remarkably Low KIR and HLA Diversity in Amerindians Reveals Signatures of Strong Purifying Selection Shaping the Centromeric KIR Region. Mol. Biol. Evol. 2022, 39, msab298. [Google Scholar] [CrossRef]
- Nakimuli, A.; Chazara, O.; Farrell, L.; Hiby, S.E.; Tukwasibwe, S.; Knee, O.; Jayaraman, J.; Traherne, J.A.; Elliott, A.M.; Kaleebu, P.; et al. Killer Cell Immunoglobulin-like Receptor (KIR) Genes and Their HLA-C Ligands in a Ugandan Population. Immunogenetics 2013, 65, 765–775. [Google Scholar] [CrossRef]
- Gentle, N.L.; Loubser, S.; Paximadis, M.; Puren, A.; Tiemessen, C.T. Killer-Cell Immunoglobulin-like Receptor (KIR) and Human Leukocyte Antigen (HLA) Class I Genetic Diversity in Four South African Populations. Hum. Immunol. 2017, 78, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Single, R.M.; Martin, M.P.; Meyer, D.; Gao, X.; Carrington, M. Methods for Assessing Gene Content Diversity of KIR with Examples from a Global Set of Populations. Immunogenetics 2008, 60, 711–725. [Google Scholar] [CrossRef]
- Yindom, L.-M.; Leligdowicz, A.; Martin, M.P.; Gao, X.; Qi, Y.; Zaman, S.M.A.; van der Loeff, M.S.; van Tienen, C.; Jaye, A.; Aveika, A.; et al. Influence of HLA Class I and HLA-KIR Compound Genotypes on HIV-2 Infection and Markers of Disease Progression in a Manjako Community in West Africa. J. Virol. 2010, 84, 8202–8208. [Google Scholar] [CrossRef]
- Hollenbach, J.A.; Nocedal, I.; Ladner, M.B.; Single, R.M.; Trachtenberg, E.A. Killer Cell Immunoglobulin-like Receptor (KIR) Gene Content Variation in the HGDP-CEPH Populations. Immunogenetics 2012, 64, 719–737. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, S.N.; Nasir, F.; Raza, A.; Khanani, R.; Uddin, S.; Kazmi, S.U. Expression Profile of KIR3DS1/KIR3DL1 Receptors in Association with Immunological Responses in TB, HIV and HIV/TB Infected Patients. Microb. Pathog. 2023, 180, 106145. [Google Scholar] [CrossRef] [PubMed]


| KIR Gene * | European Frequency (%) | African Frequency (%) | Asian Frequency (%) | Latin American Frequency (%) | References |
| KIR2DL1 | 90–100 | 90–100 | 90–100 | 90–100 | [45] |
| KIR2DL2 | 45–55 | 60–70 | 20–50 | 50–60 | |
| KIR2DL3 | 85–95 | 80–90 | 80–100 | 80–90 | |
| KIR2DL4 | 100 | NT | 100 | 100 | |
| KIR2DL5 | 40–50 | 65–70 | 40–60 | 50–60 | |
| KIR2DS1 | 30–50 | 20–30 | 30–50 | 30–50 | |
| KIR2DS2 | 50–60 | 50–60 | 20–60 | 50–60 | |
| KIR2DS3 | 20–40 | 20–30 | 10–40 | 25–35 | |
| KIR2DS4 | 90–100 | 90–100 | 90–100 | 90–100 | |
| KIR2DS5 | 20–40 | 50–60 | 20–40 | 30–40 | |
| KIR3DL1 | 90–100 | 90–100 | 90–100 | 90–100 | |
| KIR3DL2 | 100 | NT | 100 | 100 | |
| KIR3DL3 | 100 | NT | 100 | 100 | |
| KIR3DS1 | 30–50 | 10–20 | 30–40 | 30–50 | |
| KIR2DP1 | 90–100 | 90–100 | 90–100 | 90–100 | |
| KIR3DP1 | 100 | NT | 100 | 100 | |
| KIR Genotype ** | European frequency (%) | African frequency (%) | Asian frequency (%) | Latin American frequency (%) | |
| AA | 35–55 | 55–75 | 55–65 | 40–65 | [46,47,48,49,50,51,52,53,54,55] |
| Bx | 45–65 | 25–50 | 35–45 | 35–60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Lamelas, L.; Castro-Santos, P.; Carracedo, Á.; Olloquequi, J.; Díaz-Peña, R. Unveiling the Significance of HLA and KIR Diversity in Underrepresented Populations. Biomedicines 2024, 12, 1333. https://doi.org/10.3390/biomedicines12061333
Santiago-Lamelas L, Castro-Santos P, Carracedo Á, Olloquequi J, Díaz-Peña R. Unveiling the Significance of HLA and KIR Diversity in Underrepresented Populations. Biomedicines. 2024; 12(6):1333. https://doi.org/10.3390/biomedicines12061333
Chicago/Turabian StyleSantiago-Lamelas, Lucía, Patricia Castro-Santos, Ángel Carracedo, Jordi Olloquequi, and Roberto Díaz-Peña. 2024. "Unveiling the Significance of HLA and KIR Diversity in Underrepresented Populations" Biomedicines 12, no. 6: 1333. https://doi.org/10.3390/biomedicines12061333
APA StyleSantiago-Lamelas, L., Castro-Santos, P., Carracedo, Á., Olloquequi, J., & Díaz-Peña, R. (2024). Unveiling the Significance of HLA and KIR Diversity in Underrepresented Populations. Biomedicines, 12(6), 1333. https://doi.org/10.3390/biomedicines12061333







