Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Limits Tissue Damage and Promotes Tissue Regeneration and Functional Recovery in a Pediatric Piglet Traumatic-Brain-Injury Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Study Design
2.3. Controlled Cortical Impact
2.4. iNSC Culture
2.5. iNSC Transplantation
2.6. MRI Acquisition and Analysis
2.7. Neurological Assessment
2.8. Brain Tissue Collection and Processing
2.9. Immunohistochemistry
2.10. Immunofluorescent Staining
2.11. Statistical Analysis
3. Results
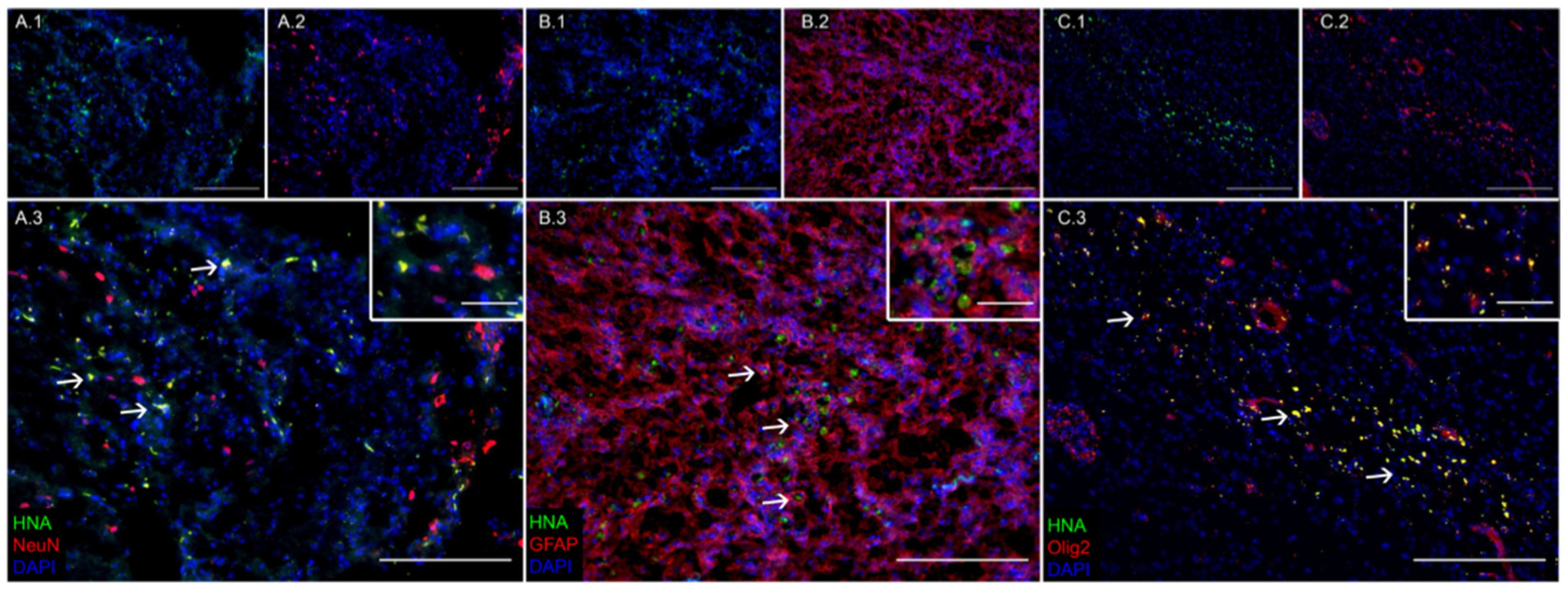
3.1. Transplanted iNSCs Survived Long-Term and Differentiated into Neurons, Astrocytes, and Oligodendrocytes
3.2. iNSC Treatment Led to Improved Neuron and Oligodendrocyte Survival and a Reduction in Immune Response 12 Weeks Post-Transplantation
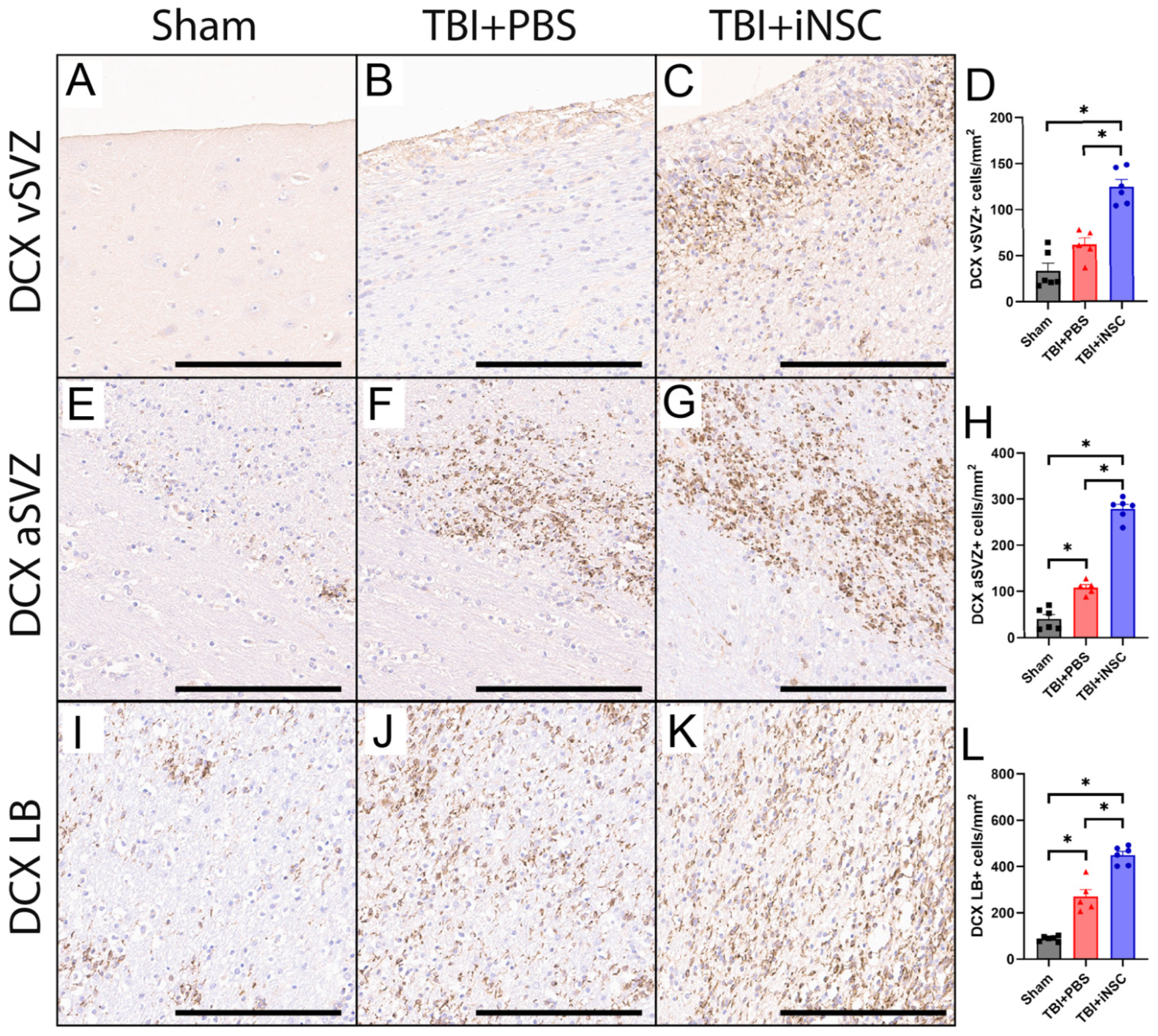
3.3. iNSC Treatment Significantly Increased Neurogenesis Compared to Untreated Piglets
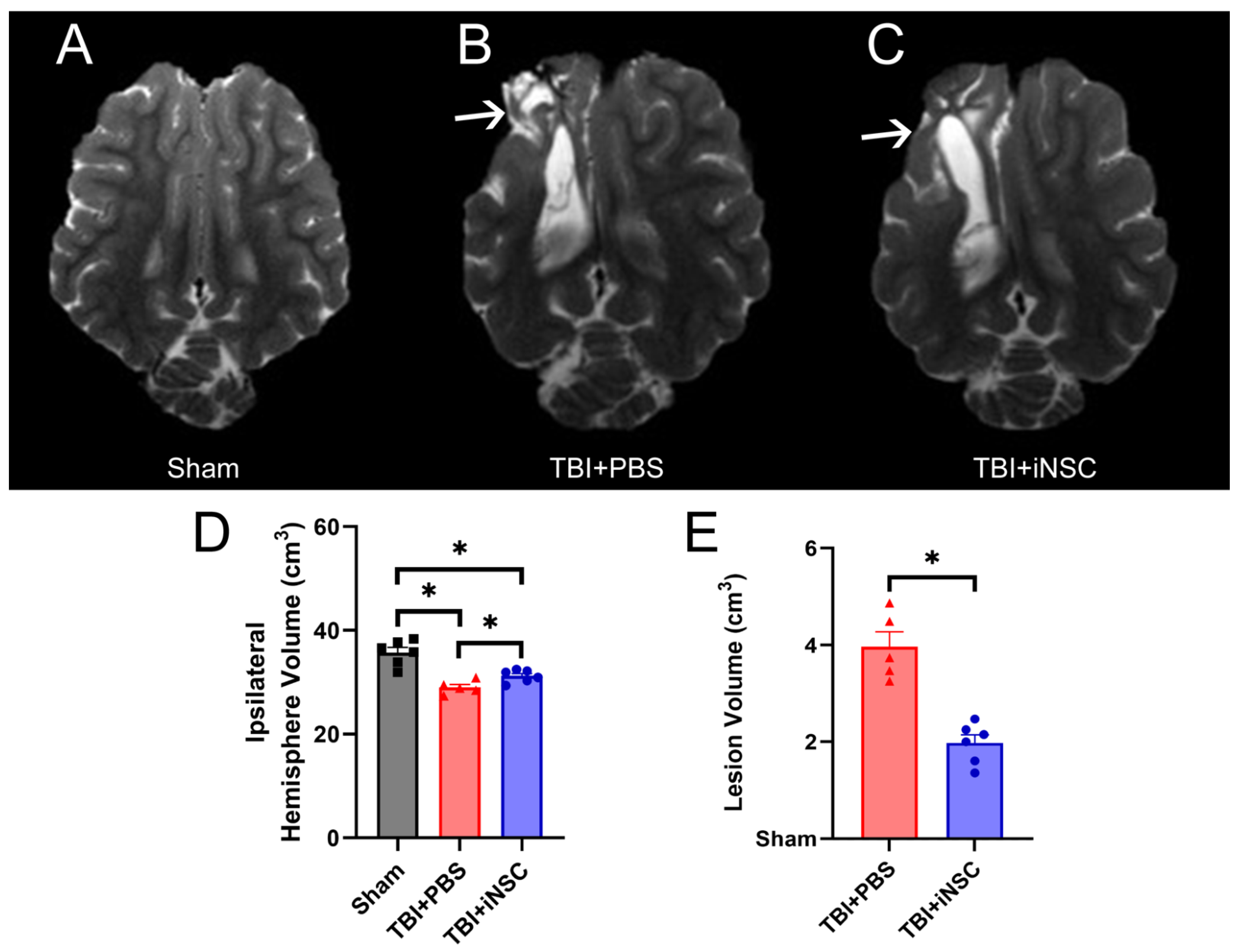
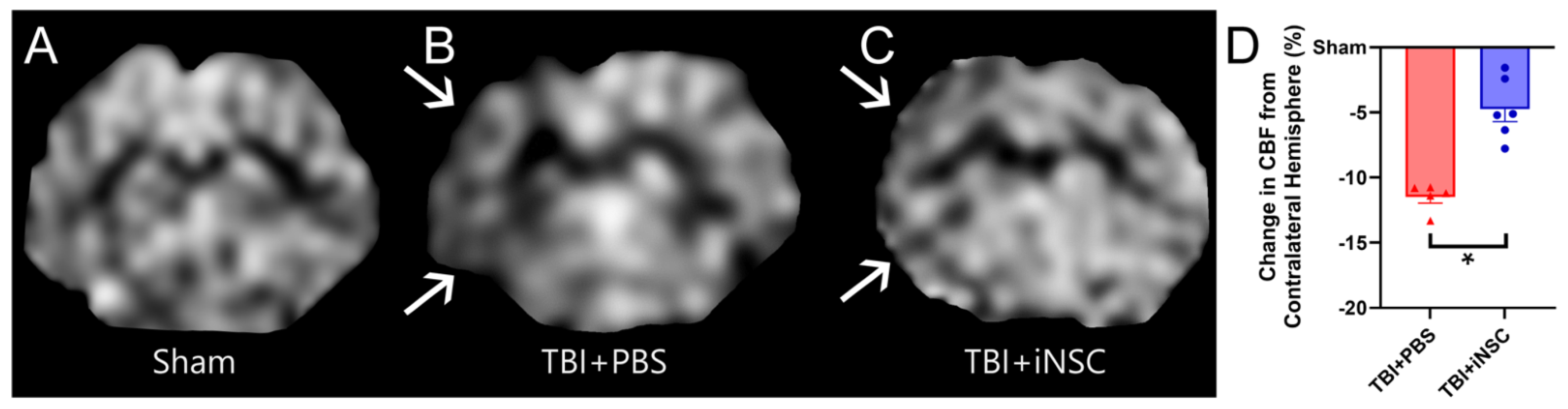
3.4. iNSC Transplantation Significantly Decreased Ipsilateral Atrophy, Lesion Volume, and Midline Shift While Preserving Cerebral Blood Flow
3.5. Treatment with iNSCs Improved Neurological Performance and Survivability Post-TBI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, L.; Zablotsky, B. Concussions and Brain Injuries in Children: United States, 2020; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Langlois, J.A.; Rutland-Brown, W.; Thomas, K.E. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2006. [Google Scholar]
- Center for Disease Control and Prevention. Report to Congress: The Management of Traumatic Brain Injury in Children, National Center for Injury Prevention and Control; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Nelson, C.A. The Neurobiological Bases of Early Intervention. In Handbook of Early Childhood Intervention, 2nd ed.; Shonkoff, J.P., Meisels, S.J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 204–229. [Google Scholar]
- Badner, A.; Reinhardt, E.K.; Nguyen, T.V.; Midani, N.; Marshall, A.T.; Lepe, C.A.; Echeverria, K.; Lepe, J.J.; Torrecampo, V.; Bertan, S.H.; et al. Freshly Thawed Cryobanked Human Neural Stem Cells Engraft within Endogenous Neurogenic Niches and Restore Cognitive Function after Chronic Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2731–2746. [Google Scholar] [CrossRef]
- Gao, J.; Grill, R.J.; Dunn, T.J.; Bedi, S.; Labastida, J.A.; Hetz, R.A.; Xue, H.; Thonhoff, J.R.; DeWitt, D.S.; Prough, D.S.; et al. Human Neural Stem Cell Transplantation-Mediated Alteration of Microglial/Macrophage Phenotypes after Traumatic Brain Injury. Cell Transplant. 2016, 25, 1863–1877. [Google Scholar] [CrossRef]
- Haus, D.L.; Lopez-Velazquez, L.; Gold, E.M.; Cunningham, K.M.; Perez, H.; Anderson, A.J.; Cummings, B.J. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp. Neurol. 2016, 281, 1–16. [Google Scholar] [CrossRef]
- Lee, J.Y.; Acosta, S.; Tuazon, J.P.; Xu, K.; Nguyen, H.; Lippert, T.; Liska, M.G.; Semechkin, A.; Garitaonandia, I.; Gonzalez, R.; et al. Human parthenogenetic neural stem cell grafts promote multiple regenerative processes in a traumatic brain injury model. Theranostics 2019, 9, 1029–1046. [Google Scholar] [CrossRef]
- Lin, G.Q.; He, X.F.; Liang, F.Y.; Guo, Y.; Sunnassee, G.; Chen, J.; Cao, X.M.; Chen, Y.Y.; Pan, G.J.; Pei, Z.; et al. Transplanted human neural precursor cells integrate into the host neural circuit and ameliorate neurological deficits in a mouse model of traumatic brain injury. Neurosci. Lett. 2018, 674, 11–17. [Google Scholar] [CrossRef]
- Narouiepour, A.; Ebrahimzadeh-Bideskan, A.; Rajabzadeh, G.; Gorji, A.; Negah, S.S. Neural stem cell therapy in conjunction with curcumin loaded in niosomal nanoparticles enhanced recovery from traumatic brain injury. Sci. Rep. 2022, 12, 3572. [Google Scholar] [CrossRef]
- Nieves, M.D.; Furmanski, O.; Doughty, M.L. Host sex and transplanted human induced pluripotent stem cell phenotype interact to influence sensorimotor recovery in a mouse model of cortical contusion injury. Brain Res. 2020, 1748, 147120. [Google Scholar] [CrossRef]
- Wennersten, A.; Meier, X.; Holmin, S.; Wahlberg, L.; Mathiesen, T. Proliferation, migration, and differentiation of human neural stem/progenitor cells after transplantation into a rat model of traumatic brain injury. J. Neurosurg. 2004, 100, 88–96. [Google Scholar] [CrossRef]
- Maas, A.I.; Menon, D.K.; Lingsma, H.F.; Pineda, J.A.; Sandel, M.E.; Manley, G.T. Re-orientation of clinical research in traumatic brain injury: Report of an international workshop on comparative effectiveness research. J. Neurotrauma 2012, 29, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.; Hicks, R.; The Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: Prospective considerations. J. Neurotrauma 2009, 26, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.K.; Michel, M.E.; Ansell, B.; Baethmann, A.; Biegon, A.; Bracken, M.B.; Bullock, M.R.; Choi, S.C.; Clifton, G.L.; Contant, C.F.; et al. Clinical trials in head injury. J. Neurotrauma 2002, 19, 503–557. [Google Scholar] [CrossRef]
- Baker, E.W.; Kinder, H.A.; Hutcheson, J.M.; Duberstein, K.J.J.; Platt, S.R.; Howerth, E.W.; West, F.D. Controlled Cortical Impact Severity Results in Graded Cellular, Tissue, and Functional Responses in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 61–73. [Google Scholar] [CrossRef]
- Fagan, M.M.; Welch, C.B.; Scheulin, K.M.; Sneed, S.E.; Jeon, J.H.; Golan, M.E.; Cheek, S.R.; Barany, D.A.; Oeltzschner, G.; Callaway, T.R.; et al. Fecal microbial transplantation limits neural injury severity and functional deficits in a pediatric piglet traumatic brain injury model. Front. Neurosci. 2023, 17, 1249539. [Google Scholar] [CrossRef]
- Kinder, H.A.; Baker, E.W.; Howerth, E.W.; Duberstein, K.J.; West, F.D. Controlled Cortical Impact Leads to Cognitive and Motor Function Deficits that Correspond to Cellular Pathology in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 2810–2826. [Google Scholar] [CrossRef]
- Kinder, H.A.; Baker, E.W.; Wang, S.; Fleischer, C.C.; Howerth, E.W.; Duberstein, K.J.; Mao, H.; Platt, S.R.; West, F.D. Traumatic Brain Injury Results in Dynamic Brain Structure Changes Leading to Acute and Chronic Motor Function Deficits in a Pediatric Piglet Model. J. Neurotrauma 2019, 36, 2930–2942. [Google Scholar] [CrossRef]
- Wang, E.; Gao, J.; Yang, Q.; Parsley, M.O.; Dunn, T.J.; Zhang, L.; DeWitt, D.S.; Denner, L.; Prough, D.S.; Wu, P. Molecular mechanisms underlying effects of neural stem cells against traumatic axonal injury. J. Neurotrauma 2012, 29, 295–312. [Google Scholar] [CrossRef]
- Baker, E.W.; Platt, S.R.; Lau, V.W.; Grace, H.E.; Holmes, S.P.; Wang, L.; Duberstein, K.J.; Howerth, E.W.; Kinder, H.A.; Stice, S.L.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Enhances Recovery in an Ischemic Stroke Pig Model. Sci. Rep. 2017, 7, 10075. [Google Scholar] [CrossRef]
- Gao, J.; Prough, D.S.; McAdoo, D.J.; Grady, J.J.; Parsley, M.O.; Ma, L.; Tarensenko, Y.I.; Wu, P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 2006, 201, 281–292. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, H.; Zhou, X.; Zhang, F.; Wang, C. Hyperbaric oxygen promotes neural stem cell proliferation by activating vascular endothelial growth factor/extracellular signal-regulated kinase signaling after traumatic brain injury. Neuroreport 2017, 28, 1232–1238. [Google Scholar] [CrossRef]
- Kinder, H.A.; Baker, E.W.; West, F.D. The pig as a preclinical traumatic brain injury model: Current models, functional outcome measures, and translational detection strategies. Neural Regen. Res. 2019, 14, 413–424. [Google Scholar] [CrossRef]
- Kaiser, E.E.; Waters, E.S.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Cheek, S.R.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; et al. Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model. Stem Cells Transl. Med. 2022, 11, 1061–1071. [Google Scholar] [CrossRef]
- Mosenthal, A.C.; Lavery, R.F.; Addis, M.; Kaul, S.; Ross, S.; Marburger, R.; Deitch, E.A.; Livingston, D.H. Isolated traumatic brain injury: Age is an independent predictor of mortality and early outcome. J. Trauma. 2002, 52, 907–911. [Google Scholar] [CrossRef]
- Burdi, A.R.; Huelke, D.F.; Snyder, R.G.; Lowrey, G.H. Infants and children in the adult world of automobile safety design: Pediatric and anatomical considerations for design of child restraints. J. Biomech. 1969, 2, 267–280. [Google Scholar] [CrossRef]
- Figaji, A.A. Anatomical and Physiological Differences between Children and Adults Relevant to Traumatic Brain Injury and the Implications for Clinical Assessment and Care. Front. Neurol. 2017, 8, 685. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Wallisch, J.S.; Bayir, H.; Clark, R.S.B. Pre-clinical models in pediatric traumatic brain injury-challenges and lessons learned. Child’s Nerv. Syst. 2017, 33, 1693–1701. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. S3), 511–533. [Google Scholar] [CrossRef]
- Chendrasekhar, A.; Kuczabski, B.; Cohen, D.; Grageda, M.; Genovese-Scullin, D.; Patwari, J.; Harris, L. Delayed Sequelae Related to Mild Traumatic Brain Injury in Children. Glob. Pediatr. Health 2020, 7, 2333794X20947988. [Google Scholar] [CrossRef]
- Serpa, R.O.; Ferguson, L.; Larson, C.; Bailard, J.; Cooke, S.; Greco, T.; Prins, M.L. Pathophysiology of Pediatric Traumatic Brain Injury. Front. Neurol. 2021, 12, 696510. [Google Scholar] [CrossRef]
- Guilhaume-Correa, F.; Cansler, S.M.; Shalosky, E.M.; Goodman, M.D.; Evanson, N.K. Greater neurodegeneration and behavioral deficits after single closed head traumatic brain injury in adolescent versus adult male mice. J. Neurosci. Res. 2020, 98, 557–570. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Michalski, S.; Zhao, S.; Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J. Neurotrauma 2016, 33, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, X.; Gao, X.; Chen, J. Delayed and progressive damages to juvenile mice after moderate traumatic brain injury. Sci. Rep. 2018, 8, 7339. [Google Scholar] [CrossRef]
- Claus, C.P.; Tsuru-Aoyagi, K.; Adwanikar, H.; Walker, B.; Manvelyan, H.; Whetstone, W.; Noble-Haeusslein, L.J. Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Dev. Neurosci. 2010, 32, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Hernandez, D.; Acosta, S.; Shinozuka, K.; Ishikawa, H.; Ehrhart, J.; Diamandis, T.; Gonzales-Portillo, C.; Borlongan, M.C.; Tan, J.; et al. Suppressed cytokine expression immediatey following traumatic brain injury in neonatal rats indicates an expeditious endogenous anti-inflammatory response. Brain Res. 2014, 1559, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.; Lee, S.M.; Cheng, C.L.; Becker, D.P.; Hovda, D.A. Fluid percussion brain injury in the developing and adult rat: A comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 1996, 95, 272–282. [Google Scholar] [CrossRef]
- Ayuso, M.; Buyssens, L.; Stroe, M.; Valenzuela, A.; Allegaert, K.; Smits, A.; Annaert, P.; Mulder, A.; Carpentier, S.; Van Ginneken, C.; et al. The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics 2020, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, N.F.; Maa, T.; Moore-Clingenpeel, M.; Rosenberg, N.; Yeates, K.O. Relationships between cerebral flow velocities and neurodevelopmental outcomes in children with moderate to severe traumatic brain injury. Child’s Nerv. Syst. 2018, 34, 663–672. [Google Scholar] [CrossRef]
- Imai, R.; Tamura, R.; Yo, M.; Sato, M.; Fukumura, M.; Takahara, K.; Kase, Y.; Okano, H.; Toda, M. Neuroprotective Effects of Genome-Edited Human iPS Cell-Derived Neural Stem/Progenitor Cells on Traumatic Brain Injury. Stem Cells 2023, 41, 603–616. [Google Scholar] [CrossRef]
- Palma-Tortosa, S.; Tornero, D.; Gronning Hansen, M.; Monni, E.; Hajy, M.; Kartsivadze, S.; Aktay, S.; Tsupykov, O.; Parmar, M.; Deisseroth, K.; et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 9094–9100. [Google Scholar] [CrossRef]
- Tatarishvili, J.; Oki, K.; Monni, E.; Koch, P.; Memanishvili, T.; Buga, A.M.; Verma, V.; Popa-Wagner, A.; Brustle, O.; Lindvall, O.; et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor. Neurol. Neurosci. 2014, 32, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Smitherman, E.; Hernandez, A.; Stavinoha, P.L.; Huang, R.; Kernie, S.G.; Diaz-Arrastia, R.; Miles, D.K. Predicting Outcome after Pediatric Traumatic Brain Injury by Early Magnetic Resonance Imaging Lesion Location and Volume. J. Neurotrauma 2016, 33, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Andreu, M.; Matti, N.; Bramlett, H.M.; Shi, Y.; Gajavelli, S.; Dietrich, W.D. Dose-dependent modulation of microglia activation in rats after penetrating traumatic brain injury (pTBI) by transplanted human neural stem cells. PLoS ONE 2023, 18, e0285633. [Google Scholar] [CrossRef]
- Hu, Z.; Gajavelli, S.; Spurlock, M.S.; Mahavadi, A.; Quesada, L.S.; Gajavelli, G.R.; Andreoni, C.B.; Di, L.; Janecki, J.; Lee, S.W.; et al. Human neural stem cell transplant location-dependent neuroprotection and motor deficit amelioration in rats with penetrating traumatic brain injury. J. Trauma. Acute Care Surg. 2020, 88, 477–485. [Google Scholar] [CrossRef]
- Barlow, K.M.; Iyer, K.; Yan, T.; Scurfield, A.; Carlson, H.; Wang, Y. Cerebral Blood Flow Predicts Recovery in Children with Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2275–2283. [Google Scholar] [CrossRef]
- Gsell, W.; De Sadeleer, C.; Marchalant, Y.; MacKenzie, E.T.; Schumann, P.; Dauphin, F. The use of cerebral blood flow as an index of neuronal activity in functional neuroimaging: Experimental and pathophysiological considerations. J. Chem. Neuroanat. 2000, 20, 215–224. [Google Scholar] [CrossRef]
- Ware, J.B.; Dolui, S.; Duda, J.; Gaggi, N.; Choi, R.; Detre, J.; Whyte, J.; Diaz-Arrastia, R.; Kim, J.J. Relationship of Cerebral Blood Flow to Cognitive Function and Recovery in Early Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2180–2187. [Google Scholar] [CrossRef]
- Barlow, K.M.; Marcil, L.D.; Dewey, D.; Carlson, H.L.; MacMaster, F.P.; Brooks, B.L.; Lebel, R.M. Cerebral Perfusion Changes in Post-Concussion Syndrome: A Prospective Controlled Cohort Study. J. Neurotrauma 2017, 34, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Biagas, K.V.; Grundl, P.D.; Kochanek, P.M.; Schiding, J.K.; Nemoto, E.M. Posttraumatic hyperemia in immature, mature, and aged rats: Autoradiographic determination of cerebral blood flow. J. Neurotrauma 1996, 13, 189–200. [Google Scholar] [CrossRef]
- Grundl, P.D.; Biagas, K.V.; Kochanek, P.M.; Schiding, J.K.; Barmada, M.A.; Nemoto, E.M. Early cerebrovascular response to head injury in immature and mature rats. J. Neurotrauma 1994, 11, 135–148. [Google Scholar] [CrossRef]
- Rostami, E.; Nilsson, P.; Enblad, P. Cerebral Blood Flow Measurement in Healthy Children and Children Suffering Severe Traumatic Brain Injury-What Do We Know? Front. Neurol. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs 2010, 11, 298–308. [Google Scholar] [PubMed]
- Skardelly, M.; Gaber, K.; Burdack, S.; Scheidt, F.; Hilbig, H.; Boltze, J.; Forschler, A.; Schwarz, S.; Schwarz, J.; Meixensberger, J.; et al. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J. Neurotrauma 2011, 28, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Rathnasamy, G.; Ling, E.A. Biology of Microglia in the Developing Brain. J. Neuropathol. Exp. Neurol. 2017, 76, 736–753. [Google Scholar] [CrossRef] [PubMed]
- Lengel, D.; Sevilla, C.; Romm, Z.L.; Huh, J.W.; Raghupathi, R. Stem Cell Therapy for Pediatric Traumatic Brain Injury. Front. Neurol. 2020, 11, 601286. [Google Scholar] [CrossRef]
- Billiards, S.S.; Haynes, R.L.; Folkerth, R.D.; Trachtenberg, F.L.; Liu, L.G.; Volpe, J.J.; Kinney, H.C. Development of microglia in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 2006, 497, 199–208. [Google Scholar] [CrossRef]
- Fan, P.; Yamauchi, T.; Noble, L.J.; Ferriero, D.M. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J. Neurotrauma 2003, 20, 437–445. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef]
- Chen, W.; Sheng, J.; Guo, J.; Peng, G.; Hong, J.; Li, B.; Chen, X.; Li, K.; Wang, S. Cytokine cascades induced by mechanical trauma injury alter voltage-gated sodium channel activity in intact cortical neurons. J. Neuroinflamm. 2017, 14, 73. [Google Scholar] [CrossRef]
- Wofford, K.L.; Harris, J.P.; Browne, K.D.; Brown, D.P.; Grovola, M.R.; Mietus, C.J.; Wolf, J.A.; Duda, J.E.; Putt, M.E.; Spiller, K.L.; et al. Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine. Exp. Neurol. 2017, 290, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001, 63, 313–319. [Google Scholar] [CrossRef]
- Villasana, L.E.; Kim, K.N.; Westbrook, G.L.; Schnell, E. Functional Integration of Adult-Born Hippocampal Neurons after Traumatic Brain Injury(1,2,3). eNeuro 2015, 2, 0056-15. [Google Scholar] [CrossRef] [PubMed]
- Niimi, Y.; Levison, S.W. Pediatric brain repair from endogenous neural stem cells of the subventricular zone. Pediatr. Res. 2018, 83, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Costine, B.A.; Missios, S.; Taylor, S.R.; McGuone, D.; Smith, C.M.; Dodge, C.P.; Harris, B.T.; Duhaime, A.C. The subventricular zone in the immature piglet brain: Anatomy and exodus of neuroblasts into white matter after traumatic brain injury. Dev. Neurosci. 2015, 37, 115–130. [Google Scholar] [CrossRef]
- Lu, K.T.; Sun, C.L.; Wo, P.Y.; Yen, H.H.; Tang, T.H.; Ng, M.C.; Huang, M.L.; Yang, Y.L. Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J. Neurotrauma 2011, 28, 441–450. [Google Scholar] [CrossRef]
- Xiong, L.L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.H.; Gao, G.D.; Zhou, X.F.; Wang, T.H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef]
- Reen, L.; Cederberg, D.; Radman, A.; Marklund, N.; Visse, E.; Siesjo, P. Low Morbidity and Mortality in Children with Severe Traumatic Brain Injury Treated According to the Lund Concept: A Population-Based Study. J. Neurotrauma 2023, 40, 720–729. [Google Scholar] [CrossRef]
- Chen, T.; Xia, Y.; Zhang, L.; Xu, T.; Yi, Y.; Chen, J.; Liu, Z.; Yang, L.; Chen, S.; Zhou, X.; et al. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater. Today Bio 2023, 19, 100606. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, Y.; Tang, L.J.; Kong, L.; Zhang, C.H.; Chu, H.Y.; Yin, L.W.; Ma, H.Y. Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites. Neural Regen. Res. 2017, 12, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ghazale, H.; Ramadan, N.; Mantash, S.; Zibara, K.; El-Sitt, S.; Darwish, H.; Chamaa, F.; Boustany, R.M.; Mondello, S.; Abou-Kheir, W.; et al. Docosahexaenoic acid (DHA) enhances the therapeutic potential of neonatal neural stem cell transplantation post-Traumatic brain injury. Behav. Brain Res. 2018, 340, 1–13. [Google Scholar] [CrossRef] [PubMed]



| Target | Species | Dilution | Manufacturer |
|---|---|---|---|
| NEUN | Rabbit | 1:750 | Novus NBP1-77686 (Centennial, CO, USA) |
| OLIG2 | Rabbit | 1:200 | GeneTex GTX132732 (Irvine, CA, USA) |
| GFAP | Rabbit | 1:500 | Novus NB300-141 |
| HNA | Mouse | 1:1000 | Abcam ab191181 (Waltham, MA, USA) |
| MS-488 | - | 1:1000 | Invitrogen A11029 (Waltham, MA, USA) |
| RB-594 | - | 1:200–750 | Invitrogen A11037 |
| DAPI prolong gold | - | - | Invitrogen P36935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schantz, S.L.; Sneed, S.E.; Fagan, M.M.; Golan, M.E.; Cheek, S.R.; Kinder, H.A.; Duberstein, K.J.; Kaiser, E.E.; West, F.D. Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Limits Tissue Damage and Promotes Tissue Regeneration and Functional Recovery in a Pediatric Piglet Traumatic-Brain-Injury Model. Biomedicines 2024, 12, 1663. https://doi.org/10.3390/biomedicines12081663
Schantz SL, Sneed SE, Fagan MM, Golan ME, Cheek SR, Kinder HA, Duberstein KJ, Kaiser EE, West FD. Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Limits Tissue Damage and Promotes Tissue Regeneration and Functional Recovery in a Pediatric Piglet Traumatic-Brain-Injury Model. Biomedicines. 2024; 12(8):1663. https://doi.org/10.3390/biomedicines12081663
Chicago/Turabian StyleSchantz, Sarah L., Sydney E. Sneed, Madison M. Fagan, Morgane E. Golan, Savannah R. Cheek, Holly A. Kinder, Kylee J. Duberstein, Erin E. Kaiser, and Franklin D. West. 2024. "Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Limits Tissue Damage and Promotes Tissue Regeneration and Functional Recovery in a Pediatric Piglet Traumatic-Brain-Injury Model" Biomedicines 12, no. 8: 1663. https://doi.org/10.3390/biomedicines12081663







