Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases
Abstract
:1. Introduction
2. Methodology
3. IL-5 and Its Receptor
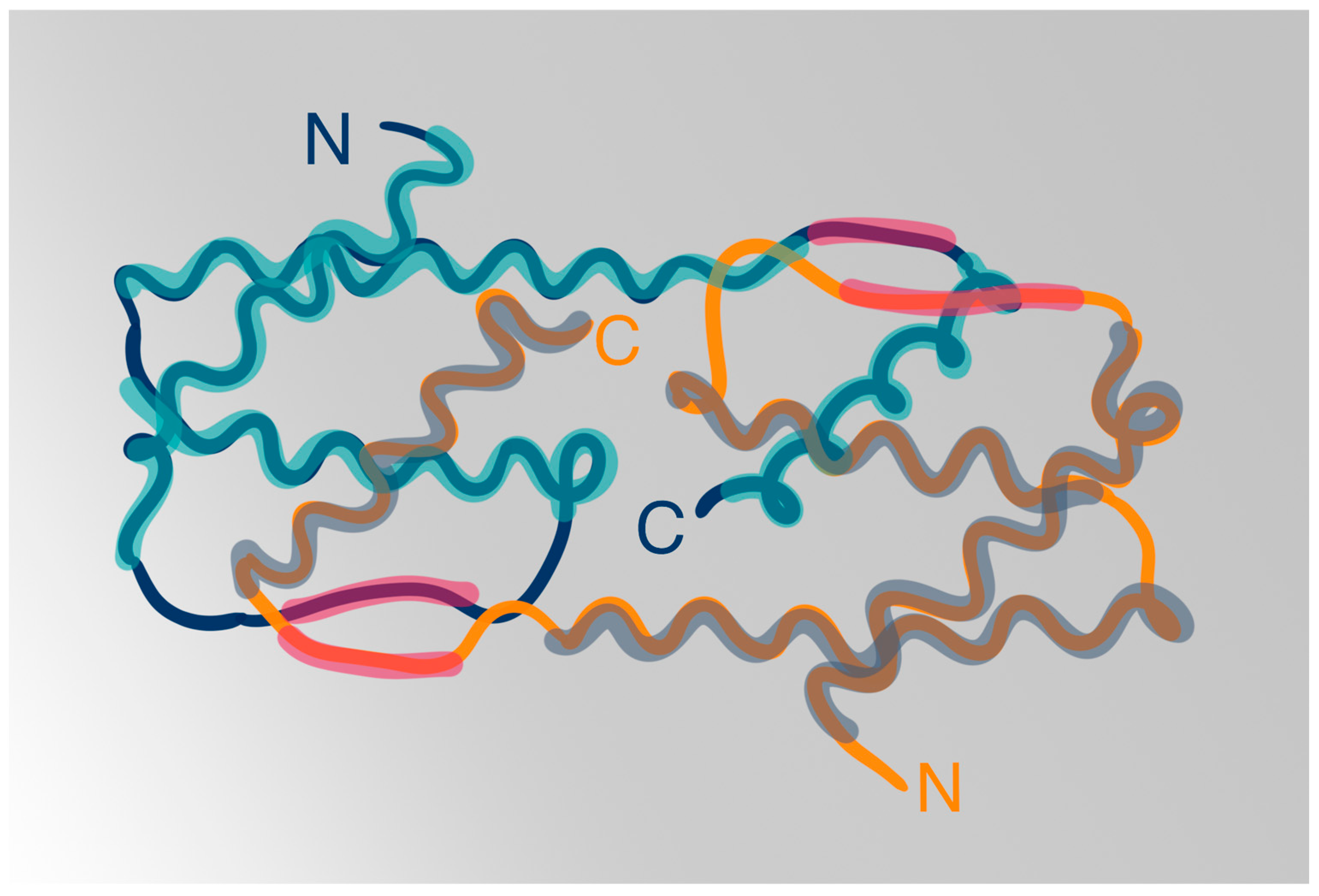
3.1. IL-5 Structure
3.2. IL-5 Receptors
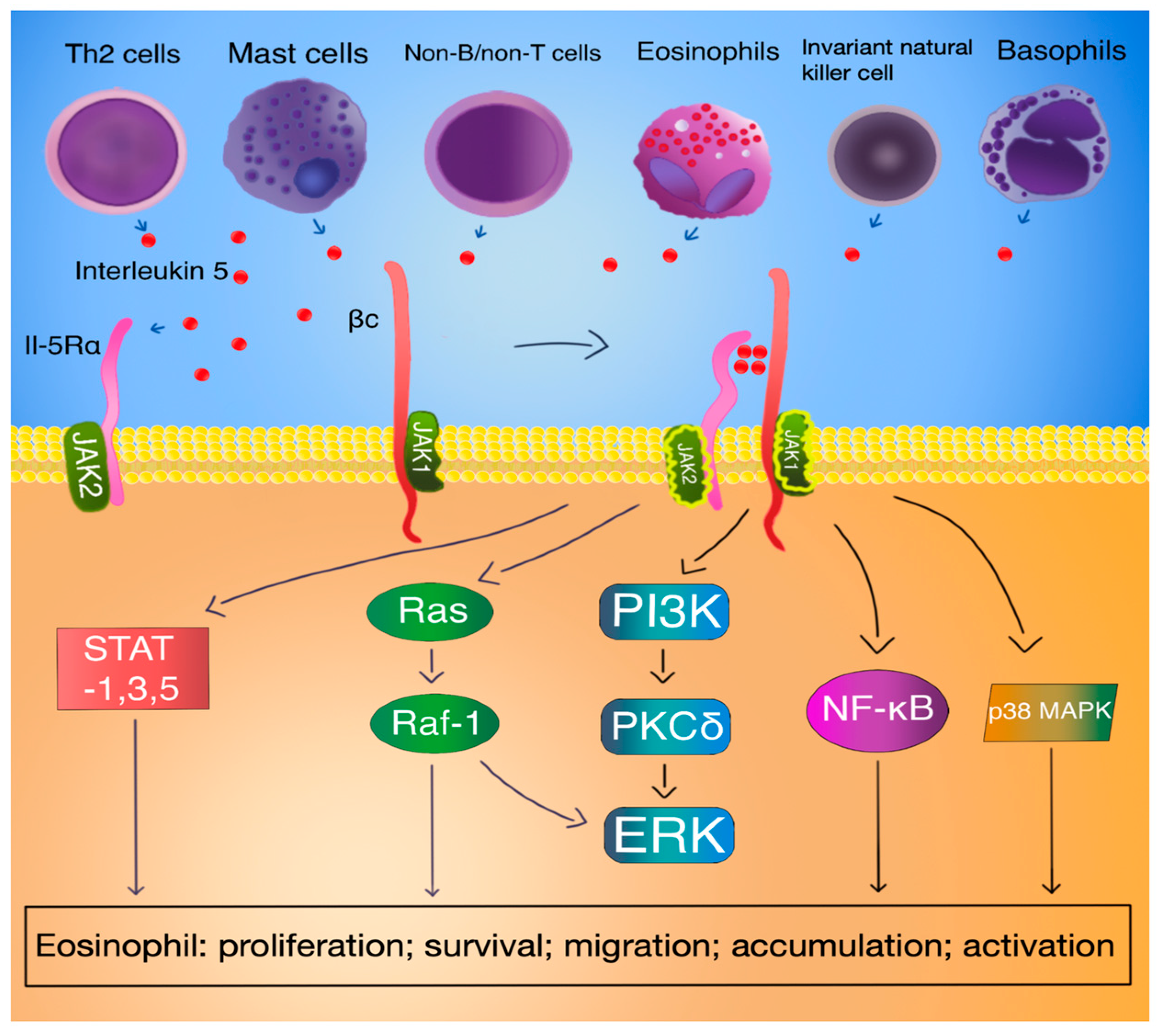
3.3. IL-5 Signal Transduction
3.4. IL-5 Function
4. Biological Treatment
5. Eosinophilic Asthma
5.1. Asthma–Basic Facts
5.2. IL-5 in Asthma
5.3. Targeting IL-5 in the Treatment of Asthma
5.4. The Role of IL-5 in Asthma-Like, Eosinophilic Diseases
6. Atopic Dermatitis
7. Chronic Spontaneous Urticaria
8. Chronic Rhinosinusitis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schimpl, A.; Wecker, E. Replacement of T-Cell Function by a T-Cell Product. Nat. New Biol. 1972, 237, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Yanagibashi, T.; Satoh, M.; Nagai, Y.; Koike, M.; Takatsu, K. Allergic diseases: From bench to clinic-Contribution of the discovery of interleukin-5. Cytokine 2017, 98, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, K.; Tominaga, A.; Harada, N.; Mita, S.; Matsumoto, M.; Takahashi, T.; Kikuchi, Y.; Yamaguchi, N. T Cell-Replacing Factor (TRF)/Interleukin 5 (IL-5): Molecular and Functional Properties. Immunol. Rev. 1988, 102, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Humbles, A.A.; Lloyd, C.M.; McMillan, S.J.; Friend, D.S.; Xanthou, G.; McKenna, E.E.; Ghiran, S.; Gerard, N.P.; Yu, C.; Orkin, S.H.; et al. A Critical Role for Eosinophils in Allergic Airways Remodeling. Science 2004, 305, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, I.; Kojima, T.; Kariyone, A.; Fukuda, T.; Makino, S.; Takatsu, K. Antibody against interleukin-5 prevents antigen-induced eosinophil infiltration and bronchial hyperreactivity in the guinea pig airways. Immunol. Lett. 1995, 45, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ramonell, R.P.; Iftikhar, I.H. Effect of Anti-IL5, Anti-IL5R, Anti-IL13 Therapy on Asthma Exacerbations: A Network Meta-analysis. Lung 2020, 198, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Wang, J.; Gao, S.; Li, C.; Wang, J.; Zhang, S.; Lin, J. Real-world Effectiveness of Mepolizumab in Severe Eosinophilic Asthma: A Systematic Review and Meta-analysis. Clin. Ther. 2021, 43, e192–e208. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Tolosa, S.; González-Gutiérrez, M.V.; Jiménez-Gálvez, G.; Sánchez-Martínez, J.A.; Pineda-Lancheros, L.E.; Gálvez-Navas, J.M.; Jiménez-Morales, A.; Pérez-Ramírez, C.; Morales-García, C. Impact of Anti-IL5 Therapies on Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study. Int. J. Mol. Sci. 2023, 24, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Liu, T.; Lan, Z.; Li, S.Y.; Mao, H. Efficacy and Safety of Anti-Interleukin-5 Therapy in Patients with Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166833. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- House, W. Global Report on Atopic Dermatitis 2022, International League of Dermatological Societies (ILDS). Available online: https://www.atopicdermatitisatlas.org/global-report-on-atopic-dermatitis-2022.pdf (accessed on 5 April 2024).
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Leiferman, K.M. A role for eosinophils in atopic dermatitis. J. Am. Acad. Dermatol. 2001, 45, S21–S24. [Google Scholar] [CrossRef]
- Oldhoff, J.M.; Darsow, U.; Werfel, T.; Katzer, K.; Wulf, A.; Laifaoui, J.; Hijnen, D.J.; Plötz, S.; Knol, E.F.; Kapp, A.; et al. Anti-IL-5 recombinant humanized monoclonal antibody (Mepolizumab) for the treatment of atopic dermatitis. Allergy 2005, 60, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.G.; Narayana, P.K.; Pouliquen, I.J.; Lopez, M.C.; Ferreira-Cornwell, M.C.; Getsy, J.A. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy 2020, 75, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Bahadori, L.; Brooks, L.; Clark, K.L.; Grindebacke, H.; Ho, C.N.; Katial, R.; Pham, T.-H.; Walton, C.; Datto, C.J. Lack of effect of benralizumab on signs and symptoms of moderate-to-severe atopic dermatitis: Results from the phase 2 randomized, double-blind, placebo-controlled HILLIER trial. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1211–e1214. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Biazus Soares, G.; Mahmoud, O. Current and Emerging Therapies for Chronic Spontaneous Urticaria: A Narrative Review. Dermatol. Ther. 2023, 13, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Benralizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2020, 383, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, L.; Tontini, C.; Garritani, M.S.; Piga, M.A.; Bilò, M.B. Efficacy of Mepolizumab in Patients With Severe Eosinophilic Asthma and Concomitant Severe Chronic Urticaria: An Example of Personalized Medicine? J. Investig. Allergol. Clin. Immunol. 2023, 33, 54–56. [Google Scholar]
- Magen, E.; Komarova, I.; Magen, I.; Phirtskhalava, S. Case of benralizumab-induced exacerbations of chronic spontaneous urticaria. Clin. Case Rep. 2022, 10, e05930. [Google Scholar] [CrossRef]
- Poblete, M.J.; Rosenbaum, A.; Winter, M. Anti-interleukin 5 therapy for chronic rhinosinusitis with polyps. Medwave 2018, 18, e7300. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031.e14. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, A.; Takahashi, T.; Kikuchi, Y.; Mita, S.; Naomi, S.; Harada, N.; Yamaguchi, N.; Takatsu, K. Role of carbohydrate moiety of IL-5. Effect of tunicamycin on the glycosylation of IL-5 and the biologic activity of deglycosylated IL-5. J. Immunol. 1990, 144, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Milburn, M.V.; Hassell, A.M.; Lambert, M.H.; Jordan, S.R.; Proudfoot, A.E.; Graber, P.; Wells, T.N. A novel dimer configuration revealed by the crystal structure at 2.4 A resolution of human interleukin-5. Nature 1993, 363, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Rozwarski, D.A.; Gronenborn, A.M.; Clore, G.M.; Bazan, J.F.; Bohm, A.; Wlodawer, A.; Hatada, M.; Karplus, P.A. Structural comparisons among the short-chain helical cytokines. Structure 1994, 2, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Molfino, N.A.; Gossage, D.; Kolbeck, R.; Parker, J.M.; Geba, G.P. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy 2012, 42, 712–737. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.L.; Peebles, R.S. INTERLEUKINS|IL-5. In Encyclopedia of Respiratory Medicine; Academic Press: Cambridge, MA, USA, 2006; pp. 359–363. [Google Scholar]
- Takatsu, K. Interleukin-5. Curr. Opin. Immunol. 1992, 4, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Shilovskiy, I.; Andreev, S.; Mazurov, D.; Barvinskaia, E.; Bolotova, S.; Nikolskii, A.; Sergeev, I.; Maerle, A.; Kudlay, D.; Khaitov, M. Identification of a novel splice variant for mouse and human interleukin-5. Heliyon 2020, 6, e03586. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Dahl, C.; Hoffmann, H.J.; Saito, H.; Schiøtz, P.O. Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy 2004, 59, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.A.; Yang, R.; Greenfeder, S.; Egan, R.W.; Pauwels, R.A.; Hey, J.A. The IL-5 receptor on human bronchus selectively primes for hyperresponsiveness. J. Allergy Clin. Immunol. 2002, 109, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Alam, R. The mechanism of IL-5 signal transduction. Am. J. Physiol.-Cell Physiol. 1998, 275, C623–C633. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Young, I.G. IL-3, IL-5, and GM-CSF Signaling: Crystal Structure of the Human Beta-Common Receptor. Vitam. Horm. 2006, 74, 1–30. [Google Scholar] [PubMed]
- He, L.; Cohen, E.B.; Edwards, A.P.B.; Xavier-Ferrucio, J.; Bugge, K.; Federman, R.S.; Absher, D.; Myers, R.M.; Kragelund, B.B.; Krause, D.S.; et al. Transmembrane Protein Aptamer Induces Cooperative Signaling by the EPO Receptor and the Cytokine Receptor β-Common Subunit. iScience 2019, 17, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Takaki, S.; Migita, M.; Kikuchi, Y.; Tominaga, A.; Takatsu, K. Molecular cloning and expression of the human interleukin 5 receptor. J. Exp. Med. 1992, 175, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Willebrand, R.; Huber, S.; Rupec, R.A.; Wu, D.; Locksley, R.; Voehringer, D. Eosinophil-specific deletion of IκBα in mice reveals a critical role of NF-κB–induced Bcl-xL for inhibition of apoptosis. Blood 2015, 125, 3896–3904. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Zaks-Zilberman, M.; Harrington, A.E.; Ishino, T.; Chaiken, I.M. Interleukin-5 receptor subunit oligomerization and rearrangement revealed by fluorescence resonance energy transfer imaging. J. Biol. Chem. 2008, 283, 13398–13406. [Google Scholar] [CrossRef]
- Kusano, S.; Kukimoto-Niino, M.; Hino, N.; Ohsawa, N.; Ikutani, M.; Takaki, S.; Sakamoto, K.; Hara-Yokoyama, M.; Shirouzu, M.; Takatsu, K.; et al. Structural basis of interleukin-5 dimer recognition by its α receptor. Protein Sci. 2012, 21, 850–864. [Google Scholar] [CrossRef]
- Kandikattu, H.K.; Upparahalli Venkateshaiah, S.; Mishra, A. Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor. Rev. 2019, 47, 83–98. [Google Scholar] [CrossRef] [PubMed]
- L Stein, M.; Munitz, A. Targeting Interleukin (IL) 5 for Asthma and Hypereosinophilic Diseases. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Stout, B.A.; Bates, M.E.; Liu, L.Y.; Farrington, N.N.; Bertics, P.J. IL-5 and Granulocyte-Macrophage Colony-Stimulating Factor Activate STAT3 and STAT5 and Promote Pim-1 and Cyclin D3 Protein Expression in Human Eosinophils. J. Immunol. 2004, 173, 6409–6417. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Kouro, T.; Yamada, A.; Koike, M.; Hanai, N.; Ishikawa, T.; Takatsu, K. JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and betac subunit, respectively, and are activated upon IL-5 stimulation. Blood 1998, 91, 2264–2271. [Google Scholar] [CrossRef]
- Pazdrak, K.; Olszewska-Pazdrak, B.; Stafford, S.; Garofalo, R.P.; Alam, R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J. Exp. Med. 1998, 188, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Kandikattu, H.K. Apoptosis of inflammatory cells in Asthma. Int. J. Cell Biol. Physiol. 2018, 1, 1–6. [Google Scholar]
- Sano, M.; Leff, A.R.; Myou, S.; Boetticher, E.; Meliton, A.Y.; Learoyd, J.; Lambertino, A.T.; Munoz, N.M.; Zhu, X. Regulation of interleukin-5-induced beta2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am. J. Respir. Cell Mol. Biol. 2005, 33, 65–70. [Google Scholar] [CrossRef]
- Adachi, T.; Choudhury, B.K.; Stafford, S.; Sur, S.; Alam, R. The Differential Role of Extracellular Signal-Regulated Kinases and p38 Mitogen-Activated Protein Kinase in Eosinophil Functions. J. Immunol. 2000, 165, 2198–2204. [Google Scholar] [CrossRef]
- Ip, W.K.; Wong, C.K.; Wang CBin Tian, Y.P.; Lam, C.W.K. Interleukin-3, -5, and Granulocyte Macrophage Colony-Stimulating Factor Induce Adhesion and Chemotaxis of Human Eosinophils via p38 Mitogen-Activated Protein Kinase and Nuclear Factor κB. Immunopharmacol. Immunotoxicol. 2005, 27, 371–393. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1263–1284. [Google Scholar] [CrossRef]
- Hall, D.J.; Cui, J.; Bates, M.E.; Stout, B.A.; Koenderman, L.; Coffer, P.J.; Bertics, P.J. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal–regulated kinase activation and interleukin-5–mediated cell viability. Blood 2001, 98, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Bachert, C.; Holtappels, G.; Novo, C.P.; Van der Heyden, J.; Fransen, L.; Depraetere, S.; Walter, H.; van Cauwenberge, P.; Tavernier, J. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy 2003, 58, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, J.; Tuypens, T.; Plaetinck, G.; Verhee, A.; Fiers, W.; Devos, R. Molecular basis of the membrane-anchored and two soluble isoforms of the human interleukin 5 receptor alpha subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 7041–7045. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, J.; Van der Heyden, J.; Verhee, A.; Brusselle, G.; Van Ostade, X.; Vandekerckhove, J.; North, J.; Rankin, S.M.; Kay, A.B.; Robinson, D.S. Interleukin 5 regulates the isoform expression of its own receptor α-subunit. Blood 2000, 95, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, J.; Devos, R.; Cornelis, S.; Tuypens, T.; Van der Heyden, J.; Fiers, W.; Plaetinck, G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell 1991, 66, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Sedgwick, J.B.; Bates, M.E.; Vrtis, R.F.; Gern, J.E.; Kita, H.; Jarjour, N.N.; Busse, W.W.; Kelly, E.A.B. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J. Immunol. 2002, 169, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Gregory, B.; Kirchem, A.; Phipps, S.; Gevaert, P.; Pridgeon, C.; Rankin, S.M.; Robinson, D.S. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J. Immunol. 2003, 170, 5359–5366. [Google Scholar] [PubMed]
- Yamaguchi, Y.; Suda, T.; Ohta, S.; Tominaga, K.; Miura, Y.; Kasahara, T. Analysis of the survival of mature human eosinophils: Interleukin-5 prevents apoptosis in mature human eosinophils. Blood 1991, 78, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Bhalla, A.; Mukherjee, M.; Nair, P. Airway Eosinophilopoietic and Autoimmune Mechanisms of Eosinophilia in Severe Asthma. Immunol. Allergy Clin. N. Am. 2018, 38, 639–654. [Google Scholar] [CrossRef]
- Johnston, L.K.; Hsu, C.L.; Krier-Burris, R.A.; Chhiba, K.D.; Chien, K.B.; McKenzie, A.; Berdnikovs, S.; Bryce, P.J. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J. Immunol. 2016, 197, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Sitkauskiene, B.; Johansson, A.K.; Sergejeva, S.; Lundin, S.; Sjöstrand, M.; Lötvall, J. Regulation of bone marrow and airway CD34+ eosinophils by interleukin-5. Am. J. Respir. Cell Mol. Biol. 2004, 30, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.D.; Marleau, S.; Griffiths-Johnson, D.A.; Jose, P.J.; Williams, T.J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 1995, 182, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Sehmi, R.; Wood, L.J.; Watson, R.; Foley, R.; Hamid, Q.; O’Byrne, P.M.; Denburg, J.A. Allergen-induced increases in IL-5 receptor alpha-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J. Clin. Investig. 1997, 100, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Stirling, R.G.; van Rensen, E.L.; Barnes, P.J.; Chung, K.F. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am. J. Respir. Crit. Care Med. 2001, 164 Pt. 1, 1403–1409. [Google Scholar] [CrossRef]
- Iwasaki, H.; Mizuno S ichi Mayfield, R.; Shigematsu, H.; Arinobu, Y.; Seed, B.; Gurish, M.F.; Takatsu, K.; Akashi, K. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 2005, 201, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Arkin, Y.; Giladi, A.; Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Winter, D.; Lara-Astiaso, D.; Gury, M.; Weiner, A.; et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell 2015, 163, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Venkatasubramanian, M.; Chaudhri, V.K.; Aronow, B.J.; Salomonis, N.; Singh, H.; Grimes, H.L. Single-cell analysis of mixed-lineage states leading to a binary cell fate choice. Nature 2016, 537, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Rådinger, M.; Lötvall, J. Eosinophil progenitors in allergy and asthma—Do they matter? Pharmacol. Ther. 2009, 121, 174–184. [Google Scholar] [CrossRef]
- Mack, E.A.; Pear, W.S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr. Opin. Hematol. 2020, 27, 27–33. [Google Scholar] [CrossRef]
- Shalit, M.; Sekhsaria, S.; Malech, H.L. Modulation of growth and differentiation of eosinophils from human peripheral blood CD34+ cells by IL5 and other growth factors. Cell Immunol. 1995, 160, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.; Christodoulopoulos, P.; Lavigne, F.; Nakamura, Y.; Eidelman, D.; McEuen, A.; Walls, A.; Tavernier, J.; Minshall, E.; Moqbel, R.; et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: Inhibition with soluble IL-5 receptor. J. Immunol. 2000, 164, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Comeau, M.R.; Smith, D.E.; Toy, D.; Endam, L.M.; Desrosiers, M.; Liu, Y.J.; Howie, K.J.; Denburg, J.A.; Gauvreau, G.M.; et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 2009, 123, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Woltmann, G.; McNulty, C.A.; Dewson, G.; Symon, F.A.; Wardlaw, A.J. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood 2000, 95, 3146–3152. [Google Scholar] [CrossRef] [PubMed]
- Sehmi, R.; Wardlaw, A.J.; Cromwell, O.; Kurihara, K.; Waltmann, P.; Kay, A.B. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood 1992, 79, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Flood-Page, P.; Sehmi, R.; Burman, J.; Hamid, Q.; Robinson, D.S.; Kay, A.B.; Denburg, J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J. Allergy Clin. Immunol. 2003, 111, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. 2017, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Ochensberger, B.; Tassera, L.; Bifrare, D.; Rihs, S.; Dahinden, C.A. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur. J. Immunol. 1999, 29, 11–22. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa J ichi Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Hoyler, T.; Klose, C.S.N.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The Transcription Factor GATA-3 Controls Cell Fate and Maintenance of Type 2 Innate Lymphoid Cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Preianò, M.; Lombardo, N.; Terracciano, R.; Maselli, R. Role of biologics in severe eosinophilic asthma – focus on reslizumab. Ther. Clin. Risk Manag. 2016, 12, 1075–1082. [Google Scholar] [PubMed]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Puggioni, F.; Passalacqua, G.; Canonica, G.W. Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma. Front. Med. 2017, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Minthorn, E.A.; Beerahee, M. Pharmacokinetics and Pharmacodynamics of Mepolizumab, an Anti-Interleukin-5 Monoclonal Antibody. Clin. Pharmacokinet. 2011, 50, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Farne, H.A.; Wilson, A.; Milan, S.; Banchoff, E.; Yang, F.; Powell, C.V. Anti-IL-5 therapies for asthma. Cochrane Database Syst. Rev. 2022, 7, CD010834. [Google Scholar] [PubMed]
- Charles, D.; Shanley, J.; Temple, S.; Rattu, A.; Khaleva, E.; Roberts, G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: A systematic review and meta-analysis. Clin. Exp. Allergy 2022, 52, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Mepolizumab: First Global Approval. Drugs 2015, 75, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marigowda, G.; Oren, E.; Israel, E.; Wechsler, M.E. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010, 125, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.R.; Nepal, G.; Mandal, S. Safety and Efficacy of Mepolizumab in Patients with Eosinophilic Granulomatosis with Polyangiitis. Pulm. Med. 2019, 2019, 4376380. [Google Scholar] [CrossRef] [PubMed]
- Revuelta-Salgado, F.; Díaz-Campos, R.M.; Hernando-Sanz, A.; Melero-Moreno, C. Mepolizumab and COPD in Real Life. Open Respir. Arch. 2022, 4, 100141. [Google Scholar] [CrossRef]
- Full Prescribing Information-Nucala. Available online: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF (accessed on 28 April 2024).
- Summary of Product Characteristics-Nucala. Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 28 April 2024).
- Nair, P.; Bardin, P.; Humbert, M.; Murphy, K.R.; Hickey, L.; Garin, M.; Vanlandingham, R.; Chanez, P. Efficacy of Intravenous Reslizumab in Oral Corticosteroid–Dependent Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 555–564. [Google Scholar] [CrossRef]
- Spergel, J.M.; Rothenberg, M.E.; Collins, M.H.; Furuta, G.T.; Markowitz, J.E.; Fuchs, G.; O’Gorman, M.A.; Abonia, J.P.; Young, J.; Henkel, T.; et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2012, 129, 456–463.e3. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Profile of reslizumab in eosinophilic disease and its potential in the treatment of poorly controlled eosinophilic asthma. Biologics 2013, 7, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.; Athwal, D.; Bodmer, M.; Carter, J.; Chapman, R.; Choua, C.C.; Coxa, M.; Emtage, S.; Fernandez, X.; Genatt, N.; et al. Effect of Sch 55700, a Humanized Monoclonal Antibody to Human Interleukin-5, on Eosinophilic Responses and Bronchial Hyperreactivity. Arzneimittelforschung 2011, 49, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Full Prescribing Information-Cinqair. Available online: https://www.cinqair.com/globalassets/cinqair/prescribinginformation.pdf (accessed on 28 April 2024).
- Manka, L.A.; Guntur, V.P.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Strand, M.J.; Wechsler, M.E. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann. Allergy Asthma Immunol. 2021, 126, 696–701.e1. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics-Cinqaero. Available online: https://www.ema.europa.eu/en/documents/product-information/cinqaero-epar-product-information_en.pdf (accessed on 28 April 2024).
- Cheung, T.T.; Mai, T.H.; Chia, Y.L.; Yap, D.Y.; Lee, C.H.; Chen, C.C.K.; Huang, Y.; Jin, Y.; Johnston, J.; Werkström, V.; et al. Safety, Tolerability, and Pharmacokinetics of Benralizumab: A Phase 1, Randomized, Single-Blind Study of Healthy Chinese Participants. Drug Des. Devel Ther. 2023, 17, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Korn, S.; Mathur, S.K.; Barker, P.; Meka, V.G.; Martin, U.J.; Zangrilli, J.G. Safety of Eosinophil-Depleting Therapy for Severe, Eosinophilic Asthma: Focus on Benralizumab. Drug Saf. 2020, 43, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Khorasanizadeh, M.; Eskian, M.; Assa’ad, A.H.; Camargo, C.A., Jr.; Rezaei, N. Efficacy and Safety of Benralizumab, a Monoclonal Antibody against IL-5Rα, in Uncontrolled Eosinophilic Asthma. Int. Rev. Immunol. 2016, 35, 294–311. [Google Scholar] [CrossRef]
- Full Prescribing Information-Fasenra. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf (accessed on 28 April 2024).
- Summary of Product Characteristics-Fasenra. Available online: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf (accessed on 28 April 2024).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. Updated July 2023. Available online: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2023-Report-WMSA.pdf (accessed on 5 April 2024).
- Maslan, J.; Mims, J.W. What is asthma? Pathophysiology, demographics, and health care costs. Otolaryngol. Clin. N. Am. 2014, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sinyor, B.; Concepcion Perez, L. Pathophysiology of Asthma; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hussain, M.; Liu, G. Eosinophilic Asthma: Pathophysiology and Therapeutic Horizons. Cells 2024, 13, 384. [Google Scholar] [CrossRef]
- Principe, S.; Porsbjerg, C.; Bolm Ditlev, S.; Kjærsgaard Klein, D.; Golebski, K.; Dyhre-Petersen, N.; van Dijk, Y.E.; van Bragt, J.J.M.H.; Dankelman, L.L.H.; Dahlen, S.; et al. Treating severe asthma: Targeting the IL-5 pathway. Clin. Exp. Allergy 2021, 51, 992–1005. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kotsimbos, A.; Hamid, Q. IL-5 and IL-5 receptor in asthma. Mem. Inst. Oswaldo Cruz 1997, 92 (Suppl. 2), 75–91. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Difficult-to-Treat & Severe Asthma in Adolescent and Adult Patients. 2023. Available online: https://ginasthma.org/severeasthma/ (accessed on 5 April 2024).
- Cabon, Y.; Molinari, N.; Marin, G.; Vachier, I.; Gamez, A.S.; Chanez, P.; Bourdin, A. Comparison of anti-interleukin-5 therapies in patients with severe asthma: Global and indirect meta-analyses of randomized placebo-controlled trials. Clin. Exp. Allergy 2017, 47, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Maglio, A.; Vitale, C.; Pelaia, C.; D’Amato, M.; Ciampo, L.; Sferra, E.; Molino, A.; Pelaia, G.; Vatrella, A. Severe Asthma Remissions Induced by Biologics Targeting IL5/IL5r: Results from a Multicenter Real-Life Study. Int. J. Mol. Sci. 2023, 24, 2455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, S.C.; Jin, L. Adverse events of anti-IL-5 drugs in patients with eosinophilic asthma: A meta-analysis of randomized controlled trials and real-world evidence-based assessments. BMC Pulm. Med. 2024, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; El-Turabi, A.; Fettelschoss-Gabriel, A.; Vogel, M. The Prospects of an Active Vaccine Against Asthma Targeting IL-5. Front. Microbiol. 2018, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, G.; Song, Z. Intrinsic Atopic Dermatitis and Extrinsic Atopic Dermatitis: Similarities and Differences. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2621–2628. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T.; Leung, D.Y.M. Immune mechanisms leading to atopic dermatitis. J. Allergy Clin. Immunol. 2003, 112 (Suppl. 6), S128–S139. [Google Scholar] [CrossRef]
- Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells 2024, 13, 425. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Stevens, S.R. Pathophysiology of atopic dermatitis. Clin. Dermatol. 2003, 21, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, H.; Sadik, C.D.; Ludwig, R.J.; Thaçi, D.; Boch, K. Dupilumab in Inflammatory Skin Diseases: A Systematic Review. Biomolecules 2023, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, Y.; Arinuma, Y.; Matsueda, Y.; Tanaka, T.; Wada, T.; Tanaka, S.; Oku, K.; Yamaoka, K. A case of dupilumab combination therapy for exacerbation of atopic dermatitis in a patient with eosinophilic granulomatosis with polyangiitis treated with mepolizumab. Mod. Rheumatol. Case Rep. 2023, 8, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.M. Chronic Urticaria. N. Engl. J. Med. 2022, 387, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Bernstein, J.A.; Maurer, M. Chronic spontaneous urticaria guidelines: What is new? J. Allergy Clin. Immunol. 2022, 150, 1249–1255. [Google Scholar] [CrossRef]
- Magerl, M.; Terhorst, D.; Metz, M.; Altrichter, S.; Zuberbier, T.; Maurer, M.; Bergmann, K.C. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. JDDG J. Der Dtsch. Dermatol. Ges. 2018, 16, 477–478. [Google Scholar] [CrossRef]
- Altrichter, S.; Giménez-Arnau, A.M.; Bernstein, J.A.; Metz, M.; Bahadori, L.; Bergquist, M.; Brooks, L.; Ho, C.N.; Jain, P.; Lukka, P.B.; et al. Benralizumab does not elicit therapeutic effect in patients with chronic spontaneous urticaria: Results from the phase 2b multinational, randomised, double-blind, placebo-controlled ARROYO trial. Br. J. Dermatol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Q.; Chen, Q.; Li, H.; Liu, D.; Wu, Q. Efficacy and Safety of Anti-Interleukin-5 Therapies in Chronic Rhinosinusitis with Nasal Polyps: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. Arch. Allergy Immunol. 2022, 183, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Gokani, S.A.; Espehana, A.; Pratas, A.C.; Luke, L.; Sharma, E.; Mattock, J.; Gavrilovic, J.; Clark, A.; Wileman, T.; Philpott, C.M. Systematic Review of Protein Biomarkers in Adult Patients With Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2023, 37, 705–729. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Ho, J.; Alvarado, R.; Smith, G.; Croucher, D.R.; Liang, S.; Grayson, J.W.; Mangussi-Gomes, J.; Van Es, S.L.; Earls, P.; et al. Mepolizumab decreases tissue eosinophils while increasing type-2 cytokines in eosinophilic chronic rhinosinusitis. Clin. Exp. Allergy 2022, 52, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Liang, J. Anti-IgE and Anti-IL5 Biologic Therapy in the Treatment of Nasal Polyposis: A Systematic Review and Meta-analysis. Ann. Otol. Rhinol. Laryngol. 2017, 126, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.Z.; Kao, S.S.; Ooi, E.H. The role of biologics in chronic rhinosinusitis: A systematic review. Int. Forum Allergy Rhinol. 2020, 10, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef]
- Guo, J.; Feng, J.; Lin, L.; Zhao, X.; Wu, H. Effect of specific immunotherapy on GM-CSF and IL-5 in the tissues of recurrent nasal polyps. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2015, 29, 2023–2025. [Google Scholar]
- Tversky, J.; Lane, A.P.; Azar, A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): A randomized double-blind placebo-controlled trial. Clin. Exp. Allergy 2021, 51, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Barroso, B.; Valverde-Monge, M.; Alobid, I.; Olaguibel, J.; Rial, M.; Quirce, S.; Arismendi, E.; Barranco, P.; Betancor, D.; Bobolea, I.; et al. Smell improvement by anti-IgE and anti-IL 5 biologics in patients with CRSwNP and severe asthma. A real life study. J. Investig. Allergy Clin. Immunol. 2022, 33, 37–44. [Google Scholar]
- Takabayashi, T.; Asaka, D.; Okamoto, Y.; Himi, T.; Haruna, S.; Yoshida, N.; Kondo, K.; Yoshikawa, M.; Sakuma, Y.; Shibata, K.; et al. A Phase II, Multicenter, Randomized, Placebo-Controlled Study of Benralizumab, a Humanized Anti-IL-5R Alpha Monoclonal Antibody, in Patients With Eosinophilic Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Sousa, A.R.; Han, J.K.; Schlosser, R.J.; Sowerby, L.J.; Hopkins, C.; Maspero, J.F.; Smith, S.G.; Kante, O.; Karidi-Andrioti, D.E.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps: Treatment efficacy by comorbidity and blood eosinophil count. J. Allergy Clin. Immunol. 2022, 149, 1711–1721.e6. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.; Alobid, I.; Lugogo, N.L.; Kariyawasam, H.H.; Bourdin, A.; Chaker, A.M.; Smith, S.G.; Sousa, A.R.; Mayer, B.; Chan, R.H.; et al. Mepolizumab Reduces Systemic Corticosteroid Use in Chronic Rhinosinusitis With Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2023, 11, 3504–3512.e2. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Han, J.K.; Lund, V.J.; Bachert, C.; Fokkens, W.J.; Diamant, Z.; Mullol, J.; Sousa, A.R.; Smith, S.G.; Yang, S.; et al. Evaluating treatment response to mepolizumab in patients with severe CRSwNP. Rhinol. J. 2023, 62, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.; Trigg, A.; Lee, S.E.; Chan, R.H.; Diamant, Z.; Hopkins, C.; Howarth, P.; Lund, V.; Mayer, B.; Sousa, A.R.; et al. Mepolizumab improvements in health-related quality of life and disease symptoms in a patient population with very severe chronic rhinosinusitis with nasal polyps: Psychometric and efficacy analyses from the SYNAPSE study. J. Patient Rep. Outcomes 2023, 7, 4. [Google Scholar] [CrossRef]
- Desrosiers, M.; Diamant, Z.; Castelnuovo, P.; Hellings, P.W.; Han, J.K.; Peters, A.T.; Silver, J.; Smith, S.G.; Fuller, A.; Sousa, A.R.; et al. Sustained efficacy of mepolizumab in patients with severe chronic rhinosinusitis with nasal polyps: SYNAPSE 24-week treatment-free follow-up. Int. Forum Allergy Rhinol. 2024, 14, 18–31. [Google Scholar] [CrossRef]



| Mepolizumab | Reslizumab | Benralizumab | |
|---|---|---|---|
| Trade name | Nucala™ by GlaxoSmithKline | Cinqair™ (USA)/Cinqaero™ (EU) by Teva Respiratory | Fasenra™ by AstraZeneca |
| Mechanism of action | Binds free IL-5, preventing its association with the IL-5 receptor | Neutralizes circulating IL-5, preventing its binding to the IL-5 receptor | Binds to the IL-5Rα on eosinophils and basophils, induces apoptosis via ADCC |
| Target | Circulating IL-5 | Circulating IL-5 | IL-5Rα on eosinophils and basophils |
| Registered use cases | Severe asthma with an eosinophilic phenotype (in children over 6 years of age, adults and adolescents), CRSwNP (adults) EGPA (EU—in children over 6 years of age, adults and adolescents; USA—adults only) and HES (adults) | Severe asthma with an eosinophilic phenotype (adults) | Severe asthma with an eosinophilic phenotype (EU—adults; USA—adults and children over 12 years of age) |
| Adverse Effects | Lower respiratory tract and urinary tract infections, pharyngitis, nasopharyngitis, arthralgia, arrythmias, hypersensitivity reactions, headache, eczema, upper abdominal pain, muscle spasms, back pain, injection site reactions, anaphylaxis | Myalgia, nasopharyngitis, exacerbation of asthma, upper respiratory tract infections, increased blood creatine phosphokinase, anaphylaxis | Pharyngitis, hypersensitivity reactions, anaphylaxis, headaches, pyrexia, injection-site reactions, and nausea |
| Disease Entity | IL-5’s Role | Effectiveness of Anti-IL5 Therapy | Extra Comments |
|---|---|---|---|
| Asthma | Airway eosinophilia and bronchial hyperresponsiveness induced by allergen challenge, mucus production, airway remodeling, and tissue damage [106,110,113] | It was found that therapies targeting IL-5 and its receptor are effective when it comes to the improvement of lung function, decreased eosinophil blood and sputum counts, and reduction of asthma exacerbations [8,40,110,116] | There are studies suggesting that vaccination against IL-5 could be an effective option for treatment [43]. |
| Atopic dermatitis (AD) | Higher expression in extrinsic AD, therefore causing higher Eozynophil level and their migration. However, this is not the most important role in pathogenesis [119,120,121] | It appears to be ineffective in stopping the progression and recurrence of the disease [14,15,16,122,125] | Much better results and effective therapies are directed against IL-31, IL-4, or IL-13 [126] |
| Chronic Spontaneous Urticaria (CSU) | Both mast cells and eosinophils act synergistically in pathways, where IL-5 plays an important role [18] | The results are uncertain, single case reports confirm the effectiveness of the therapy in patients with a strong eosinophilic profile. However, cases of exacerbations or lack of clinical effect have also been described [19,20,131,132] | So far, only omalizumab is officially used in therapy of CSU [130] |
| Chronic rhinosinusitis (CRS) | Types of CRS are created based on different endotypes. IL-5 is one of the most common biomarkers in CRS [135]. The IL-5-depended pathway stimulates eosinophil action, chemotaxis, differentiation, and survival, increasing inflammation [22,135,136] | The data seem promising, the therapy can efficiently help with the limitation of the complications of CRS. The therapy risks and side effects also appear to be low [22,139,140]. | Initially, most reports were based on patients suffering from both asthma and CSR. Recently, more and more studies have been available confirming the effectiveness of the therapy [22,139,140,148,149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosz, K.; Batko, J.; Błażejewska, M.; Gawor, A.; Sleziak, J.; Gomułka, K. Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases. Biomedicines 2024, 12, 1531. https://doi.org/10.3390/biomedicines12071531
Antosz K, Batko J, Błażejewska M, Gawor A, Sleziak J, Gomułka K. Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases. Biomedicines. 2024; 12(7):1531. https://doi.org/10.3390/biomedicines12071531
Chicago/Turabian StyleAntosz, Katarzyna, Joanna Batko, Marta Błażejewska, Antoni Gawor, Jakub Sleziak, and Krzysztof Gomułka. 2024. "Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases" Biomedicines 12, no. 7: 1531. https://doi.org/10.3390/biomedicines12071531
APA StyleAntosz, K., Batko, J., Błażejewska, M., Gawor, A., Sleziak, J., & Gomułka, K. (2024). Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases. Biomedicines, 12(7), 1531. https://doi.org/10.3390/biomedicines12071531






