Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review
Abstract
1. Introduction
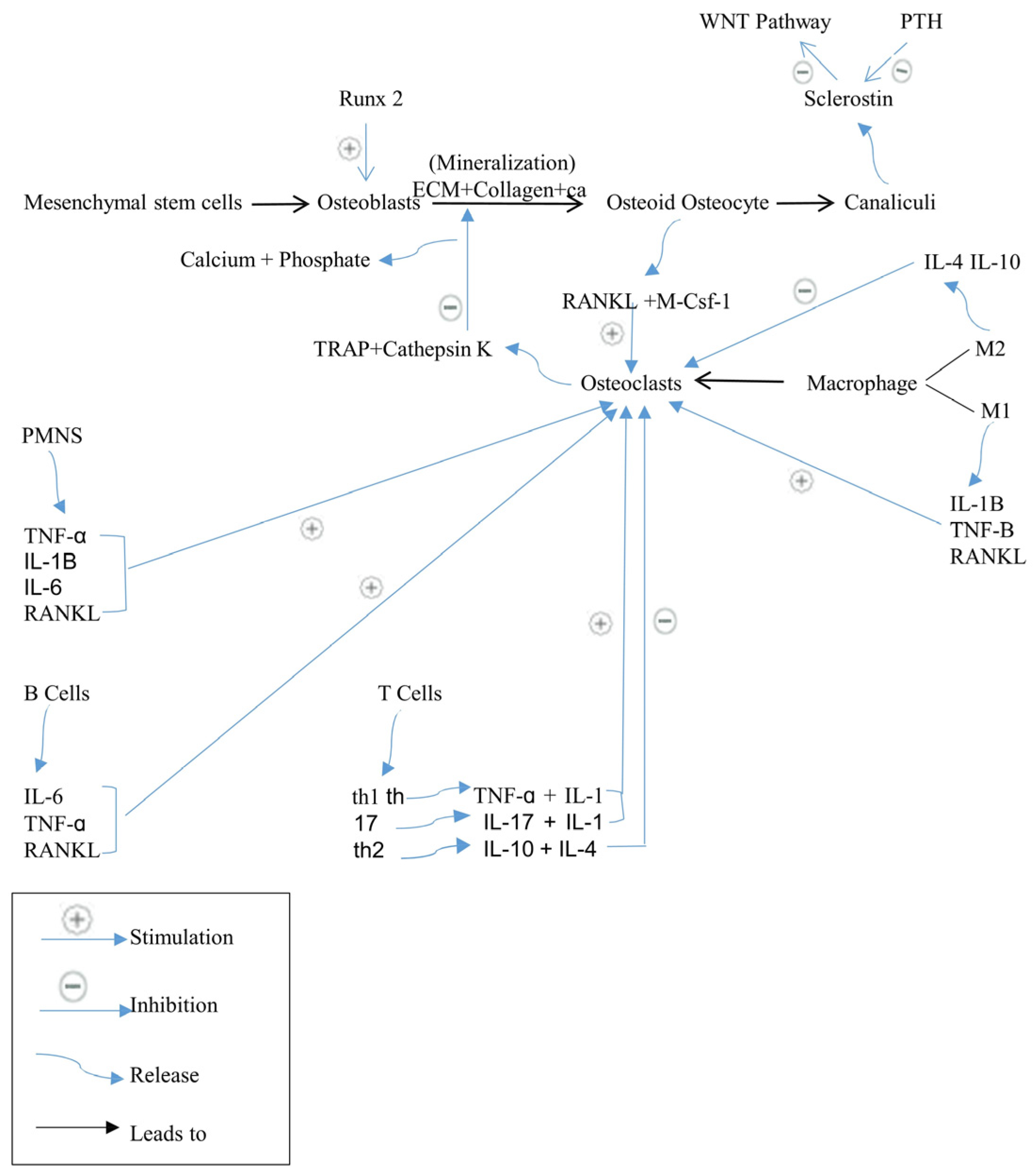
2. Bone Remodeling
2.1. Cells Involved in Bone Remodeling
2.2. Regulation of Bone Remodeling
2.2.1. RANK/RANKL/OPG Signaling Pathway
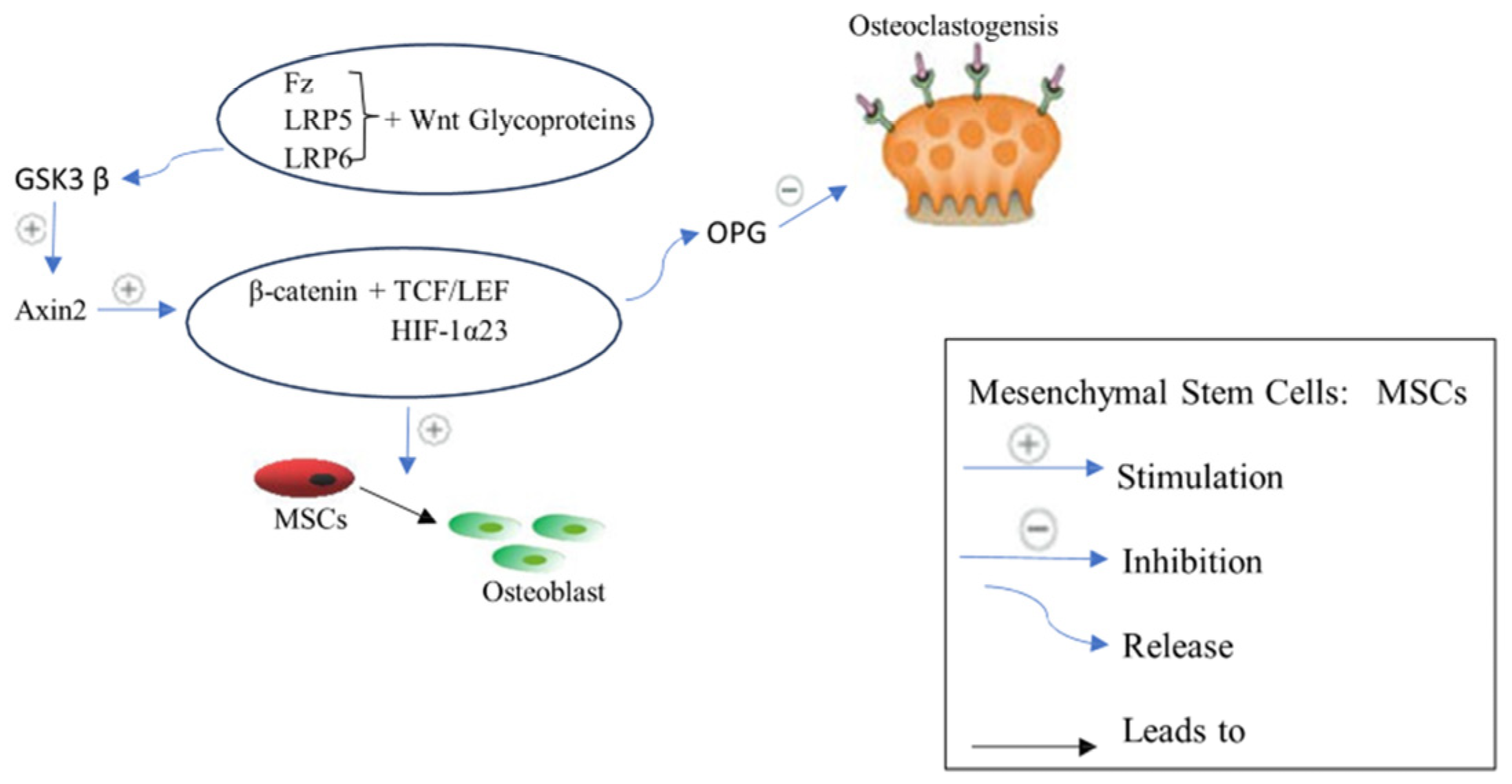
2.2.2. Wnt/β-Catenin Signaling Pathway or Canonical Pathway
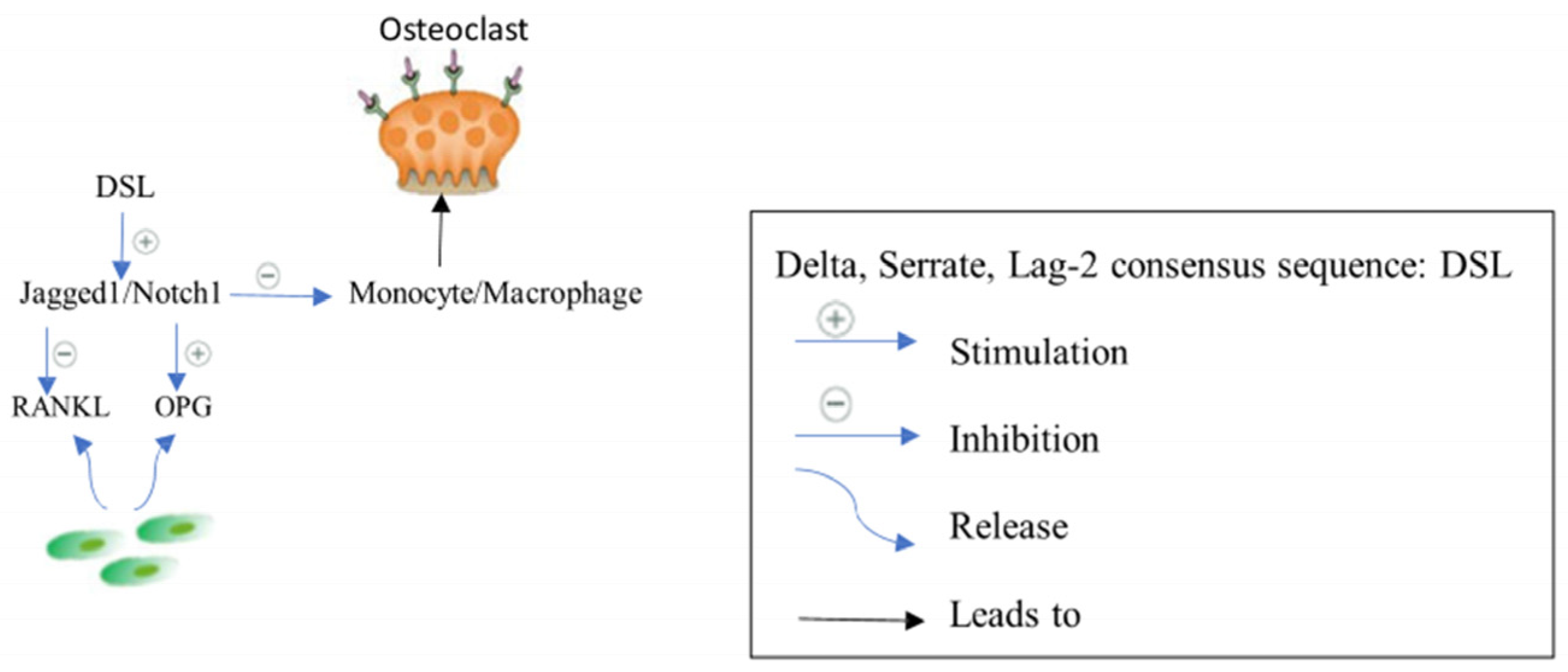
2.2.3. Jagged1/Notch1 Signaling
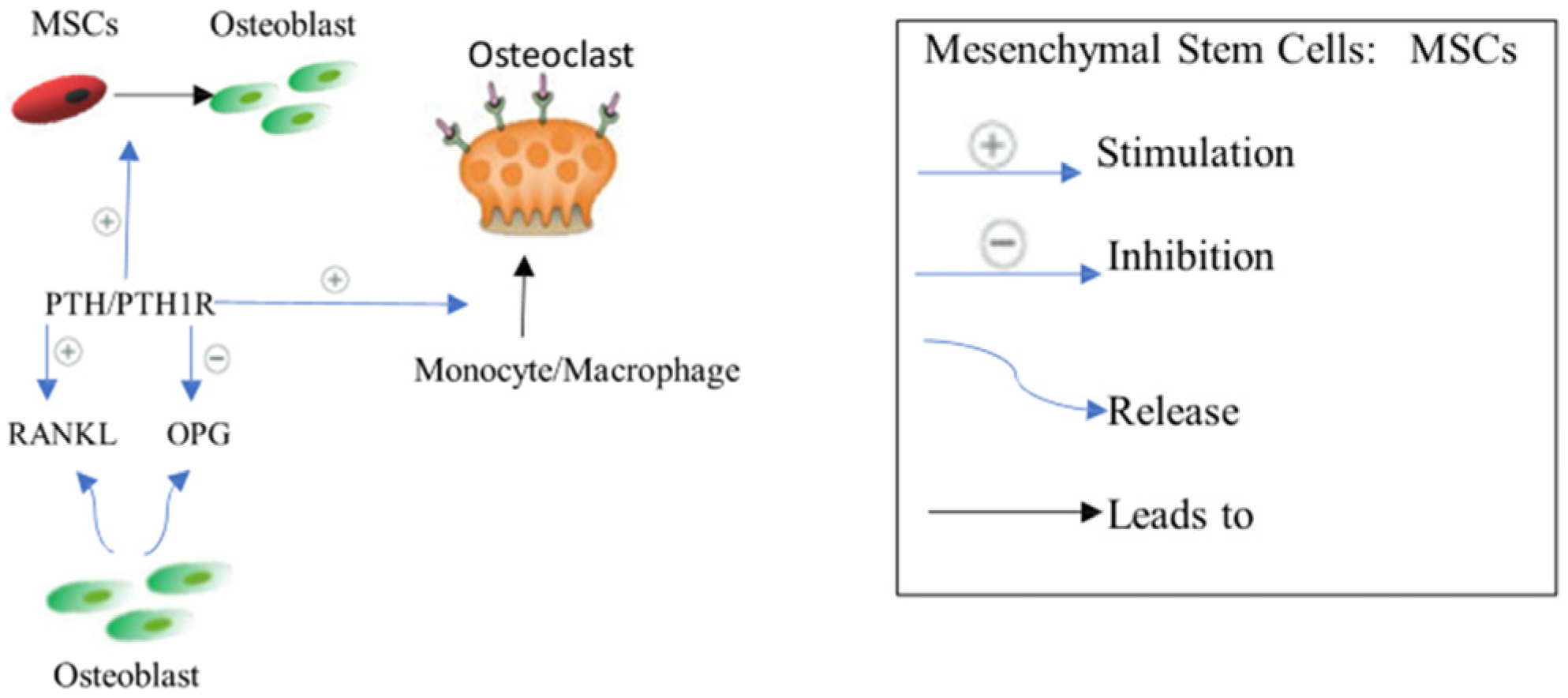
2.2.4. Parathyroid Hormone PTH Signaling
2.2.5. Pro-Inflammatory Cytokines Complex Network
2.2.6. Kynurenine (KYN) Pathway
3. Current Pharmacological Treatment
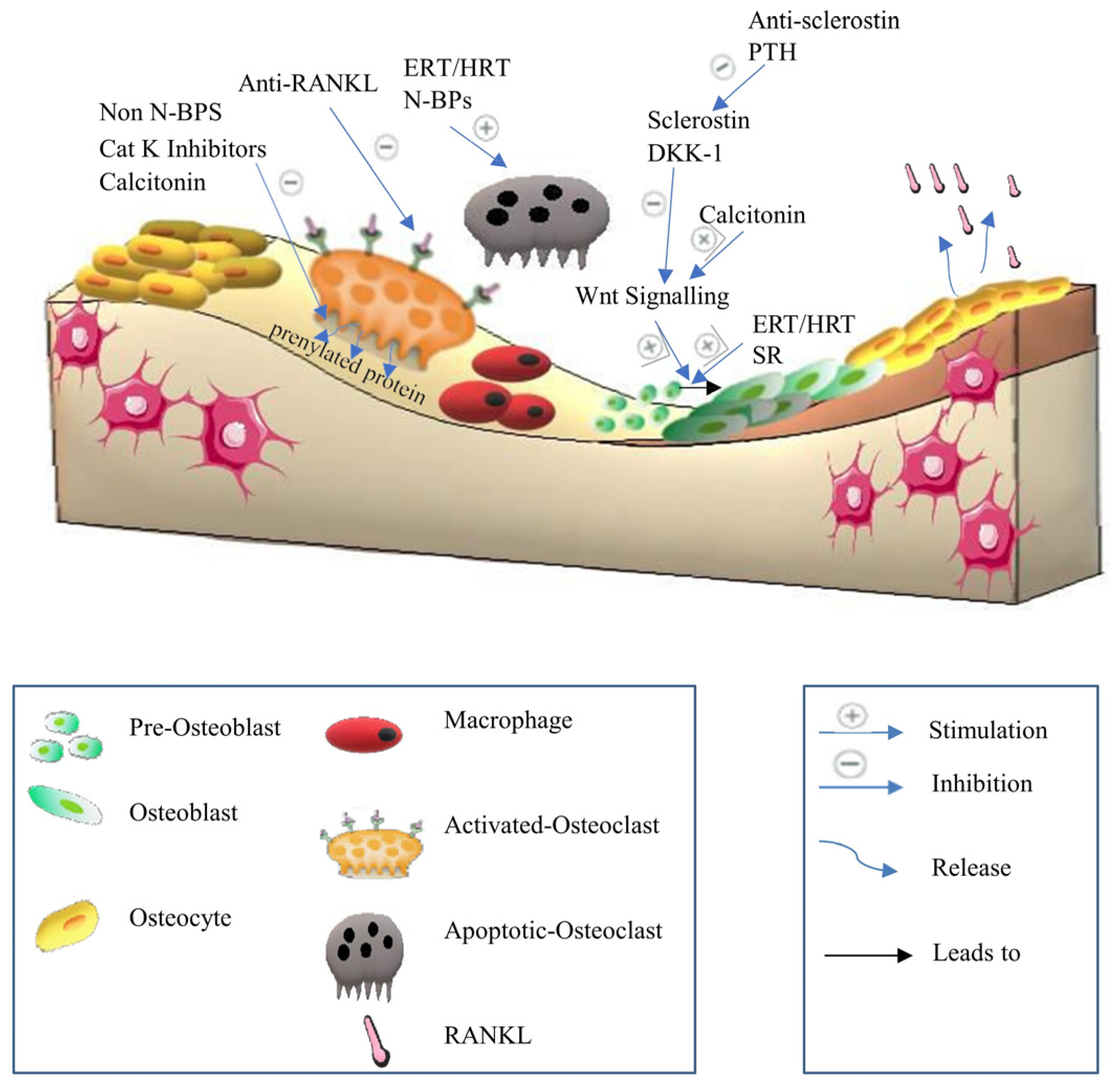
3.1. Anti-Resorptive Agents
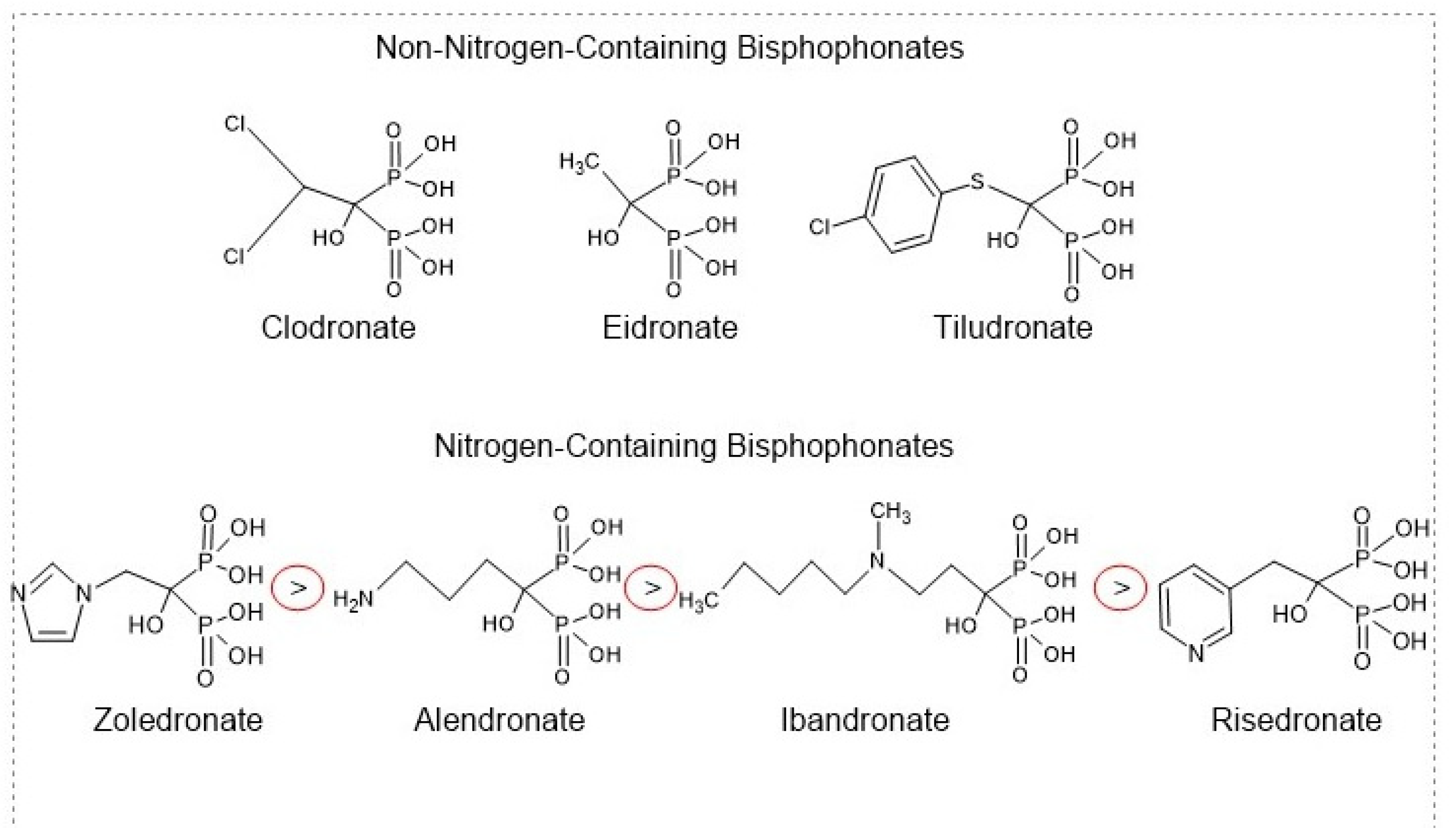
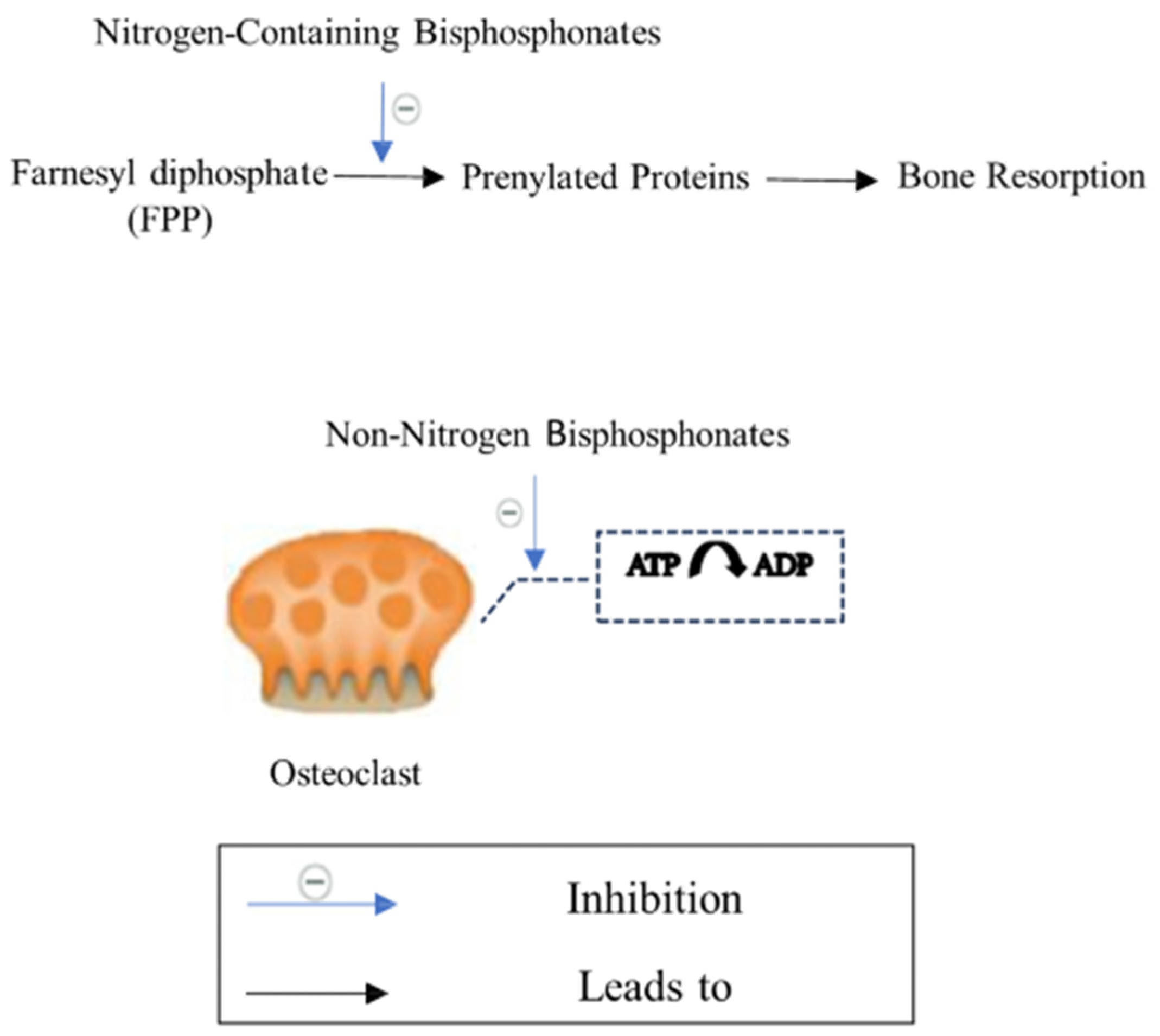
3.1.1. Bisphosphonates
3.1.2. Estrogen Replacement Therapy/Hormone Replacement Therapy (ERT/HRT)
3.1.3. Selective Estrogen Receptor Modulators (SERMs)
3.1.4. Calcitonin
3.1.5. Anti-RANKL Antibody
3.1.6. Cathepsin K Inhibitors
3.2. Anabolic Drugs
3.2.1. Parathyroid Hormone PTH
3.2.2. Anti-Sclerostin Antibody
3.2.3. Strontium Ranelate
3.2.4. Pro-Inflammatory Cytokines Complex Network Inhibitors
- Tumor Necrosis Factor-alpha (TNF-α) Inhibitors:
- Interleukin-6 Inhibitors:
- Interleukin 17 Inhibitor:
3.3. Nutritional Supplements
3.3.1. Calcium
3.3.2. Vitamin D
3.3.3. Vitamin K2
3.3.4. Vitamin E
3.4. Stem Cells
3.5. Combination Therapy
3.6. Sequential Therapy
3.7. Alternative Therapy
4. Conclusions
Funding
Conflicts of Interest
References
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A mini review on osteoporosis: From biology to pharmacological management of bone loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health-Care Level; Technical Report; University of Sheffield Medical School: Sheffield, UK, 2007. [Google Scholar]
- Kim, H.-Y.; Kim, Y. Associations of obesity with osteoporosis and metabolic syndrome in Korean postmenopausal women: A cross-sectional study using national survey data. Arch. Osteoporos. 2019, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Subramaniam, S.; Chin, K.-Y.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Aini, J.N.; Aziz, N.A.; Mohamed, N. Effect of a screening and education programme on knowledge, beliefs, and practices regarding osteoporosis among Malaysians. Int. J. Environ. Res. Public Health 2022, 19, 6072. [Google Scholar] [CrossRef]
- Leong, J.; Teh, J.; Zainodin, A.; Belani, L.; Ashraff, M.; Ariff, M. Osteoporosis care gap following fragility fracture in a tertiary teaching hospital. J. Community Med. Public Health Rep. 2021, 2, 1–5. [Google Scholar]
- Feng, Q.; Zheng, S.; Zheng, J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci. Rep. 2018, 38, BSR20180453. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.-L.; Ang, S.B.; Chadha, M.; Chow, E.S.-L.; Chung, Y.-S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.-H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Sarafrazi, N.; Wambogo, E.A.; Shepherd, J.A. Osteoporosis or Low Bone Mass in Older Adults: United States, 2017–2018; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. [Google Scholar]
- Lorentzon, M.; Johansson, H.; Harvey, N.; Liu, E.; Vandenput, L.; McCloskey, E.; Kanis, J. Osteoporosis and fractures in women: The burden of disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Chan, D.C.; Lau, T.C.; Lee, J.K.; Songpatanasilp, T.; Wong, S.H.; Hew, F.L.; Sethi, R.; Williams, M. Secondary prevention of fragility fractures in Asia Pacific: An educational initiative. Osteoporos. Int. 2020, 31, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.W.; Mohd Tahir, N.A.; Mohamed Said, M.S.; Makmor Bakry, M.; Li, S.C. Budget impact of increasing uptake of denosumab for the treatment of postmenopausal osteoporosis in Malaysia. Arch. Osteoporos. 2023, 18, 145. [Google Scholar] [CrossRef]
- Brown, C. Staying strong. Nature 2017, 550, S15–S17. [Google Scholar] [CrossRef]
- Xiao, W.; Li, S.; Pacios, S.; Wang, Y.; Graves, D.T. Bone remodeling under pathological conditions. Tooth Mov. 2016, 18, 17–27. [Google Scholar]
- Painter, S.E.; Kleerekoper, M.; Camacho, P.M. Secondary osteoporosis: A review of the recent evidence. Endocr. Pract. 2006, 12, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Yeap, S.; Lee, E.; Chee, W.; Hew, F.; Lee, J.; Lim, H.; Damodaran, P.; Mumtaz, M. Clinical Guidance on Management of Osteoporosis; Malaysian Osteoporosis Society, Academy of Medicine & Ministry of Health: Petaling Jaya, Malaysia, 2015.
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-based treatment strategies for osteoporosis: A literature review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological bone remodeling: Systemic regulation and growth factor involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, V.; Kadian, S.; Detwiler, D.A.; Zareei, A.; Woodhouse, I.; Qi, Z.; Peana, S.; Alcaraz, A.M.; Wang, H.; Rahimi, R. Laser-assisted nanotexturing and silver immobilization on titanium implant surfaces to enhance bone cell mineralization and antimicrobial properties. Langmuir 2022, 38, 4014–4027. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Zhou, Q.; Kubo, K.Y. Morphological and molecular characterization of the senile osteoporosis in senescence-accelerated mouse prone 6 (SAMP6). Med. Mol. Morphol. 2018, 51, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Moriishi, T.; Komori, T. Osteocytes: Their lacunocanalicular structure and mechanoresponses. Int. J. Mol. Sci. 2022, 23, 4373. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Subramaniam, S.; Mohamed, N.; Muhammad, N.; Ramli, F.F.; Ima-Nirwana, S.; Chin, K.-Y. Circulating Biomarkers Related to Osteocyte and Calcium Homeostasis Between Postmenopausal Women with and without Osteoporosis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, D.; Davison, N.; Habibović, P. Human osteoclast formation and resorptive function on biomineralized collagen. Bioact. Mater. 2022, 8, 241–252. [Google Scholar] [CrossRef]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of innate immune cells in osteoporosis. Front. Immunol. 2021, 12, 687037. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Peake, C.; Shah, K.; Solan, M.C. Bone metabolism and the receptor activator of nuclear factor-κB ligand (RANKL) pathway: A comprehensive review. Orthop. Trauma. 2021, 35, 297–304. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Osteoporosis: Current and Emerging Therapies Targeted to Immunological Checkpoints. Curr. Med. Chem. 2020, 27, 6356–6372. [Google Scholar] [CrossRef]
- Feehan, J.; Al Saedi, A.; Duque, G. Targeting fundamental aging mechanisms to treat osteoporosis. Expert. Opin. Ther. Targets 2019, 23, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Refaey, M.E.; McGee-Lawrence, M.E.; Fulzele, S.; Kennedy, E.J.; Bollag, W.B.; Elsalanty, M.; Zhong, Q.; Ding, K.H.; Bendzunas, N.G.; Shi, X.m. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J. Bone Miner. Res. 2017, 32, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168. [Google Scholar] [PubMed]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone—The functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Glasnović, A.; Stojić, M.; Dežmalj, L.; Tudorić-Đeno, I.; Romić, D.; Jeleč, V.; Vrca, A.; Vuletić, V.; Grčević, D. RANKL/RANK/OPG axis is deregulated in the cerebrospinal fluid of multiple sclerosis patients at clinical onset. Neuroimmunomodulation 2018, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Dulewicz, M.; Mroczko, B. A disintegrin and metalloproteinase (ADAM) family: Their significance in malignant tumors of the central nervous system (CNS). Int. J. Mol. Sci. 2021, 22, 10378. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, J.; Buckland, G.; Chan, P.E.; Ong, A.; Ramos, A.S.; Baxter, M.; Laskou, F.; Dennison, E.M.; Cooper, C.; Patel, H.P. Pathophysiology and treatment of osteoporosis: Challenges for clinical practice in older people. Aging Clin. Exp. Res. 2021, 33, 759–773. [Google Scholar] [CrossRef]
- Karim, K.; Giribabu, N.; Salleh, N. Marantodes pumilum Var Alata (Kacip Fatimah) ameliorates derangement in RANK/RANKL/OPG pathway and reduces inflammation and oxidative stress in the bone of estrogen-deficient female rats with type-2 diabetes. Phytomedicine 2021, 91, 153677. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Salari, P.; Khashayar, P.; Larijani, B. New horizons in treatment of osteoporosis. DARU J. Pharm. Sci. 2017, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- BMJ Group. Strontium ranelate discontinued. Drug Ther. Bull. 2017, 55, 86. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Li, L.; Yang, P.; Ma, Y. Menaquinone 4 reduces bone loss in ovariectomized mice through dual regulation of bone remodeling. Nutrients 2021, 13, 2570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-e.; Yang, Z.; Zhang, H.; Yao, G.; Liu, J.; Wei, Q.; Ma, B. Resveratrol promotes osteogenic differentiation of canine bone marrow mesenchymal stem cells through wnt/beta-catenin signaling pathway. Cell. Reprogram. 2018, 20, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shi, H.; Zhang, W.; Gu, B. The crosstalk between canonical and noncanonical Wnt signaling pathway in osteoblast differentiation of periodontal ligament stem cells in inflammatory microenvironments. Zhonghua Kou Qiang Yi Xue Za Zhi = Zhonghua Kouqiang Yixue Zazhi = Chin. J. Stomatol. 2016, 51, 673–679. [Google Scholar]
- Hua, Y.; Yang, Y.; Li, Q.; He, X.; Zhu, W.; Wang, J.; Gan, X. Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway. J. Biol. Chem. 2018, 293, 19710–19724. [Google Scholar] [CrossRef] [PubMed]
- Tortelote, G.G.; Reis, R.R.; de Almeida Mendes, F.; Abreu, J.G. Complexity of the Wnt/β-catenin pathway: Searching for an activation model. Cell. Signal. 2017, 40, 30–43. [Google Scholar] [CrossRef] [PubMed]
- García de Herreros, A.; Duñach, M. Intracellular signals activated by canonical Wnt ligands independent of GSK3 inhibition and β-catenin stabilization. Cells 2019, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-W.; Duan, L.-X.; Yu, Y.; Wang, P.; Feng, J.-L.; Feng, G.-Z.; Liu, Y. Bone marrow mesenchymal stem cells promote prostate cancer cell stemness via cell–cell contact to activate the Jagged1/Notch1 pathway. Cell Biosci. 2021, 11, 87. [Google Scholar] [CrossRef]
- Song, L. Calcium and bone metabolism indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [PubMed]
- Tabacco, G.; Bilezikian, J.P. Osteoanabolic and dual action drugs. Br. J. Clin. Pharmacol. 2019, 85, 1084–1094. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Michopoulos, A.; Yovos, J.G. PTH and PTHR1 in osteocytes. New insights into old partners. Hormones 2017, 16, 150–160. [Google Scholar] [PubMed]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of osteoporosis—Role of T cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M.; Saitta, S.; Sirufo, M.M.; Mannucci, C.; Casciaro, M.; Ciccarelli, F.; Gangemi, S. Interleukin-33 serum levels in postmenopausal women with osteoporosis. Sci. Rep. 2019, 9, 3786. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk, I.; Jasiecka-Mikołajczyk, A.; Maślanka, T. Blockade of NF-κB translocation and of RANKL/RANK interaction decreases the frequency of Th2 and Th17 cells capable of IL-4 and IL-17 production, respectively, in a mouse model of allergic asthma. Molecules 2021, 26, 3117. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shi, P.; Liu, M.; Chen, H.; Tu, M.; Lu, W.; Du, M. Lactoferrin preserves bone homeostasis by regulating the RANKL/RANK/OPG pathway of osteoimmunology. Food Funct. 2018, 9, 2653–2660. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Tyagi, A.M.; Vaccaro, C.; Hsu, E.; Adams, J.; Bellido, T.; Weitzmann, M.N.; Pacifici, R. IL-17 receptor signaling in osteoblasts/osteocytes mediates PTH-induced bone loss and enhances osteocytic RANKL production. J. Bone Miner. Res. 2019, 34, 349–360. [Google Scholar] [CrossRef] [PubMed]
- DeSelm, C.J.; Takahata, Y.; Warren, J.; Chappel, J.C.; Khan, T.; Li, X.; Liu, C.; Choi, Y.; Kim, Y.F.; Zou, W.; et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J. Cell. Biochem. 2012, 113, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Soh, G.T.; Mohammad, A.H.; Syed Isa, S.N.L.; Chin, K.-Y.; Mohamed, N. Comparison of Cytokine Profile between Postmenopausal Women with and Without Osteoporosis–A Case-Control Study. Endocr. Metab. Immune Disord.-Drug Targets 2023, 23, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Li, F.; Li, X.; Wang, Z.G.; Zhang, B. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Rep. 2018, 17, 6605–6611. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hurchla, M.A.; Deng, H.; Uluçkan, O.; Bu, F.; Berdy, A.; Eagleton, M.C.; Heller, E.A.; Floyd, D.H.; Dirksen, W.P. Interferon-γ targets cancer cells and osteoclasts to prevent tumor-associated bone loss and bone metastases. J. Biol. Chem. 2009, 284, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-induced tryptophan breakdown is related with anemia, fatigue, and depression in cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Hamrick, M.W.; Yoo, H.J.; Lee, S.H.; Kim, S.J.; Koh, J.-M.; Isales, C.M. The detrimental effects of kynurenine, a tryptophan metabolite, on human bone metabolism. J. Clin. Endocrinol. Metab. 2019, 104, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E. Bisphosphonates for Postmenopausal Osteoporosis. JAMA 2021, 325, 96. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Veliceasa, B.; Puha, B.; Filip, C.; Popescu, D.; Alexa, O. Bisphosphonates influence and pain assessment in mobilization of patients with fragility fracture of the pelvis. Rev. Chim. 2019, 70, 1094–1097. [Google Scholar] [CrossRef]
- Rosen, H.N.; Rosenblatt, M. Pharmacology of bisphosphonates. Bone 2019, 49, 42–49. [Google Scholar]
- Barbosa, J.S.; Braga, S.S.; Almeida Paz, F.A. Empowering the medicinal applications of bisphosphonates by unveiling their synthesis details. Molecules 2020, 25, 2821. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, molecular target and current status on drug development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef] [PubMed]
- National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation; National Osteoporosis Foundation: Washington, DC, USA, 2010; pp. 1–55. [Google Scholar]
- Qaseem, A.; Forciea, M.; McLean, R.; Denberg, T. Clinical guidelines committee of the American college of P. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American college of physicians. Ann. Intern. Med. 2017, 166, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef]
- Wiesner, A.; Szuta, M.; Galanty, A.; Paśko, P. Optimal dosing regimen of osteoporosis drugs in relation to food intake as the key for the enhancement of the treatment effectiveness—A concise literature review. Foods 2021, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A review of treatment options. Pharm. Ther. 2018, 43, 92. [Google Scholar]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef]
- Lorentzon, M. Treating osteoporosis to prevent fractures: Current concepts and future developments. J. Intern. Med. 2019, 285, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Pavone, V.; Testa, G.; Giardina, S.M.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological therapy of osteoporosis: A systematic current review of literature. Front. Pharmacol. 2017, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, S.; Inoue, K.; Igarashi, K.; Sugizaki, H.; Shirode-Fukuda, Y.; Inoue, E.; Yu, T.; Takeuchi, J.K.; Kanno, J.; Bonewald, L.F. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone 2014, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Wu, T.; Cai, C.; Song, W.; Wang, J.; Yu, Q.C.; Zeng, Y.A. A novel function of R-spondin1 in regulating estrogen receptor expression independent of Wnt/β-catenin signaling. eLife 2020, 9, e56434. [Google Scholar] [CrossRef] [PubMed]
- Bagger, Y.Z.; Tankó, L.B.; Alexandersen, P.; Hansen, H.B.; Møllgaard, A.; Ravn, P.; Qvist, P.; Kanis, J.A.; Christiansen, C. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: The PERF study. Bone 2004, 34, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Delgado, L.; Castelán-Martínez, O.D.; Clark, P.; Garduño-Espinosa, J.; Mendoza-Núñez, V.M.; Sánchez-Rodríguez, M.A. Effect of Tibolone on Bone Mineral Density in Postmenopausal Women: Systematic Review and Meta-Analysis. Biology 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Zhang, H.; Yang, J.; Lin, W.; Wang, K.; Su, M.; Peng, W.; Li, Y.; Lai, Y.; Liu, C. Increased risk of cardiovascular death in breast cancer patients without chemotherapy or (and) radiotherapy: A large population-based study. Front. Oncol. 2021, 10, 619622. [Google Scholar] [CrossRef] [PubMed]
- Guañabens, N.; Moro-Álvarez, M.J.; Casado, E.; Blanch-Rubió, J.; Gómez-Alonso, C.; Díaz-Guerra, G.M.; del Pino-Montes, J.; Valero Díaz de Lamadrid, C.; Peris, P.; Muñoz-Torres, M. The next step after anti-osteoporotic drug discontinuation: An up-to-date review of sequential treatment. Endocrine 2019, 64, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Mohamad, N.-V.; Jayusman, P.A.; Shuid, A.N.; Ima-Nirwana, S.; Chin, K.-Y. The use of selective estrogen receptor modulators on bone health in men. Aging Male 2018, 22, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Ohlsson, C. Estrogens as regulators of bone health in men. Nat. Rev. Endocrinol. 2009, 5, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, N.D.; Nguyen, T.V. Quantification of the relative contribution of estrogen to bone mineral density in men and women. BMC Musculoskelet. Disord. 2013, 14, 366. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Zhang, M.; Bockman, R. Bone mineral density response from teriparatide in patients with osteoporosis. HSS J. 2017, 13, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Phipps, R. Selective Estrogen Receptor Modulators (SERMs). In Osteoporotic Fracture and Systemic Skeletal Disorders: Mechanism, Assessment, and Treatment; Takahashi, H.E., Burr, D.B., Yamamoto, N., Eds.; Springer: Singapore, 2022; pp. 399–411. [Google Scholar]
- Hsiao, C.-Y.; Chen, T.-H.; Chu, T.-H.; Ting, Y.-N.; Tsai, P.-J.; Shyu, J.-F. Calcitonin induces bone formation by increasing expression of Wnt10b in osteoclasts in ovariectomy-induced osteoporotic rats. Front. Endocrinol. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, J.; Kanwal, Z.; Wu, M.; Lv, X.; Ibrahim, N.A.; Li, P.; Buabeid, M.A.; Arafa, E.-S.A.; Sun, Q. Calcitonin and bone physiology: In vitro, in vivo, and clinical investigations. Int. J. Endocrinol. 2020, 2020, 3236828. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, E.; Dagistan, Y.; Kukner, A.; Yilmaz, B.; Agus, S.; Soyler, G.; Tore, F. Salmon calcitonin ameliorates migraine pain through modulation of CGRP release and dural mast cell degranulation in rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the calcium-parathyroid hormone-vitamin D axis. Vitam. D Clin. Med. 2018, 50, 1–13. [Google Scholar]
- Gambardella, C.; Offi, C.; Patrone, R.; Clarizia, G.; Mauriello, C.; Tartaglia, E.; Di Capua, F.; Di Martino, S.; Romano, R.M.; Fiore, L. Calcitonin negative Medullary Thyroid Carcinoma: A challenging diagnosis or a medical dilemma? BMC Endocr. Disord. 2019, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Portal-Nunez, S.; Mediero, A.; Esbrit, P.; Sanchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Unexpected bone formation produced by RANKL blockade. Trends Endocrinol. Metab. 2017, 28, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, T.; Miyazaki, A.; Saito, M.; Suzuki, T.; Takahashi, J.; Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci. Rep. 2021, 11, 11801. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Ominsky, M.S.; Rachner, T.D.; Hofbauer, L.C.; Kostenuik, P.J. Pharmacological mechanisms of therapeutics: Receptor activator of nuclear factor–kappa B ligand inhibition. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1689–1710. [Google Scholar]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 57, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, C.; Cai, X.Z.; Zhao, X.; Shi, M.M.; Ying, Z.M.; Yuan, F.Z.; Guo, C.; Yan, S.G. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: A meta-analysis. Int. J. Clin. Pract. 2012, 66, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumoto, T.; Sugimoto, T.; Hosoi, T.; Miki, T.; Gorai, I.; Yoshikawa, H.; Tanaka, Y.; Tanaka, S.; Sone, T. Clinical Trials Express: Fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: Denosumab fracture intervention randomized placebo controlled trial (DIRECT). J. Clin. Endocrinol. Metab. 2014, 99, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Libanati, C.; Lin, C.J.F.; Brown, J.; Cosman, F.; Czerwiński, E.; de Gregόrio, L.; Malouf-Sierra, J.; Reginster, J.Y.; Wang, A. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J. Bone Miner. Res. 2019, 34, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Pazianas, M.; Abrahamsen, B. Osteoporosis treatment: Bisphosphonates reign to continue for a few more years, at least? Ann. N. Y. Acad. Sci. 2016, 1376, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guañabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Yavropoulou, M.P.; Makras, P.; Sakellariou, G.T.; Papadopoulou, F.; Gerou, S.; Papapoulos, S.E. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur. J. Endocrinol. 2017, 176, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.-J.; Gonzalez Rodriguez, E.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N. Fracture risk and management of discontinuation of denosumab therapy: A systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 2021, 106, 264–281. [Google Scholar] [CrossRef]
- Panwar, P.; Law, S.; Jamroz, A.; Azizi, P.; Zhang, D.; Ciufolini, M.; Brömme, D. Tanshinones that selectively block the collagenase activity of cathepsin K provide a novel class of ectosteric antiresorptive agents for bone. Br. J. Pharmacol. 2018, 175, 902–923. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Benhamou, C.-L.; Halse, J.; Miller, P.; Reid, I.; Rodriguez Portales, J.; DaSilva, C.; Kroon, R.; Verbruggen, N.; Leung, A. Continuous treatment with odanacatib for up to 8 years in postmenopausal women with low bone mineral density: A phase 2 study. Osteoporos. Int. 2016, 27, 2099–2107. [Google Scholar] [CrossRef]
- Boggild, M.K.; Gajic-Veljanoski, O.; McDonald-Blumer, H.; Ridout, R.; Tile, L.; Josse, R.; Cheung, A.M. Odanacatib for the treatment of osteoporosis. Expert. Opin. Pharmacother. 2015, 16, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Libouban, H.; Mindeholm, L.; Baslé, M.F.; Legrand, E.; Audran, M. The cathepsin K inhibitor AAE581 induces morphological changes in osteoclasts of treated patients. Microsc. Res. Tech. 2010, 73, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Oursler, M.J.; Khosla, S. Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned. Endocr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Sølling, A.S.K.; Harsløf, T.; Langdahl, B. Current status of bone-forming therapies for the management of osteoporosis. Drugs Aging 2019, 36, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Hofbauer, L.C.; Göbel, A.; Tsourdi, E. Novel therapies in osteoporosis: PTH-related peptide analogs and inhibitors of sclerostin. J. Mol. Endocrinol. 2019, 62, R145–R154. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; McAndrews, K.; Wu, G.; Orr, A.L.; Ferrari, A.; Tu, X.; Srinivasan, V.; Roodman, G.D.; Ebetino, F.H.; Boeckman, R.K., Jr.; et al. The Notch pathway regulates the bone gain induced by PTH anabolic signaling. FASEB J. 2022, 36, e22196. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, G.; Bilezikian, J.P. PTH and PTHrP analogs: Treatment of osteoporosis. In Osteoporosis: Pathophysiology and Clinical Management; Humana: Cham, Switzerland, 2020; pp. 349–362. [Google Scholar]
- Makino, A.; Hasegawa, T.; Takagi, H.; Takahashi, Y.; Hase, N.; Amizuka, N. Frequent administration of abaloparatide shows greater gains in bone anabolic window and bone mineral density in mice: A comparison with teriparatide. Bone 2021, 142, 115651. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.; Hu, M.-Y.; Harris, A.G. Effect of abaloparatide vs. placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 2016, 157, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Stoltenborg, F.E.; Brüel, A.; Thomsen, J.S. Teriparatide and Abaloparatide Have a Similar Effect on Bone in Mice. Front. Endocrinol. 2021, 12, 628994. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Matsumoto, T. Recent advances in the management of osteoporosis. F1000Research 2017, 6, 625. [Google Scholar] [CrossRef] [PubMed]
- Boyce, E.G.; Mai, Y.; Pham, C. Abaloparatide: Review of a Next-Generation Parathyroid Hormone Agonist. Ann. Pharmacother. 2018, 52, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z. Optimizing sequential and combined anabolic and antiresorptive osteoporosis therapy. JBMR Plus 2018, 2, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, B.; Smertka, M.; Chudek, J. Sclerostin: Intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv. Clin. Exp. Med. 2017, 26, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, M.K.; Mishra, S.P. Study of correlation of level of expression of Wnt signaling pathway inhibitors sclerostin and dickkopf-1 with disease activity and severity in rheumatoid arthritis patients. Drug Discov. Ther. 2019, 13, 22–27. [Google Scholar] [CrossRef]
- Florio, M.; Gunasekaran, K.; Stolina, M.; Li, X.; Liu, L.; Tipton, B.; Salimi-Moosavi, H.; Asuncion, F.J.; Li, C.; Sun, B. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016, 7, 11505. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E. Management of endocrine disease: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44. [Google Scholar] [CrossRef]
- Vahe, C.; Benomar, K.; Espiard, S.; Coppin, L.; Jannin, A.; Odou, M.-F.; Vantyghem, M.-C. Diseases associated with calcium-sensing receptor. Orphanet J. Rare Dis. 2017, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, T.J. The quest for new drugs to prevent osteoporosis-related fractures. Climacteric J. Int. Menopause Soc. 2017, 20, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Diepenhorst, N.A.; Leach, K.; Keller, A.N.; Rueda, P.; Cook, A.E.; Pierce, T.L.; Nowell, C.; Pastoureau, P.; Sabatini, M.; Summers, R.J.; et al. Divergent effects of strontium and calcium-sensing receptor positive allosteric modulators (calcimimetics) on human osteoclast activity. Br. J. Pharmacol. 2018, 175, 4095–4108. [Google Scholar] [CrossRef]
- Noh, J.-Y.; Yang, Y.; Jung, H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Tuffour, A.; Kosiba, A.A.; Zhang, Y.; Peprah, F.A.; Gu, J.; Shi, H. Role of the calcium-sensing receptor (CaSR) in cancer metastasis to bone: Identifying a potential therapeutic target. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188528. [Google Scholar] [CrossRef] [PubMed]
- Berencsi, K.; Ali, M.; Marinier, K.; Deltour, N.; Hawley, S.; Pedersen, L.; Rijnbeek, P.; Duijnhoven, R.; Van Der Lei, J.; Lapi, F.; et al. Impact of risk minimisation measures on the use of strontium ranelate: A multi-national cohort study in 5 EU countries by the EU-ADR Alliance. Pharmacoepidemiol. Drug Saf. 2017, 26, 483. [Google Scholar]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, W.; Xu, N.; Guan, H.; Fang, Z.; Liao, H.; Zhang, Y.; Gao, B.; Xiao, X.; Fu, J. Strontium-loaded titania nanotube arrays repress osteoclast differentiation through multiple signalling pathways: In vitro and in vivo studies. Sci. Rep. 2017, 7, 2328. [Google Scholar] [CrossRef] [PubMed]
- Saidenberg-Kermanac’h, N.; Corrado, A.; Lemeiter, D.; Devernejoul, M.; Boissier, M.; Cohen-Solal, M. TNF-α antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone 2004, 35, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Korczowska, I.; Lacki, J.K.; Hrycaj, P. Influence of infliximab on cytokines network and markers of bone remodeling in rheumatoid arthritis patients. Yonsei Med. J. 2013, 54, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.; Voskuyl, A.; Wolbink, G.; Dijkmans, B.; Lems, W. Bone mineral density in patients with rheumatoid arthritis treated with infliximab. Ann. Rheum. Dis. 2005, 64, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Teichmann, J.; Muller-Ladner, U.; Strunk, J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-α antibody: A prospective open-label pilot study. Rheumatology 2005, 44, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Thompson, E.; Woodworth, T.; Smolen, J.S. Rapid and sustained improvement in bone and cartilage turnover markers with the anti–interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: Results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2010, 62, 33–43. [Google Scholar] [PubMed]
- Karsdal, M.A.; Schett, G.; Emery, P.; Harari, O.; Byrjalsen, I.; Kenwright, A.; Bay-Jensen, A.C.; Platt, A. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: Biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study (NCT00106522). Semin. Arthritis Rheum. 2012, 42, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Chen, H.-H.; Huang, W.-N.; Liao, T.-L.; Chen, J.-P.; Chao, W.-C.; Lin, C.-T.; Hung, W.-T.; Hsieh, C.-W.; Hsieh, T.-Y.; et al. Tocilizumab potentially prevents bone loss in patients with anticitrullinated protein antibody-positive rheumatoid arthritis. PLoS ONE 2017, 12, e0188454. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Amano, K.; Yamada, S.; Kanazawa, T.; Ohta, H.; Hatta, K.; Amano, K.; Kuwaba, N. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology 2014, 53, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, A.M.; Lim, M.J.; Lane, N.E. Osteoporosis in Rheumatic Diseases: Anti-rheumatic Drugs and the Skeleton. Calcif. Tissue Int. 2018, 102, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-TNF monoclonal antibodies in inflammatory bowel disease: Pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.B.; Furst, D.E. Problems encountered during anti-tumour necrosis factor therapy. Best. Pract. Res. Clin. Rheumatol. 2006, 20, 757–790. [Google Scholar] [CrossRef] [PubMed]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthritis—Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kahwati, L.C.; Weber, R.P.; Pan, H.; Gourlay, M.; LeBlanc, E.; Coker-Schwimmer, M.; Viswanathan, M. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018, 319, 1600–1612. [Google Scholar] [CrossRef]
- Weaver, C.; Alexander, D.; Boushey, C.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.; Liu, S.; Looker, A.; Wallace, T.; Wang, D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Subramaniam, S.; Chin, K.-Y.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Jamil, N.A.; Abd Aziz, N.; Mohamed, N. Levels of knowledge, beliefs, and practices regarding osteoporosis and the associations with bone mineral density among populations more than 40 years old in Malaysia. Int. J. Environ. Res. Public Health 2019, 16, 4115. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Mohamad, S.; Mohamed, N.; Fadzilah, F.M.; Mokhtar, S.A.; Abdullah, S.; Othman, F.; Suhaimi, F.; Muhammad, N.; Soelaiman, I.N. Effects of calcium supplements on fracture healing in a rat osteoporotic model. J. Orthop. Res. 2010, 28, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Reid, I.R. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric 2015, 18, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shaker, J.L.; Deftos, L. Calcium and Phosphate Homeostasis. Endotext. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279023/ (accessed on 15 July 2024).
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rovito, D.; Belorusova, A.Y.; Chalhoub, S.; Rerra, A.-I.; Guiot, E.; Molin, A.; Linglart, A.; Rochel, N.; Laverny, G.; Metzger, D. Cytosolic sequestration of the vitamin D receptor as a therapeutic option for vitamin D-induced hypercalcemia. Nat. Commun. 2020, 11, 6249. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Fuleihan, G.E.-H.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Yamazaki, Y.; Shiraki, Y.; Hosoi, T.; Tsugawa, N.; Okano, T. High level of serum undercarboxylated osteocalcin in patients with incident fractures during bisphosphonate treatment. J. Bone Miner. Metab. 2010, 28, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J. Vitamin K2 therapy for postmenopausal osteoporosis. Nutrients 2014, 6, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Nazrun, A.; Norazlina, M.; Norliza, M.; Nirwana, S. The anti-inflammatory role of vitamin E in prevention of osteoporosis. Adv. Pharmacol. Pharm. Sci. 2012, 2012, 142702. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The effects of tocotrienol on bone peptides in a rat model of osteoporosis induced by metabolic syndrome: The possible communication between bone cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, P.; Zhang, X.; Lv, L.; Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021, 54, e12956. [Google Scholar] [CrossRef]
- Aghebati-Maleki, L.; Dolati, S.; Zandi, R.; Fotouhi, A.; Ahmadi, M.; Aghebati, A.; Nouri, M.; Kazem Shakouri, S.; Yousefi, M. Prospect of mesenchymal stem cells in therapy of osteoporosis: A review. J. Cell Physiol. 2019, 234, 8570–8578. [Google Scholar] [CrossRef] [PubMed]
- Paspaliaris, V.; Kolios, G. Stem cells in osteoporosis: From biology to new therapeutic approaches. Stem Cells Int. 2019, 2019, 1730978. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Lv, H.; Yin, P.; Li, Z.; Tang, P.; Wang, Y. Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2019, 30, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Garapati, S.S. Combination therapies in the treatment of osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Burnett-Bowie, S.-A.M.; Zhu, Y.; Foley, K.; Lee, H.; Neer, R.M. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): A randomized controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, K.; Kashii, M.; Ebina, K.; Kaito, T.; Okada, R.; Makino, T.; Noguchi, T.; Ishimoto, T.; Nakano, T.; Yoshikawa, H. Effects of single or combination therapy of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in mice. Bone 2018, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Yavropoulou, M.P.; Makras, P. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert. Opin. Pharmacother. 2020, 21, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Shiau, S.; Recker, R.R.; Lappe, J.M.; Agarwal, S.; Kamanda-Kosseh, M.; Bucovsky, M.; Stubby, J.; Cohen, A. Denosumab after teriparatide in premenopausal women with idiopathic osteoporosis. J. Clin. Endocrinol. Metab. 2022, 107, e1528–e1540. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.K.; Singh, D.K.A.; Zubir, K.; Chua, Y.Y.; Rajaratnam, B.S.; Mokhtar, S.A. Relationship between muscle strength, physical performance, quality of life and bone mineral density among postmenopausal women at risk of osteoporotic fractures. Sci. Eng. Health Stud. 2020, 14, 8–21. [Google Scholar]
- Kuan, C.S.; Yian, C.Y.; Singh, A.; Kaur, D.; Mokhtar, S.A. Effectiveness of Physiotherapeutic Group Education in Improving Quality of Life, Physical Performance and Back Extensor Muscle Strength among Postmenopausal Women with Osteoporosis. Malays. J. Med. Health Sci. 2022, 18, 269–277. [Google Scholar]
- Wong, S.K.; Mohamad, N.-V.; Ibrahim, N.I.; Chin, K.-Y.; Shuid, A.N.; Ima-Nirwana, S. The molecular mechanism of vitamin E as a bone-protecting agent: A review on current evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [PubMed]
- Hairi, H.A.; Jamal, J.A.; Aladdin, N.A.; Husin, K.; Sofi, N.S.M.; Mohamed, N.; Mohamed, I.N.; Shuid, A.N. Demethylbelamcandaquinone B Isolated from Labisia pumila Enhanced Proliferation and Differentiation of Osteoblast Cells. J. Appl. Pharm. Sci. 2018, 8, 012–020. [Google Scholar]
- M Calderon-Montano, J.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The osteoprotective effects of kaempferol: The evidence from in vivo and in vitro studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed]



| Agent | Drug Name | Drug Class | Mechanism of Action | Side Effects |
|---|---|---|---|---|
| Bisphosphonates | Ibandronate Alendronate Zoledronic acid Risedronate Clodronate Etidronate Tiludronate [64,65] | Anti-resorptive drug [62] | Induces osteoclast apoptosis Inhibits protons release from osteoclasts [36]. | Oral administration causes dysphagia, nausea, abdominal pain, constipation or diarrhea, flatulence, acid regurgitation, esophageal ulcers, taste distortion, and gastritis. Rare side effects such as osteonecrosis of the jaw (ONJ), atypical femoral fractures (AFFs), influenza-like symptoms, uveitis, hypocalcemia, and episcleritis [69,70,71,72,73]. |
| ERT/HRT | Estrogen/progestin along with estrogen | Anti-resorptive drug [75] | Induces osteoclast apoptosis. Decreases apoptosis in osteoblasts and osteocytes. Inhibits RANKL and boosts the synthesis of OPG. Stimulates Wnt/β-catenin signaling [37,75,76,77,78]. | ERT increases the chance of cerebrovascular accidents, endometrial cancer, and venous thromboembolic disorders. HRT increases the risk of cerebrovascular accidents, cardiovascular diseases, breast cancer, and venous thromboembolic disorders [72]. |
| SERMs | Raloxifene Bazedoxifene [12] | Anti-resorptive drug [38] | Induces osteoclast apoptosis. Decreases apoptosis in osteoblasts and osteocytes. Inhibits RANKL and boosts the synthesis of OPG. Stimulates Wnt/β-catenin signaling [38]. | SERMs raise the risk of thromboembolic disorder, muscular spasms, and cerebrovascular accident [88]. |
| Anti-RANKL antibody | Denosumab [96] | Anti-resorptive drug [95] | Blocks RANKL pathway [96]. | Rebound enhancement in osteoclastogenesis, atypical femoral fractures (AFFs), osteonecrosis of the jaw (ONJ), musculoskeletal discomfort, hypocalcemia, gastrointestinal problems, and impairs the immune system in long-term use [38,98]. |
| Cathepsin K inhibitors | Odanacatib Balicatib [107,110] | Anti-resorptive drug [106] | Enlarges osteoclasts and reduces collagen degradation via cathepsin K inhibition [106,109,110]. | AFF, pycnodysostosis, and a higher risk of cerebrovascular accident [38]. |
| Strontium ranelate | Ranelic acid Distrontium salt [134] | Anabolic and anti-resorptive drug [134] | Stimulates the ERK signaling improves osteoblast proliferation, and AKT signaling reduces osteoblast apoptosis. Stimulates IGF-1 and IGF-2, causing stimulation of osteoblast proliferation and differentiation. Activates PLC and NF-κB, causing osteoclast apoptosis. Activates OPG. Increasing osteoclast apoptosis and osteoblast proliferation and differentiation directly [128,129,130,131,132,133]. | Venous thromboembolism, cardiovascular disorders, symptoms of the nervous system, and myocardial infarction, including allergic reactions like systemic symptoms syndrome and drug rash with eosinophilia [136]. |
| Vitamin K2 | Vitamin K2 | Nutrition intake Anabolic effect [40] | Aids γ-carboxylation of osteocalcin, which is released via osteoblasts to form bone matrix [40]. | Safe [158]. |
| Calcitonin | Miacalcin [18] | Anti-resorptive drug [90] | Increases calcium uptake in the bone. Stimulates Wnt10b in osteoclasts, leading to bone formation induction. Limits osteoclast motility and capacity to resorb bone via transcription regulation. Inhibits the maturation of osteoclast precursors, leading to inhibit bone loss [18,89,90]. | Hypocalcemia, loss of appetite, abdominal pain, nausea, diarrhea, and increasing incidence of prostate cancer [38,93]. |
| Anti-sclerostin antibody | Romosozumab [125] | Anabolic drug [112] | Activates the canonical Wnt signaling pathway, increases bone formation, and reduces bone resorption. Suppresses the BMP7 signaling [112,122]. | Myocardial infarction, cerebrovascular accidents, cardiovascular events, and malignant tumors [122,124,126,127]. |
| PTH | Teriparatide Abaloparatide [116] | Anabolic drug [47] | Inhibits sclerostin and stimulates Wnt signaling pathway. Promotes osteoblast proliferation and differentiation [18,112,113,114,115]. | Osteosarcoma risk, cephalgia, dizziness, limb cramps, nausea, and hypercalcemia [119,120]. |
| Calcium | Calcium | Nutrition intake Anti-resorptive drug [149] | Lower PTH secretion [149]. | Gastrointestinal disorders and hypercalcemia [149]. |
| Vitamin D | Vitamin D | Nutrition intake Anti-resorptive drug [154] | Promotes the intestinal intake of calcium [154]. | Hypercalcemia, gastrointestinal adverse effects, renal calculus, and myocardial infarction, which exceed the limited advantages of the therapy [156]. |
| TNF-α inhibitors | Infliximab [138] | Anti-resorptive drug [137] | Leads to serum level changes for bone resorption and formation markers, but further studies are needed [138,139,140]. | Congestive heart failure and aplastic anemia, low tissue penetrating capacity, and oral availability [146,147,148]. |
| IL-6 inhibitors | Tocilizumab [141] | Anti-resorptive drug [141] | Stimulates PINP, which is a bone formation marker, and inhibits CTX-1 and ICTP, which are bone resorption markers [141,142,143]. | Congestive heart failure and aplastic anemia, low tissue penetrating capacity, and oral availability [146,147,148]. |
| Stem cells | Stem cells | Source of osteoblasts [165] | MSCs directly cover the affected area in bones and differentiate into osteoblasts. MSCs secrete a variety of growth factors that inhibit osteoclastic differentiation and promote angiogenesis indirectly [165,166]. | Not well documented [167]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elahmer, N.R.; Wong, S.K.; Mohamed, N.; Alias, E.; Chin, K.-Y.; Muhammad, N. Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines 2024, 12, 1635. https://doi.org/10.3390/biomedicines12081635
Elahmer NR, Wong SK, Mohamed N, Alias E, Chin K-Y, Muhammad N. Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines. 2024; 12(8):1635. https://doi.org/10.3390/biomedicines12081635
Chicago/Turabian StyleElahmer, Nyruz Ramadan, Sok Kuan Wong, Norazlina Mohamed, Ekram Alias, Kok-Yong Chin, and Norliza Muhammad. 2024. "Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review" Biomedicines 12, no. 8: 1635. https://doi.org/10.3390/biomedicines12081635
APA StyleElahmer, N. R., Wong, S. K., Mohamed, N., Alias, E., Chin, K.-Y., & Muhammad, N. (2024). Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines, 12(8), 1635. https://doi.org/10.3390/biomedicines12081635






