From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry
Abstract
:1. Introduction
2. The Immune System throughout the Lifespan
3. Decoding the Genesis of the Immune System
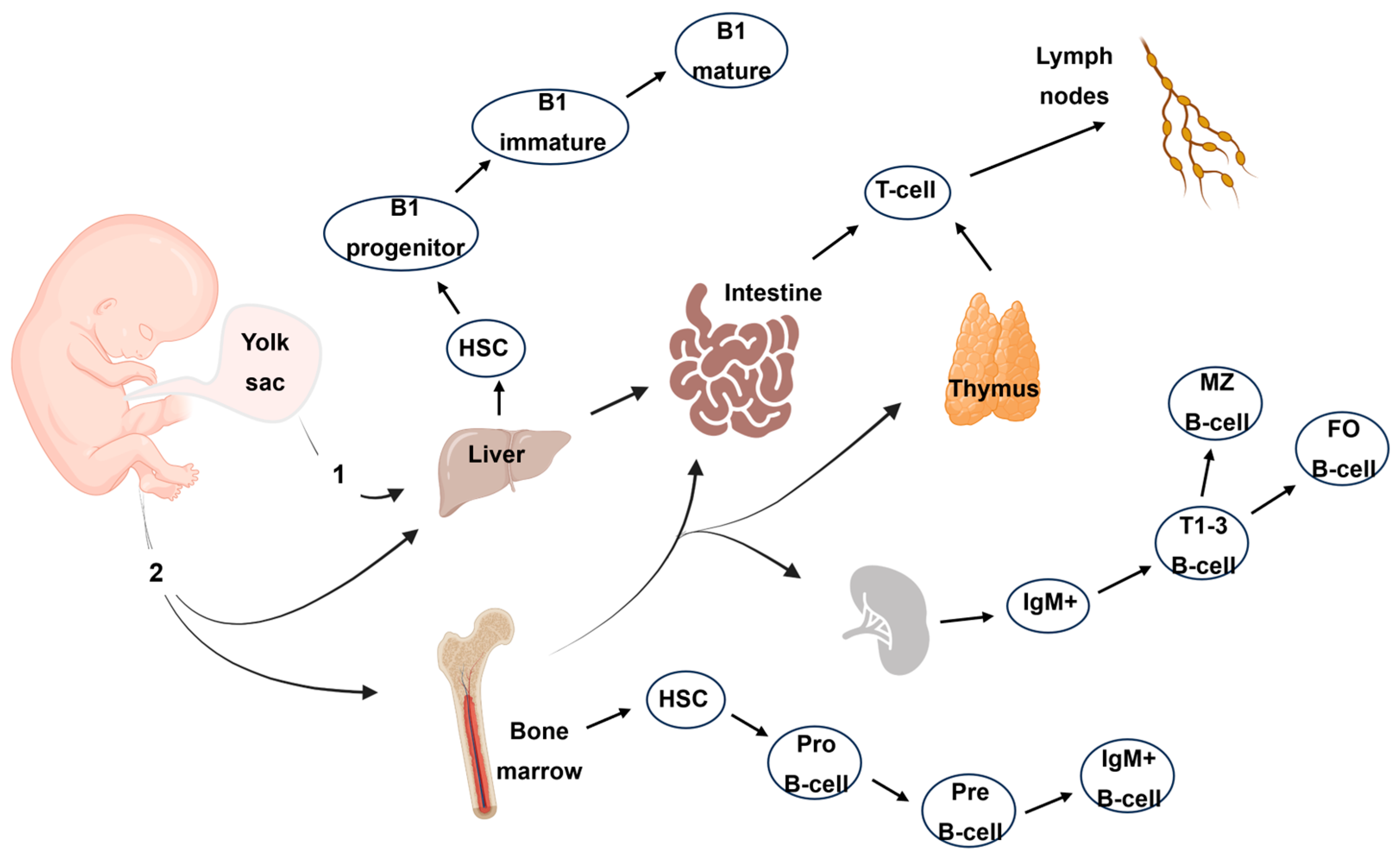
3.1. Development of the Fetal Immune System
3.2. Myeloid Cell Ontogeny
3.3. Lymphoid Cell Ontogeny
3.4. Soluble Factors of Immunity during Fetal Development
4. The Temporal Dimension for the Immune System
5. The Contribution of Flow Cytometry to Understanding Immunity during Lifespan
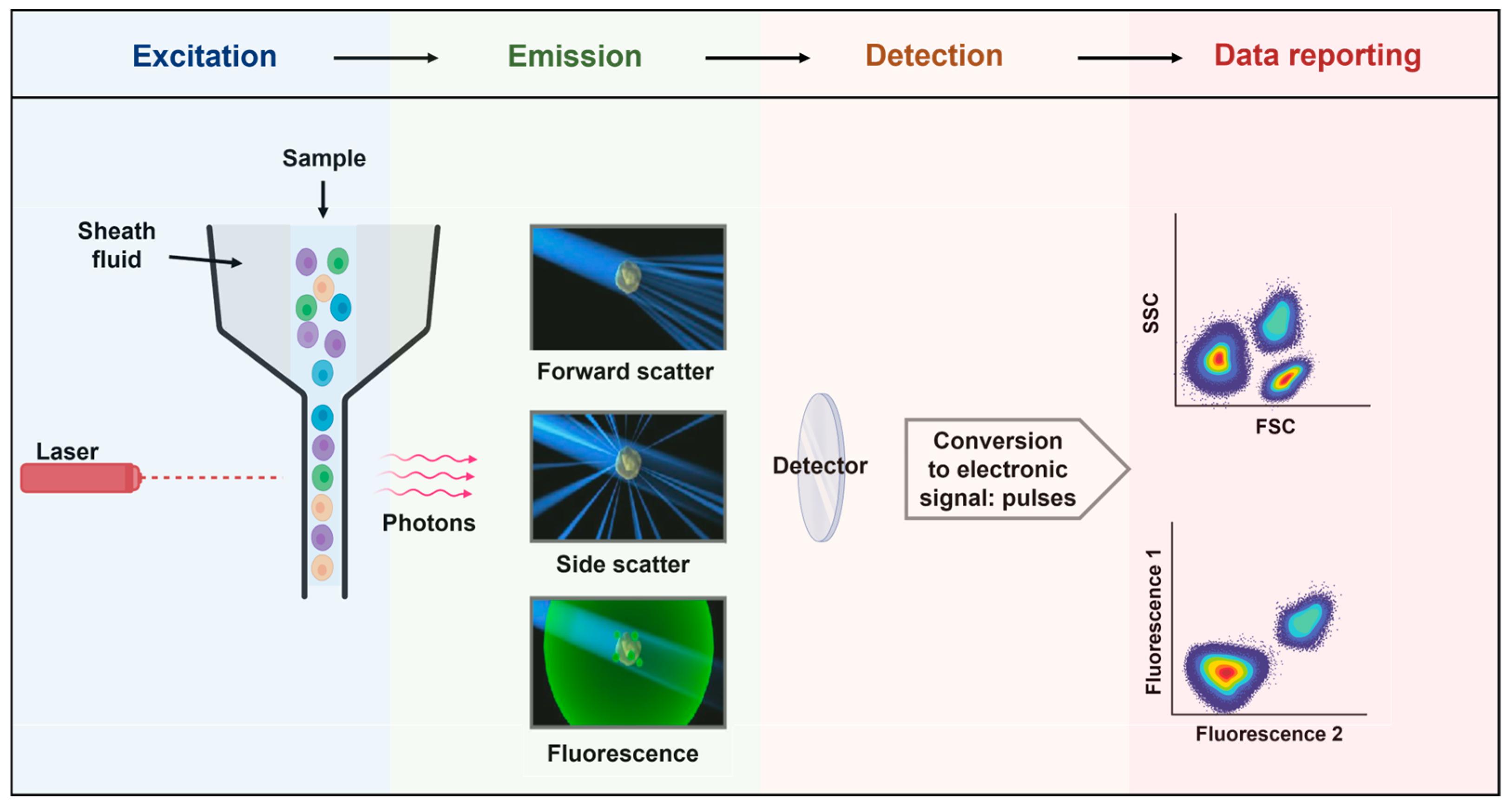
5.1. Principles of Flow Cytometry
5.2. The Contribution of Flow Cytometry in Immunology
- Naïve T-cells that recently emigrated from the thymus: TRTE cells express CD31.
- Antigen-naïve T-cells: TN with no antigenic experience but homeostatic replicative history.
- Virtual-memory T-cells: TVM have a memory phenotype prior to antigenic contact.
- T-cells with stem cell-like properties: TSCM cells possess the highest proliferative capacity of memory cells; they express CD95, CXCR3, CD45RA, CCR7, and CD27.
- Central memory T-cells: TCM express the lymph node homing molecules and have limited effector functions.
- Effector Memory T-cells: TEM preferentially traffic to peripheral tissues and mediate rapid effector functions.
- Transitional Memory T-cells: TTM, defined as CD45RA-CCR7-CD28+, have an intermediate differentiation status between CM and EM.
- Terminal Effector T-cells: TTE are memory cells re-expressing CD45RA.
- T helper 1: Th1 producing IFN-γ.
- T helper 2: Th2 producing IL-4, IL-5, IL-13, and IL-9.
- T helper 17: Th17 producing IL-17 and IL-22.
- Regulatory T-cells: Treg expressing FoxP3 which are suppressor functions.
- Follicular T-cells: Tfh producing IL-21 and expressing CXCR5.
5.3. The Analysis of the Human Immune System in Aging
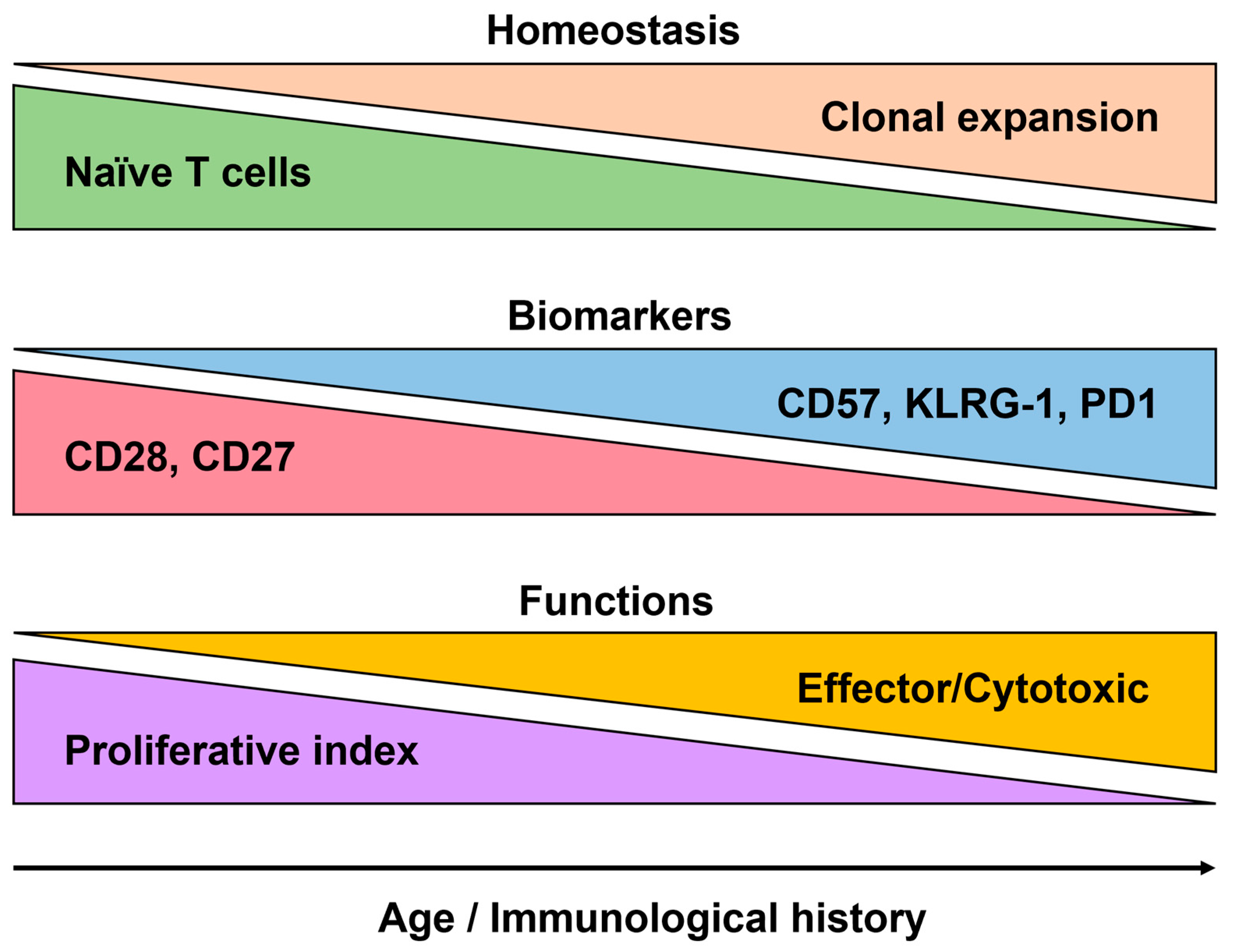
5.3.1. Immunosenescence
5.3.2. Markers of Immunosenescence
5.3.3. Exploring Aging through Extracellular Vesicles (EVs)
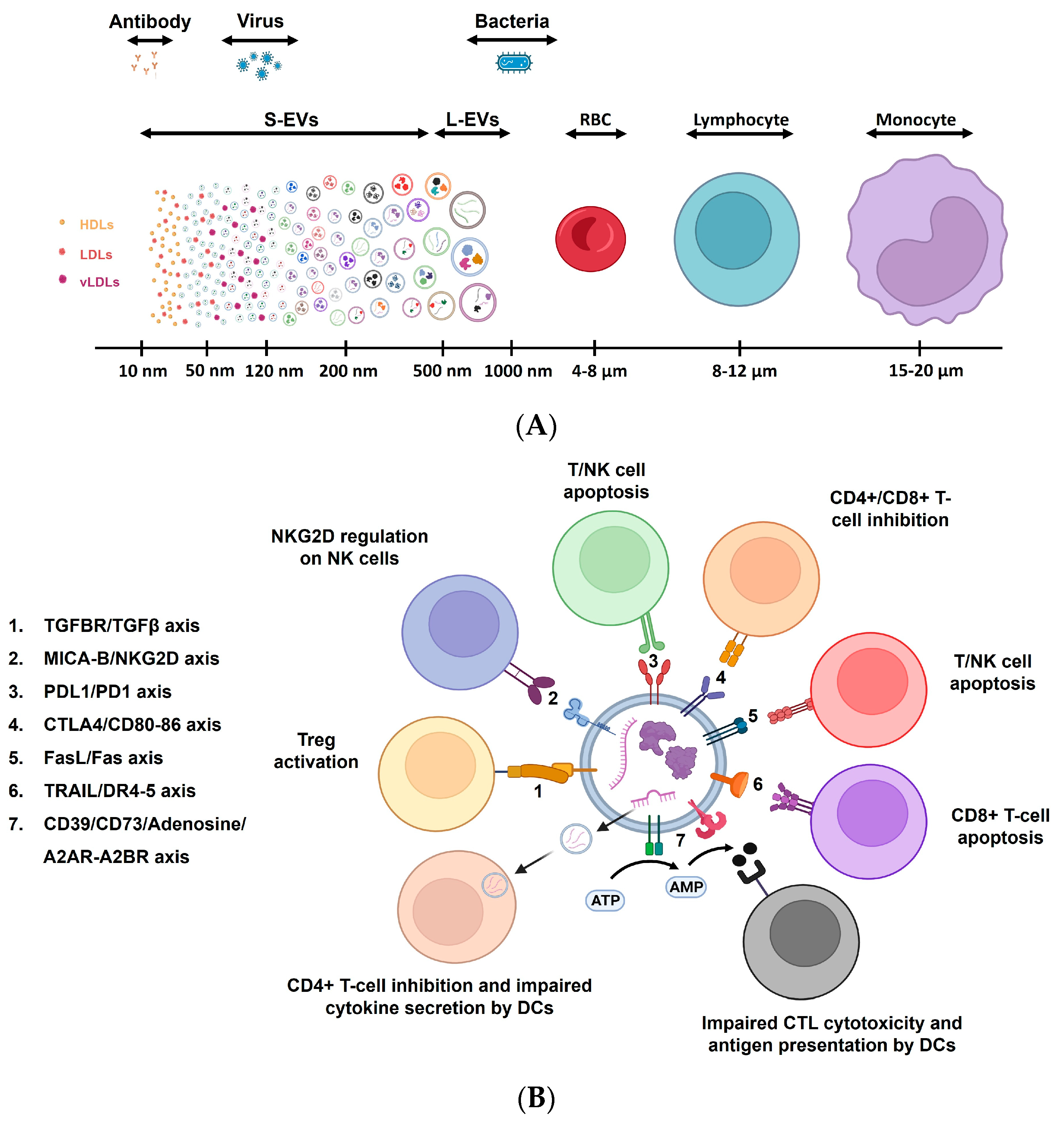
5.3.4. Immunity, Aging, and Extracellular Vesicles
6. Conclusions
Funding
Conflicts of Interest
References
- Jardine, L.; Schim van der Loeff, I.; Haq, I.J.; Sproat, T.D.R. Gestational Development of the Human Immune System. Immunol. Allergy Clin. N. Am. 2023, 43, 1–15. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef]
- Xu, W.; Wong, G.; Hwang, Y.Y.; Larbi, A. The untwining of immunosenescence and aging. Semin. Immunopathol. 2020, 42, 559–572. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Aprile, S.; Caldarella, R.; Carru, C.; Ciaccio, M.; De Vivo, I.; Gambino, C.M.; Ligotti, M.E.; Vasto, S.; et al. Age and Gender-related Variations of Molecular and Phenotypic Parameters in A Cohort of Sicilian Population: From Young to Centenarians. Aging Dis. 2021, 12, 1773–1793. [Google Scholar] [CrossRef]
- Borgoni, S.; Kudryashova, K.S.; Burka, K.; de Magalhães, J.P. Targeting immune dysfunction in aging. Ageing Res. Rev. 2021, 70, 101410. [Google Scholar] [CrossRef]
- Robinson, J.P.; Ostafe, R.; Iyengar, S.N.; Rajwa, B.; Fischer, R. Flow Cytometry: The Next Revolution. Cells 2023, 12, 1875. [Google Scholar] [CrossRef]
- Lewis, J.E.; Hergott, C.B. The Immunophenotypic Profile of Healthy Human Bone Marrow. Clin. Lab. Med. 2023, 43, 323–332. [Google Scholar] [CrossRef]
- Yi, J.S.; Rosa-Bray, M.; Staats, J.; Zakroysky, P.; Chan, C.; Russo, M.A.; Dumbauld, C.; White, S.; Gierman, T.; Weinhold, K.J.; et al. Establishment of normative ranges of the healthy human immune system with comprehensive polychromatic flow cytometry profiling. PLoS ONE 2019, 14, e0225512. [Google Scholar] [CrossRef]
- Hurabielle, C.; LaFlam, T.N.; Gearing, M.; Ye, C.J. Functional genomics in inborn errors of immunity. Immunol. Rev. 2024, 322, 53–70. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.J.; Tesselaar, K.; Borghans, J.A.M. Better safe than sorry: Naive T-cell dynamics in healthy ageing. Semin. Immunol. 2023, 70, 101839. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies (Translated from the French by P. Chalmers Mitchell); William Heineman: London, UK, 1907. [Google Scholar]
- Härtel, C.; Adam, N.; Strunk, T.; Temming, P.; Müller-Steinhardt, M.; Schultz, C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin. Exp. Immunol. 2005, 142, 446–453. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Nino, G.; Hong, X.; Wang, X. Epigenomics and Early Life Human Humoral Immunity: Novel Paradigms and Research Opportunities. Front. Immunol. 2020, 11, 1766. [Google Scholar] [CrossRef]
- Moossavi, S.; Miliku, K.; Sepehri, S.; Khafipour, E.; Azad, M.B. The Prebiotic and Probiotic Properties of Human Milk: Implications for Infant Immune Development and Pediatric Asthma. Front. Pediatr. 2018, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Ying, K.; Justice, J.N.; Belsky, D.W.; Chen, A.T.H.; Chen, B.H.; Cohen, A.A.; Fuellen, G.; et al. Validation of biomarkers of aging. Nat. Med. 2024, 30, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Biomarkers of Aging Consortium; Justice, J.; Belsky, D.W.; Higgins-Chen, A.; Moskalev, A.; Fuellen, G.; Cohen, A.A.; et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 2023, 186, 3758–3775. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Fulop, T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytom. A 2014, 85, 25–35. [Google Scholar] [CrossRef]
- Miles, D.J.; van der Sande, M.; Jeffries, D.; Kaye, S.; Ismaili, J.; Ojuola, O.; Sanneh, M.; Touray, E.S.; Waight, P.; Rowland-Jones, S.; et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J. Virol. 2007, 81, 5766–5776. [Google Scholar] [CrossRef]
- Sauce, D.; Larsen, M.; Fastenackels, S.; Duperrier, A.; Keller, M.; Grubeck-Loebenstein, B.; Ferrand, C.; Debré, P.; Sidi, D.; Appay, V. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Investig. 2009, 119, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Reza, A.M.; Qasem, W.A.; Friel, J.K.; Omri, A. Development of the immune system in the human embryo. Pediatr. Res. 2022, 92, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front. Immunol. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Brousseau, N.; Angers-Goulet, M.E.; Bastien, R.; Ye, L.; Sadarangani, M.; Halperin, S.A. Vaccination during pregnancy and modulation of IgG response to pertussis vaccines in infants: The impact of different vaccine formulations. Vaccine 2024, 42, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Ono, S.; Michihata, N.; Yamana, H.; Yasunaga, H. Duration of influenza vaccine effectiveness in the elderly in Japan: A retrospective cohort study using large-scale population-based registry data. Vaccine 2023, 41, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Dirks, J.; Viemann, D.; Beyersdorf, N.; Härtel, C.; Morbach, H. Insights into B-cell ontogeny inferred from human immunology. Eur. J. Immunol. 2023, 53, e2250116. [Google Scholar] [CrossRef] [PubMed]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Wang, J.; Han, T.; Zhu, X. Role of maternal-fetal immune tolerance in the establishment and maintenance of pregnancy. Chin. Med. J. 2024, 137, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C. Immunological Consequences of In Utero Exposure to Foreign Antigens. Front. Immunol. 2021, 12, 638435. [Google Scholar] [CrossRef]
- Gu, W.; Eke, C.; Gonzalez Santiago, E.; Olaloye, O.; Konnikova, L. Single-cell atlas of the small intestine throughout the human lifespan demonstrates unique features of fetal immune cells. Mucosal Immunol. 2024. [Google Scholar] [CrossRef]
- Koren, O.; Konnikova, L.; Brodin, P.; Mysorekar, I.U.; Collado, M.C. The maternal gut microbiome in pregnancy: Implications for the developing immune system. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 35–45. [Google Scholar] [CrossRef] [PubMed]
- McGovern, N.; Shin, A.; Low, G.; Low, D.; Duan, K.; Yao, L.J.; Msallam, R.; Low, I.; Shadan, N.B.; Sumatoh, H.R.; et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 2017, 546, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.; Zhang, L.; Breslin, S.P.; Zhang, J. The Fetal-to-Adult Hematopoietic Stem Cell Transition and its Role in Childhood Hematopoietic Malignancies. Stem Cell Rev. Rep. 2021, 17, 2059–2080. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Kapoor, S.; Carvalho, C.; Bajénoff, M.; Gentek, R. Mast cell ontogeny: From fetal development to life-long health and disease. Immunol. Rev. 2023, 315, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Liu, Z.; Kwok, I.; Ginhoux, F. Origin and Heterogeneity of Tissue Myeloid Cells: A Focus on GMP-Derived Monocytes and Neutrophils. Annu. Rev. Immunol. 2023, 41, 375–404. [Google Scholar] [CrossRef]
- Patel, A.A.; Ginhoux, F.; Yona, S. Monocytes, macrophages, dendritic cells and neutrophils: An update on lifespan kinetics in health and disease. Immunology 2021, 163, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Hematopoietic stem cell-independent hematopoiesis: Emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. FEBS Lett. 2016, 590, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Feyaerts, D.; Urbschat, C.; Gaudillière, B.; Stelzer, I.A. Establishment of tissue-resident immune populations in the fetus. Semin. Immunopathol. 2022, 44, 747–766. [Google Scholar] [CrossRef]
- Hou, S.; Liu, C.; Yao, Y.; Bai, Z.; Gong, Y.; Wang, C.; He, J.; You, G.; Zhang, G.; Liu, B.; et al. Hematopoietic Stem Cell Development in Mammalian Embryos. Adv. Exp. Med. Biol. 2023, 1442, 1–16. [Google Scholar] [CrossRef]
- Haynes, B.F.; Heinly, C.S. Early human T cell development: Analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J. Exp. Med. 1995, 181, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Alhaj Hussen, K.; Louis, V.; Canque, B. A new model of human lymphopoiesis across development and aging. Trends Immunol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, C.; Gong, Y.; Bai, Z.; Hou, S.; He, J.; Bian, Z.; Li, Z.; Ni, Y.; Yan, J.; et al. Single-Cell RNA Sequencing Resolves Spatiotemporal Development of Pre-thymic Lymphoid Progenitors and Thymus Organogenesis in Human Embryos. Immunity 2019, 51, 930–948.e6. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, J.; Mold, J.E.; McCune, J.M.; Nixon, D.F. Regulation of T cell responses in the developing human fetus. J. Immunol. 2006, 176, 5741–5748. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, R.R.C.E.; Baumdick, M.E.; Sagebiel, A.F.; Kaufmann, M.; Mokry, M.; Klarenbeek, P.L.; Schaltenberg, N.; Steinert, F.L.; van Rijn, J.M.; Drewniak, A.; et al. Human Fetal TNF-α-Cytokine-Producing CD4+ Effector Memory T Cells Promote Intestinal Development and Mediate Inflammation Early in Life. Immunity 2019, 50, 462–476.e8. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P.; Tavian, M.; Humeau, L.; Péault, B. Early ontogeny of the human marrow from long bones: An immunohistochemical study of hematopoiesis and its microenvironment. Blood 1996, 87, 4109–4119. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, C.; Nishimoto, N.; Gartland, G.L.; Billips, L.G.; Burrows, P.D.; Kubagawa, H.; Cooper, M.D. B cells are generated throughout life in humans. J. Immunol. 1996, 156, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Herzenberg, L.A.; Tung, J.W. B cell lineages: Documented at last! Nat. Immunol. 2006, 7, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, D.; Biasucci, A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J. Clin. Investig. 1969, 48, 1433–1446. [Google Scholar] [CrossRef]
- Miller, D.L.; Hiravonen, T.; Gitlin, D. Synthesis of IgE by the human conceptus. J. Allergy Clin. Immunol. 1973, 52, 182–188. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Palettas, M.; Christian, L.M. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav. Immun. 2017, 60, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Kurian, N.K.; Rao, K.A. Cytokines, NK cells and regulatory T cell functions in normal pregnancy and reproductive failures. Am. J. Reprod. Immunol. 2023, 89, e13667. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.H.; Arroyave, C.M. The complement system of the newborn infant. Biol. Neonatol. 1980, 37, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; Penix, L.; Weaver, W.M.; Melvin, A.; Lewis, D.B. Ontogeny of T lymphocyte function in the neonate. Am. J. Reprod. Immunol. 1992, 28, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Variability of vaccine responsiveness in early life. Cell Immunol. 2023, 393–394, 104777. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Marzollo, A.; Michev, A.; Fellay, J. Susceptibility to infection in early life: A growing role for human genetics. Hum. Genet. 2020, 139, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Connors, T.J.; Matsumoto, R.; Verma, S.; Szabo, P.A.; Guyer, R.; Gray, J.; Wang, Z.; Thapa, P.; Dogra, P.; Poon, M.M.L.; et al. Site-specific development and progressive maturation of human tissue-resident memory T cells over infancy and childhood. Immunity 2023, 56, 1894–1909.e5. [Google Scholar] [CrossRef]
- Jameson, S.C. The Naming of Memory T-Cell Subsets. Cold Spring Harb. Perspect. Biol. 2021, 13, a037788. [Google Scholar] [CrossRef]
- Larbi, A.; Franceschi, C.; Mazzatti, D.; Solana, R.; Wikby, A.; Pawelec, G. Aging of the immune system as a prognostic factor for human longevity. Physiology 2008, 23, 64–74. [Google Scholar] [CrossRef]
- Pardieck, I.N.; Beyrend, G.; Redeker, A.; Arens, R. Cytomegalovirus infection and progressive differentiation of effector-memory T cells. F1000Research 2018, 7, 1554. [Google Scholar] [CrossRef] [PubMed]
- Cicin-Sain, L. Cytomegalovirus memory inflation and immune protection. Med. Microbiol. Immunol. 2019, 208, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Faint, J.M.; Annels, N.E.; Curnow, S.J.; Shields, P.; Pilling, D.; Hislop, A.D.; Wu, L.; Akbar, A.N.; Buckley, C.D.; Moss, P.A.; et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J. Immunol. 2001, 167, 212–220. [Google Scholar] [CrossRef]
- Tedeschi, V.; Paldino, G.; Kunkl, M.; Paroli, M.; Sorrentino, R.; Tuosto, L.; Fiorillo, M.T. CD8+ T Cell Senescence: Lights and Shadows in Viral Infections, Autoimmune Disorders and Cancer. Int. J. Mol. Sci. 2022, 23, 3374. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Shin, K.S.; Kim, G.Y.; Song, Y.C.; Bae, E.A.; Kim, I.K.; Koh, C.H.; Kang, C.Y. Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell. 2016, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Martelli, S.; Tan, S.W.; Simoni, Y.; Chong, M.L.; Yap, S.H.; Newell, E.W.; Pender, S.L.F.; Kamarulzaman, A.; Rajasuriar, R.; et al. Adaptive NKG2C+CD57+ Natural Killer Cell and Tim-3 Expression During Viral Infections. Front. Immunol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Van Avondt, K.; Strecker, J.K.; Tulotta, C.; Minnerup, J.; Schulz, C.; Soehnlein, O. Neutrophils in aging and aging-related pathologies. Immunol. Rev. 2023, 314, 357–375. [Google Scholar] [CrossRef]
- McQuattie-Pimentel, A.C.; Ren, Z.; Joshi, N.; Watanabe, S.; Stoeger, T.; Chi, M.; Lu, Z.; Sichizya, L.; Aillon, R.P.; Chen, C.I.; et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Investig. 2021, 131, e140299. [Google Scholar] [CrossRef]
- Pence, B.D.; Yarbro, J.R. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp. Gerontol. 2018, 108, 112–117. [Google Scholar] [CrossRef]
- Gupta, S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp. Gerontol. 2014, 54, 47–52. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leuk. Biol. 2017, 102, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Thakolwiboon, S.; Mills, E.A.; Yang, J.; Doty, J.; Belkin, M.I.; Cho, T.; Schultz, C.; Mao-Draayer, Y. Immunosenescence and multiple sclerosis: Inflammaging for prognosis and therapeutic consideration. Front. Aging. 2023, 4, 1234572. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Khavinson, V.; Linkova, N.; Dyatlova, A.; Kantemirova, R.; Kozlov, K. Senescence-Associated Secretory Phenotype of Cardiovascular System Cells and Inflammaging: Perspectives of Peptide Regulation. Cells 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Practical Flow Cytometry, 4th ed.; Wiley-Liss: New York, NY, USA, 2003; ISBN 978-0-471-41125-3. [Google Scholar]
- Sharpless, T.; Traganos, F.; Darzynkiewicz, Z.; Melamed, M.R. Flow cytofluorimetry: Discrimination between single cells and cell aggregates by direct size measurements. Acta Cytol. 1975, 19, 577–581. [Google Scholar] [PubMed]
- Hardy, R.R.; Hayakawa, K.; Haaijman, J.; Herzenberg, L.A. B-cell subpopulations identified by two-colour fluorescence analysis. Nature 1982, 297, 589–591. [Google Scholar] [CrossRef]
- Shapiro, H.M. Flow Cytometry: The Glass Is Half Full. Methods Mol. Biol. 2018, 1678, 1–10. [Google Scholar] [CrossRef]
- Lacombe, F.; Bernal, E.; Bloxham, D.; Couzens, S.; Porta, M.G.; Johansson, U.; Kern, W.; Macey, M.; Matthes, T.; Morilla, R.; et al. Harmonemia: A universal strategy for flow cytometry immunophenotyping-A European LeukemiaNet WP10 study. Leukemia 2016, 30, 1769–1772. [Google Scholar] [CrossRef]
- Giudice, V.; Serio, B.; Bertolini, A.; Mettivier, L.; D’Alto, F.; Pezzullo, L.; D’Addona, M.; Fumo, R.; Zeppa, P.; Gorrese, M.; et al. Implementation of International Prognostic Index with flow cytometry immunophenotyping for better risk stratification of chronic lymphocytic leukemia. Eur. J. Haematol. 2022, 109, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Park, L.M.; Lannigan, J.; Jaimes, M.C. OMIP-069: Forty-Color Full Spectrum Flow Cytometry Panel for Deep Immunophenotyping of Major Cell Subsets in Human Peripheral Blood. Cytom. A 2020, 97, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Andrä, I.; Ulrich, H.; Dürr, S.; Soll, D.; Henkel, L.; Angerpointner, C.; Ritter, J.; Przibilla, S.; Stadler, H.; Effenberger, M.; et al. An Evaluation of T-Cell Functionality After Flow Cytometry Sorting Revealed p38 MAPK Activation. Cytom. A 2020, 97, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J. Evaluation of Cytokine Production at the Single Cell Level by Flow Cytometry Upon Polyclonal Stimulation. Methods Mol. Biol. 2021, 2285, 111–119. [Google Scholar] [CrossRef]
- de Neergaard, T.; Nordenfelt, P. Quantification of Phagocytosis Using Flow Cytometry. Methods Mol. Biol. 2023, 2674, 221–234. [Google Scholar] [CrossRef]
- Perfetto, S.P.; Chattopadhyay, P.K.; Roederer, M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004, 4, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Feng, X.; Xu, L.; Li, C.; Ma, Y.; Peng, M. Within- and between-subject biological variation estimates for the enumeration of lymphocyte deep immunophenotyping and monocyte subsets. Clin. Chem. Lab. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Schimke, L.F.; de Oliveira, E.B., Jr.; El Khawanky, N.; Ramos, R.N.; Al-Ramadi, B.K.; Segundo, G.R.S.; Ochs, H.D.; Condino-Neto, A. Flow Cytometry Contributions for the Diagnosis and Immunopathological Characterization of Primary Immunodeficiency Diseases with Immune Dysregulation. Front. Immunol. 2019, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Mulroney, K.; Kopczyk, M.; Carson, C.; Paton, T.; Inglis, T.; Chakera, A. Same-day confirmation of infection and antimicrobial susceptibility profiling using flow cytometry. eBioMedicine 2022, 82, 104145. [Google Scholar] [CrossRef]
- Ohno, T.; Kanoh, T.; Suzuki, T.; Masuda, T.; Kuribayashi, K.; Araya, S.; Arai, H.; Uchino, H. Comparative analysis of lymphocyte phenotypes between carriers of human immunodeficiency virus (HIV) and adult patients with primary immunodeficiency using two-color immunofluorescence flow cytometry. Tohoku J. Exp. Med. 1988, 154, 157–172. [Google Scholar] [CrossRef]
- Kestens, L.; Mandy, F. Thirty-five years of CD4 T-cell counting in HIV infection: From flow cytometry in the lab to point-of-care testing in the field. Cytom. B Clin. Cytom. 2017, 92, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gossez, M.; Malcus, C.; Demaret, J.; Frater, J.; Poitevin-Later, F.; Monneret, G. Evaluation of a novel automated volumetric flow cytometer for absolute CD4+ T lymphocyte quantitation. Cytom. B Clin. Cytom. 2017, 92, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Schmit, T.; Klomp, M.; Khan, M.N. The Application of Flow Cytometry for Simultaneous and Multi-parametric Analysis of Heterogenous Cell Populations in Basic and Clinical Research. Methods Mol. Biol. 2021, 2223, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mai, H.; Wang, L.; Chen, S.; Chen, F.; Li, T.; Liu, Y.; Zhou, G.; Liu, S.; Wang, Y.; et al. Diagnostic significance of cerebrospinal fluid flow cytometry in Chinese children with B lineage acute lymphoblastic leukemia. BMC Pediatr. 2024, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Westers, T.M.; Saft, L.; van der Velden, V.H.J.; Te Marvelde, J.G.; Dunlop, A.; Ireland, R.; Valent, P.; Porwit, A.; Béné, M.C.; van de Loosdrecht, A.A. A series of case studies illustrating the role of flow cytometry in the diagnostic work-up of myelodysplastic syndromes. Cytom. B Clin. Cytom. 2023, 104, 87–97. [Google Scholar] [CrossRef]
- Felgentreff, K.; Baumann, U.; Klemann, C.; Schuetz, C.; Viemann, D.; Wetzke, M.; Pannicke, U.; von Hardenberg, S.; Auber, B.; Debatin, K.M.; et al. Biomarkers of DNA Damage Response Enable Flow Cytometry-Based Diagnostic to Identify Inborn DNA Repair Defects in Primary Immunodeficiencies. J. Clin. Immunol. 2022, 42, 286–298. [Google Scholar] [CrossRef] [PubMed]
- David, J.A.; Huang, J.Z. Diagnostic Utility of Flow Cytometry Analysis of Reactive T Cells in Nodular Lymphocyte-Predominant Hodgkin Lymphoma. Am. J. Clin. Pathol. 2016, 145, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T memory stem cells in health and disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef]
- Hunt, R.M.; Elzayat, M.T.; Markofski, M.M.; Laughlin, M.; LaVoy, E.C. Characterization of transitional memory CD4+ and CD8+ T-cell mobilization during and after an acute bout of exercise. Front. Sports Act. Living. 2023, 5, 1120454. [Google Scholar] [CrossRef]
- Yoshitomi, H. Peripheral helper T cells, mavericks of peripheral immune responses. Int. Immunol. 2024, 36, 9–16. [Google Scholar] [CrossRef]
- Pérez-Lanzón, M.; Plantureux, C.; Paillet, J.; Sotty, J.; Soussan, P.; Kroemer, G.; Maiuri, M.C.; Pol, J. Flow Cytometry Assessment of Lymphocyte Populations Infiltrating Liver Tumors. Methods Mol. Biol. 2024, 2769, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Villanueva, A.; Korangy, F.; Ruf, B.; Yarchoan, M.; Ma, L.; Ruppin, E.; Wang, X.W. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Huerta, R.F.; Mandujano-López, V.; Velásquez-Ortiz, G.; Alcalá-Carmona, B.; Ostos-Prado, M.J.; Reyna-Juárez, Y.; Meza-Sánchez, D.E.; Juárez-Vega, G.; Mejía-Domínguez, N.R.; Torres-Ruiz, J.; et al. Novel B cell subsets as potential biomarkers in Idiopathic Inflammatory Myopathies: Insights into disease pathogenesis and disease activity. J. Leukoc. Biol. 2024, 116, 84–94. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Attwood, K.M.; Liu, X.; Chen, G.L.; Minderman, H.; Alousi, A.; Bashey, A.; Lowsky, R.; Miklos, D.B.; Hansen, J.; et al. Galectin-3 predicts acute GvHD and overall mortality post reduced intensity allo-HCT: A BMT-CTN biorepository study. Bone Marrow Transplant. 2024, 59, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cenariu, M.; Grewal, R.; Bumbea, H.; Sauma, D.; Tomuleasa, C. Editorial: Flow cytometry—A powerful tool for diagnosis and therapy monitoring in hematology and immunology. Front Med. 2023, 10, 1282060. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, R.; Gatt, P.N.; Smith, S.; Fernandez, M.A.; Vaughan, L.; Blyth, E.; Curnow, J.; Brown, D.A.; Tegg, E.; Sasson, S.C. Measurable Residual Disease (MRD) by Flow Cytometry in Adult B-Acute Lymphoblastic Leukaemia (B-ALL) and Acute Myeloid Leukaemia (AML): Correlation with Molecular MRD Testing and Clinical Outcome at One Year. Cancers 2023, 15, 5064. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Fernández, R.; Sánchez, B.; Ruiz, L.; Margolles, A. Convergence of flow cytometry and bacteriology. Current and future applications: A focus on food and clinical microbiology. Crit. Rev. Microbiol. 2023, 49, 556–577. [Google Scholar] [CrossRef]
- Mazzoni, A.; Annunziato, F.; Maggi, L. T lymphocytes-related cell network in the pathogenesis of juvenile idiopathic arthritis: A key point for personalized treatment. Curr. Opin. Rheumatol. 2024, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Q.; Goronzy, J.J.; Weyand, C.M. Immune aging—A mechanism in autoimmune disease. Semin. Immunol. 2023, 69, 101814. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Ng, T.P.; Pender, S.L.; Larbi, A. CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol. Immunother. 2016, 65, 441–452. [Google Scholar] [CrossRef]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Liao, T.; Qian, H.; Liu, Y. Immunosenescence and macrophages: From basics to therapeutics. Int. J. Biochem. Cell Biol. 2023, 165, 106479. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Cohen, A.A.; Provost, G.; Khalil, A.; Lacombe, G.; Rodrigues, S.; Desroches, M.; Hirokawa, K.; et al. Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change. Vaccines 2022, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting Cellular Senescence in Aging and Age-Related Diseases: Challenges, Considerations, and the Emerging Role of Senolytic and Senomorphic Therapies. Aging Dis. 2024. [Google Scholar] [CrossRef]
- Cevirgel, A.; Shetty, S.A.; Vos, M.; Nanlohy, N.M.; Beckers, L.; Bijvank, E.; Rots, N.; van Beek, J.; Buisman, A.M.; van Baarle, D. Identification of aging-associated immunotypes and immune stability as indicators of post-vaccination immune activation. Aging Cell. 2022, 21, e13703. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Antonangeli, F.; Sozzani, S.; Santoni, A.; Cippitelli, M.; Soriani, A. The senescence journey in cancer immunoediting. Mol. Cancer 2024, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, Y.; Goronzy, J.J.; Weyand, C.M. T cell aging as a risk factor for autoimmunity. J. Autoimmun. 2023, 137, 102947. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.L.; Godarova, A.; Wayman, J.A.; Miraldi, E.R.; Hildeman, D.A.; Chougnet, C.A. Accumulation of immune-suppressive CD4 + T cells in aging—Tempering inflammaging at the expense of immunity. Semin. Immunol. 2023, 70, 101836. [Google Scholar] [CrossRef]
- Brzezińska, A.; Magalska, A.; Szybińska, A.; Sikora, E. Proliferation and apoptosis of human CD8+CD28+ and CD8+CD28− lymphocytes during aging. Exp. Gerontol. 2004, 39, 539–544. [Google Scholar] [CrossRef]
- Bowyer, G.; Sharpe, H.; Venkatraman, N.; Ndiaye, P.B.; Wade, D.; Brenner, N.; Mentzer, A.; Mair, C.; Waterboer, T.; Lambe, T.; et al. Reduced Ebola vaccine responses in CMV+ young adults is associated with expansion of CD57+KLRG1+ T cells. J. Exp. Med. 2020, 217, e20200004. [Google Scholar] [CrossRef]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, C.; Liang, Z.; Hu, T.; Yin, Z.; Liang, Y.; Zhang, T.; Ding, Y.; Li, X.; Gai, X.; et al. Elevated CD4+ T Cell Senescence Associates with Impaired Immune Responsiveness in Severe COVID-19. Aging Dis. 2024. [Google Scholar] [CrossRef]
- Quinn, K.M.; Vicencio, D.M.; La Gruta, N.L. The paradox of aging: Aging-related shifts in T cell function and metabolism. Semin. Immunol. 2023, 70, 101834. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Liu, J.; Purba, C.M.; Christians, A.J.; Kibbie, J.J.; Castleman, M.J.; McCarter, M.D.; Wilson, C.C. Age-related alterations in human gut CD4 T cell phenotype, T helper cell frequencies, and functional responses to enteric bacteria. J. Leukoc. Biol. 2020, 107, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Fann, M.; Chiu, W.K.; Wood WH 3rd Levine, B.L.; Becker, K.G.; Weng, N.P. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol. Rev. 2005, 205, 190–206. [Google Scholar] [CrossRef]
- Hong, M.S.; Dan, J.M.; Choi, J.Y.; Kang, I. Age-associated changes in the frequency of naïve, memory and effector CD8+ T cells. Mech. Ageing Dev. 2004, 125, 615–618. [Google Scholar] [CrossRef]
- Miles, D.J.; van der Sande, M.; Jeffries, D.; Kaye, S.; Ojuola, O.; Sanneh, M.; Cox, M.; Palmero, M.S.; Touray, E.S.; Waight, P.; et al. Maintenance of large subpopulations of differentiated CD8 T-cells two years after cytomegalovirus infection in Gambian infants. PLoS ONE 2008, 3, e2905. [Google Scholar] [CrossRef] [PubMed]
- Fastenackels, S.; Sauce, D.; Vigouroux, C.; Avettand-Fènoël, V.; Bastard, J.P.; Fellahi, S.; Nailler, L.; Arezes, E.; Rouzioux, C.; Warszawski, J.; et al. HIV-mediated immune aging in young adults infected perinatally or during childhood. AIDS 2019, 33, 1705–1710. [Google Scholar] [CrossRef]
- Kared, H.; Tan, S.W.; Lau, M.C.; Chevrier, M.; Tan, C.; How, W.; Wong, G.; Strickland, M.; Malleret, B.; Amoah, A.; et al. Immunological history governs human stem cell memory CD4 heterogeneity via the Wnt signaling pathway. Nat. Commun. 2020, 11, 821. [Google Scholar] [CrossRef]
- Jain, A.; Sturmlechner, I.; Weyand, C.M.; Goronzy, J.J. Heterogeneity of memory T cells in aging. Front. Immunol. 2023, 14, 1250916. [Google Scholar] [CrossRef]
- Quinn, K.M.; Fox, A.; Harland, K.L.; Russ, B.E.; Li, J.; Nguyen, T.H.O.; Loh, L.; Olshanksy, M.; Naeem, H.; Tsyganov, K.; et al. Age-Related Decline in Primary CD8+ T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8+ T Cells. Cell Rep. 2018, 23, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Marusina, A.I.; Ono, Y.; Merleev, A.A.; Shimoda, M.; Ogawa, H.; Wang, E.A.; Kondo, K.; Olney, L.; Luxardi, G.; Miyamura, Y.; et al. CD4+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity. J. Autoimmun. 2017, 77, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Canzonieri, V.; Rizzolio, F. The history of small extracellular vesicles and their implication in cancer drug resistance. Front. Oncol. 2022, 12, 948843. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Alique, M. Extracellular Vesicles as “Very Important Particles” (VIPs) in Aging. Int. J. Mol. Sci. 2023, 24, 4250. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, H.; Wang, Y.; Zhang, L.; Wang, X. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J. Extracell. Vesicles 2021, 10, e12154. [Google Scholar] [CrossRef]
- Huber, J.; Longaker, M.T.; Quarto, N. Circulating and extracellular vesicle-derived microRNAs as biomarkers in bone-related diseases. Front Endocrinol. 2023, 14, 1168898. [Google Scholar] [CrossRef]
- Robbins, P.D. Extracellular vesicles and aging. Stem Cell Investig. 2017, 4, 98. [Google Scholar] [CrossRef]
- Manni, G.; Buratta, S.; Pallotta, M.T.; Chiasserini, D.; Di Michele, A.; Emiliani, C.; Giovagnoli, S.; Pascucci, L.; Romani, R.; Bellezza, I.; et al. Extracellular Vesicles in Aging: An Emerging Hallmark? Cells 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Lazzarini, R.; Nicolai, M.; Pirani, V.; Mariotti, C.; Di Primio, R. Effects of senescent secretory phenotype acquisition on human retinal pigment epithelial stem cells. Aging 2018, 10, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.A.; Rodríguez-Navarro, J.A.; O’Loghlen, A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020, 32, 71–86.e5. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Jeon, J.H.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Potential Benefits of Allogeneic Haploidentical Adipose Tissue-Derived Stromal Vascular Fraction in a Hutchinson-Gilford Progeria Syndrome Patient. Front. Bioeng. Biotechnol. 2020, 8, 574010. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Huda, M.N.; Nurunnabi, M. Potential Application of Exosomes in Vaccine Development and Delivery. Pharm. Res. 2022, 39, 2635–2671. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Rodrigues, M.; Fan, J.; Lyon, C.; Wan, M.; Hu, Y. Role of Extracellular Vesicles in Viral and Bacterial Infections: Pathogenesis, Diagnostics, and Therapeutics. Theranostics 2018, 8, 2709–2721. [Google Scholar] [CrossRef]
- Tankov, S.; Petrovic, M.; Lecoultre, M.; Espinoza, F.; El-Harane, N.; Bes, V.; Chliate, S.; Bedoya, D.M.; Jordan, O.; Borchard, G.; et al. Hypoxic glioblastoma-cell-derived extracellular vesicles impair cGAS-STING activity in macrophages. Cell Commun. Signal. 2024, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.; Lesende-Rodriguez, I.; Fernández-Pernas, P.; Sangiao-Alvarellos, S.; Monserrat, L.; Arntz, O.J.; van de Loo, F.J.; Mateos, J.; Arufe, M.C. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci. Rep. 2017, 7, 43923. [Google Scholar] [CrossRef] [PubMed]
- Roig-Carles, D.; Willms, E.; Fontijn, R.D.; Martinez-Pacheco, S.; Mäger, I.; de Vries, H.E.; Hirst, M.; Sharrack, B.; Male, D.K.; Hawkes, C.A.; et al. Endothelial-Derived Extracellular Vesicles Induce Cerebrovascular Dysfunction in Inflammation. Pharmaceutics 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Fujita, Y.; Yoshioka, Y.; Araya, J.; Kuwano, K.; Ochiya, T. Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol. Aspects Med. 2018, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Zhu, Y.; Chen, P.; Yang, K.; Chen, Y.; Wang, Y.; Dai, Z.; Huang, Z.; Zhong, P.; Zhao, X.; et al. Biological functions and biomedical applications of extracellular vesicles derived from blood cells. Free Rad. Biol. Med. 2024, 222, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. Therapeutic Potential of Extracellular Vesicles in Aging and Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 14632. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Ruan, L.; Oh, J.; Dong, X.; Zhuge, Q.; Su, D.M. Extracellular vesicles extracted from young donor serum attenuate inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. 2018, 32, 5899–5912. [Google Scholar] [CrossRef]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles. 2024, 13, e12404. [Google Scholar] [CrossRef]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Görgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles. 2023, 12, e12299. [Google Scholar] [CrossRef]
- Nolan, J.P. Flow Cytometry of Extracellular Vesicles: Potential, Pitfalls, and Prospects. Curr. Protoc. Cytom. 2015, 73, 13–14. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Libregts, S.F.W.M.; Arkesteijn, G.J.A.; Németh, A.; Nolte-‘t Hoen, E.N.M.; Wauben, M.H.M. Flow cytometric analysis of extracellular vesicle subsets in plasma: Impact of swarm by particles of non-interest. J. Thromb. Haemost. 2018, 16, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Renner, T.M.; Tang, V.A.; Burger, D.; Langlois, M.A. Intact Viral Particle Counts Measured by Flow Virometry Provide Insight into the Infectivity and Genome Packaging Efficiency of Moloney Murine Leukemia Virus. J. Virol. 2020, 94, e01600-19. [Google Scholar] [CrossRef] [PubMed]
- Brittain, G.C., 4th; Chen, Y.Q.; Martinez, E.; Tang, V.A.; Renner, T.M.; Langlois, M.A.; Gulnik, S. A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles. Sci. Rep. 2019, 9, 16039. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.K.; Zamzow, P.; Kramer, M.W.; Merseburger, A.S.; Gorantla, S.P.; Klinger, M.; Cramer, L.; Sauer, T.; Gemoll, T.; von Bubnoff, N.; et al. Targeting cancer-derived extracellular vesicles by combining CD147 inhibition with tissue factor pathway inhibitor for the management of urothelial cancer cells. Cell Commun. Signal. 2024, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Mary, B.; Asokan, N.; Jerabkova-Roda, K.; Larnicol, A.; Busnelli, I.; Stemmelen, T.; Pichot, A.; Molitor, A.; Carapito, R.; Lefebvre, O.; et al. Blood flow diverts extracellular vesicles from endothelial degradative compartments to promote angiogenesis. EMBO Rep. 2023, 24, e57042. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.I.; Loudig, O. Communicator Extraordinaire: Extracellular Vesicles in the Tumor Microenvironment Are Essential Local and Long-Distance Mediators of Cancer Metastasis. Biomedicines 2023, 11, 2534. [Google Scholar] [CrossRef]
- Wong, S.W.K.; Tey, S.K.; Mao, X.; Fung, H.L.; Xiao, Z.J.; Wong, D.K.H.; Mak, L.Y.; Yuen, M.F.; Ng, I.O.; Yun, J.P.; et al. Small Extracellular Vesicle-Derived vWF Induces a Positive Feedback Loop between Tumor and Endothelial Cells to Promote Angiogenesis and Metastasis in Hepatocellular Carcinoma. Adv. Sci. 2023, 10, e2302677. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Goo, J.; Lee, Y.; Lee, J.; Kim, I.S.; Jeong, C. Extracellular Vesicles in Therapeutics: A Comprehensive Review on Applications, Challenges, and Clinical Progress. Pharmaceutics 2024, 16, 311. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larbi, A. From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry. Biomedicines 2024, 12, 1469. https://doi.org/10.3390/biomedicines12071469
Larbi A. From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry. Biomedicines. 2024; 12(7):1469. https://doi.org/10.3390/biomedicines12071469
Chicago/Turabian StyleLarbi, Anis. 2024. "From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry" Biomedicines 12, no. 7: 1469. https://doi.org/10.3390/biomedicines12071469
APA StyleLarbi, A. (2024). From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry. Biomedicines, 12(7), 1469. https://doi.org/10.3390/biomedicines12071469




