Inflammaging: The Next Challenge—Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences
Abstract
:1. Introduction
2. Immunosenescence and Inflammaging
3. Gut Microbiota and Inflammaging
4. Environmental Factors and Inflammaging
4.1. Nutrition and Metabolism
4.2. Pollutants
5. Therapy Goals and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, J.; Han, Z.; Ding, L.; Wang, P.; He, X.; Lin, L. The Molecular Mechanism of Aging and the Role in Neurodegenerative Diseases. Heliyon 2024, 10, e24751. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M.; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2023, 14, a041197. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and Inflammaging in the Aging Process: Age-Related Diseases or Longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Frigé, C.; Pellegrini, V.; Scisciola, L.; Santoro, A.; Monti, D.; Rippo, M.R.; Ivanchenko, M.; Olivieri, F.; Franceschi, C. Organ-Specific Biological Clocks: Ageotyping for Personalized Anti-Aging Medicine. Ageing Res. Rev. 2024, 96, 102253. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.; Duggal, N.A. Ageing of the Gut Microbiome: Potential Influences on Immune Senescence and Inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular Mechanisms and Diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Lima-Silva, M.L.; Torres, K.C.L.; Mambrini, J.V.D.M.; Brot, N.C.; Santos, S.O.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Lima-Costa, M.F.; Peixoto, S.V. A Nationwide Study on Immunosenescence Biomarkers Profile in Older Adults: ELSI-Brazil. Exp. Gerontol. 2024, 191, 112433. [Google Scholar] [CrossRef] [PubMed]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and Age-Related Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Olivieri, F.; Marchegiani, F.; Matacchione, G.; Giuliani, A.; Ramini, D.; Fazioli, F.; Sabbatinelli, J.; Bonafè, M. Sex/Gender-Related Differences in Inflammaging. Mech. Ageing Dev. 2023, 211, 111792. [Google Scholar] [CrossRef]
- Rio, P.; Caldarelli, M.; Chiantore, M.; Ocarino, F.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Immune Cells, Gut Microbiota, and Vaccines: A Gender Perspective. Cells 2024, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R. Sex, the Aging Immune System, and Chronic Disease. Cell. Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Layug, P.J.; Vats, H.; Kannan, K.; Arsenio, J. Sex Differences in CD8 + T Cell Responses during Adaptive Immunity. WIREs Mech. Dis. 2024, e1645. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Poisson, J.; El-Sissy, C.; Serret-Larmande, A.; Smith, N.; Lebraud, M.; Augy, J.-L.; Conti, C.; Gonnin, C.; Planquette, B.; Arlet, J.-B.; et al. Increased Levels of GM-CSF and CXCL10 and Low CD8+ Memory Stem T Cell Count Are Markers of Immunosenescence and Severe COVID-19 in Older People. Immun. Ageing 2024, 21, 28. [Google Scholar] [CrossRef]
- Kumar, S.J.; Shukla, S.; Kumar, S.; Mishra, P. Immunosenescence and Inflamm-Aging: Clinical Interventions and the Potential for Reversal of Aging. Cureus 2024, 16, e53297. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, V.; Jang, I.H.; Camell, C.D. Inflammaging and Fatty Acid Oxidation in Monocytes and Macrophages. Immunometabolism 2024, 6, e00038. [Google Scholar] [CrossRef]
- Martínez De Toda, I.; González-Sánchez, M.; Díaz-Del Cerro, E.; Valera, G.; Carracedo, J.; Guerra-Pérez, N. Sex Differences in Markers of Oxidation and Inflammation. Implications for Ageing. Mech. Ageing Dev. 2023, 211, 111797. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Tissue Fibroblasts Are Versatile Immune Regulators: An Evaluation of Their Impact on the Aging Process. Ageing Res. Rev. 2024, 97, 102296. [Google Scholar] [CrossRef]
- Vijg, J. From DNA Damage to Mutations: All Roads Lead to Aging. Ageing Res. Rev. 2021, 68, 101316. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- Schellnegger, M.; Hofmann, E.; Carnieletto, M.; Kamolz, L.-P. Unlocking Longevity: The Role of Telomeres and Its Targeting Interventions. Front. Aging 2024, 5, 1339317. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Sex Differences in Telomere Length, Lifespan, and Embryonic Dyskerin Levels. Aging Cell 2022, 21, e13614. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiong, Y.; Li, R.; Zhang, J.; Zhang, S. Shorter Telomere Length Increases the Risk of Lymphocyte Immunodeficiency: A Mendelian Randomization Study. Immun. Inflamm. Dis. 2024, 12, e1251. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A Key Player in Cancer Development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Snijckers, R.P.M.; Foks, A.C. Adaptive Immunity and Atherosclerosis: Aging at Its Crossroads. Front. Immunol. 2024, 15, 1350471. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef]
- Verma, K.; Ogonek, J.; Varanasi, P.R.; Luther, S.; Bünting, I.; Thomay, K.; Behrens, Y.L.; Mischak-Weissinger, E.; Hambach, L. Human CD8+ CD57-TEMRA Cells: Too Young to Be Called “Old”. PLoS ONE 2017, 12, e0177405. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Xue, L.; Guo, H. Senescent T Cells: A Potential Biomarker and Target for Cancer Therapy. eBioMedicine 2021, 68, 103409. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Coppola, G.; Rio, P.; Caldarelli, M.; Borriello, R.; Gambassi, G.; Gasbarrini, A.; Cianci, R. Factors Influencing Microbiota in Modulating Vaccine Immune Response: A Long Way to Go. Vaccines 2023, 11, 1609. [Google Scholar] [CrossRef]
- Allen, J.C.; Toapanta, F.R.; Chen, W.; Tennant, S.M. Understanding Immunosenescence and Its Impact on Vaccination of Older Adults. Vaccine 2020, 38, 8264–8272. [Google Scholar] [CrossRef]
- Caldarelli, M.; Rio, P.; Marrone, A.; Ocarino, F.; Chiantore, M.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut–Brain Axis: Focus on Sex Differences in Neuroinflammation. Int. J. Mol. Sci. 2024, 25, 5377. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological Mechanisms of Inflammatory Diseases Caused by Gut Microbiota Dysbiosis: A Review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Zhang, C.; Feng, S.; Zhan, Z.; Bao, Y.; Zhang, S.; Chao, G. Improving Intestinal Inflammaging to Delay Aging? A New Perspective. Mech. Ageing Dev. 2023, 214, 111841. [Google Scholar] [CrossRef]
- Khaledi, M.; Poureslamfar, B.; Alsaab, H.O.; Tafaghodi, S.; Hjazi, A.; Singh, R.; Alawadi, A.H.; Alsaalamy, A.; Qasim, Q.A.; Sameni, F. The Role of Gut Microbiota in Human Metabolism and Inflammatory Diseases: A Focus on Elderly Individuals. Ann. Microbiol. 2024, 74, 1. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.; Zeng, G.; Pang, B.; Wang, Q.; Wei, J. Gut Microbiome-Mediated Mechanisms in Aging-Related Diseases: Are Probiotics Ready for Prime Time? Front. Pharmacol. 2023, 14, 1178596. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Ye, C.; Li, Z.; Ye, C.; Yuan, L.; Wu, K.; Zhu, C. Association between Gut Microbiota and Biological Aging: A Two-Sample Mendelian Randomization Study. Microorganisms 2024, 12, 370. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Telhig, S.; Ben Said, L.; Zirah, S.; Fliss, I.; Rebuffat, S. Bacteriocins to Thwart Bacterial Resistance in Gram Negative Bacteria. Front. Microbiol. 2020, 11, 586433. [Google Scholar] [CrossRef]
- Dong, L.; Yang, H.; Wang, Z.; Jiang, N.; Zhang, A. Antimicrobial Peptide CC34 Attenuates Intestinal Inflammation via Downregulation of the NF-κB Signaling Pathway. 3 Biotech 2021, 11, 397. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging Increases the Severity of Colitis and the Related Changes to the Gut Barrier and Gut Microbiota in Humans and Mice. J. Gerontol. Ser. A 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Man, A.L.; Bertelli, E.; Rentini, S.; Regoli, M.; Briars, G.; Marini, M.; Watson, A.J.M.; Nicoletti, C. Age-Associated Modifications of Intestinal Permeability and Innate Immunity in Human Small Intestine. Clin. Sci. 2015, 129, 515–527. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut Microbiota Alterations Affect Glioma Growth and Innate Immune Cells Involved in Tumor Immunosurveillance in Mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef]
- Bouskra, D.; Brézillon, C.; Bérard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid Tissue Genesis Induced by Commensals through NOD1 Regulates Intestinal Homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef]
- Watnick, P.I.; Jugder, B.-E. Microbial Control of Intestinal Homeostasis via Enteroendocrine Cell Innate Immune Signaling. Trends Microbiol. 2020, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged With Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- Kim, K.S. Regulation of T Cell Repertoires by Commensal Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 1004339. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, C.; Wang, Q.; Feng, S.; Fang, Y.; Zhang, S. The Impact of Aging on Intestinal Mucosal Immune Function and Clinical Applications. Front. Immunol. 2022, 13, 1029948. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential Implications of Polyphenols on Aging Considering Oxidative Stress, Inflammation, Autophagy, and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-Y.; Lin, C.-H.; Fang, J.-Y. Natural Compounds and Aging: Between Autophagy and Inflammasome. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Jeffery, V.; Goldson, A.J.; Dainty, J.R.; Chieppa, M.; Sobolewski, A. IL-6 Signaling Regulates Small Intestinal Crypt Homeostasis. J. Immunol. 2017, 199, 304–311. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Sakamuri, A.; Bardhan, P.; Tummala, R.; Mauvais-Jarvis, F.; Yang, T.; Joe, B.; Ogola, B.O. Sex Hormones, Sex Chromosomes, and Microbiota: Identification of Akkermansia Muciniphila as an Estrogen-Responsive Microbiota. Microbiota Host 2023, 1, e230010. [Google Scholar] [CrossRef]
- Bucurica, S.; Lupanciuc, M.; Ionita-Radu, F.; Stefan, I.; Munteanu, A.E.; Anghel, D.; Jinga, M.; Gaman, E.L. Estrobolome and Hepatocellular Adenomas—Connecting the Dots of the Gut Microbial β-Glucuronidase Pathway as a Metabolic Link. Int. J. Mol. Sci. 2023, 24, 16034. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, C.; Yao, W.; Zhu, D.; Lu, H.; Li, L.; Ma, B.; Sun, B.; Xue, D.; Zhang, W. Intestinal Flora Imbalance Affects Bile Acid Metabolism and Is Associated with Gallstone Formation. BMC Gastroenterol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Shen, X.; Miao, J.; Wan, Q.; Wang, S.; Li, M.; Pu, F.; Wang, G.; Qian, W.; Yu, Q.; Marotta, F.; et al. Possible Correlation between Gut Microbiota and Immunity among Healthy Middle-Aged and Elderly People in Southwest China. Gut Pathog. 2018, 10, 4. [Google Scholar] [CrossRef]
- Kawamoto, S.; Hara, E. Crosstalk between Gut Microbiota and Cellular Senescence: A Vicious Cycle Leading to Aging Gut. Trends Cell Biol. 2024. [Google Scholar] [CrossRef]
- Kawamoto, S.; Uemura, K.; Hori, N.; Takayasu, L.; Konishi, Y.; Katoh, K.; Matsumoto, T.; Suzuki, M.; Sakai, Y.; Matsudaira, T.; et al. Bacterial Induction of B Cell Senescence Promotes Age-Related Changes in the Gut Microbiota. Nat. Cell Biol. 2023, 25, 865–876. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating Host and Microbiome Contributions to Drug Pharmacokinetics and Toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton Pump Inhibitors Alter the Composition of the Gut Microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Evans, A.K.; Saw, N.L.; Woods, C.E.; Vidano, L.M.; Blumenfeld, S.E.; Lam, R.K.; Chu, E.K.; Reading, C.; Shamloo, M. Impact of High-Fat Diet on Cognitive Behavior and Central and Systemic Inflammation with Aging and Sex Differences in Mice. Brain. Behav. Immun. 2024, 118, 334–354. [Google Scholar] [CrossRef]
- Dunbar, C.L.; Aukema, H.M.; Calder, P.C.; Gibson, D.L.; Henrickson, S.E.; Khan, S.; Mailhot, G.; Panahi, S.; Tabung, F.K.; Tom, M.; et al. Nutrition and Immunity: Perspectives on Key Issues and next Steps. Appl. Physiol. Nutr. Metab. 2023, 48, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak-Gramacka, E.; Hertmanowska, N.; Tylutka, A.; Morawin, B.; Wacka, E.; Gutowicz, M.; Zembron-Lacny, A. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients 2021, 13, 3696. [Google Scholar] [CrossRef]
- Eder, P.; Niezgódka, A.; Krela-Kaźmierczak, I.; Stawczyk-Eder, K.; Banasik, E.; Dobrowolska, A. Dietary Support in Elderly Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 1421. [Google Scholar] [CrossRef]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Gialluisi, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Increased Adherence to a Mediterranean Diet Is Associated With Reduced Low-Grade Inflammation after a 12.7-Year Period: Results From the Moli-Sani Study. J. Acad. Nutr. Diet. 2023, 123, 783–795.e7. [Google Scholar] [CrossRef]
- Waqas, K.; Chen, J.; Lu, T.; van der Eerden, B.C.J.; Rivadeneira, F.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Dietary Advanced Glycation End-Products (dAGEs) Intake and Its Relation to Sarcopenia and Frailty—The Rotterdam Study. Bone 2022, 165, 116564. [Google Scholar] [CrossRef]
- Cao, Y.; Li, P.; Zhang, Y.; Qiu, M.; Li, J.; Ma, S.; Yan, Y.; Li, Y.; Han, Y. Dietary Inflammatory Index and All-Cause Mortality in Older Adults with Hypertension: Results from NHANES. J. Clin. Med. 2023, 12, 506. [Google Scholar] [CrossRef]
- De Nucci, S.; Bonfiglio, C.; Donvito, R.; Di Chito, M.; Cerabino, N.; Rinaldi, R.; Sila, A.; Shahini, E.; Giannuzzi, V.; Pesole, P.L.; et al. Effects of an Eight Week Very Low-Calorie Ketogenic Diet (VLCKD) on White Blood Cell and Platelet Counts in Relation to Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Subjects with Overweight and Obesity. Nutrients 2023, 15, 4468. [Google Scholar] [CrossRef]
- Peng, M.; Yuan, S.; Lu, D.; Ling, Y.; Huang, X.; Lyu, J.; Xu, A. Dietary Inflammatory Index, Genetic Susceptibility and Risk of Incident Dementia: A Prospective Cohort Study from UK Biobank. J. Neurol. 2024, 271, 1286–1296. [Google Scholar] [CrossRef]
- Martínez, C.F.; Esposito, S.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Hébert, J.R.; Cerletti, C.; Donati, M.B.; et al. Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort. Nutrients 2023, 15, 1503. [Google Scholar] [CrossRef]
- Vettoretti, S.; Molinari, P.; Armelloni, S.; Castellano, G.; Caldiroli, L. Spontaneous Low-Protein Intake in Older CKD Patients: One Diet May Not Fit All. Front. Nutr. 2024, 11, 1328939. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN Guideline on Clinical Nutrition and Hydration in Geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed]
- Majidi, A.; Hughes, M.C.B.; Webb, I.K.; Miura, K.; van der Pols, J.C. Inflammatory Potential of Diet and Mortality in Australian Adults. Public Health Nutr. 2024, 27, e129. [Google Scholar] [CrossRef]
- Memili, A.; Lulla, A.; Liu, H.; Shikany, J.M.; Jacobs, D.R.; Langsetmo, L.; North, K.E.; Jones, C.; Launer, L.J.; Meyer, K.A. Physical Activity and Diet Associations with the Gut Microbiota in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Nutr. 2023, 153, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, D.; Giuffrè, A.M. Mediterranean Diet and Health in the Elderly. AIMS Public Health 2023, 10, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; Barbagallo, M. Mediterranean Diet and Mitochondria: New Findings. Exp. Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef]
- Georgoulis, M.; Damigou, E.; Chrysohoou, C.; Barkas, F.; Anastasiou, G.; Kravvariti, E.; Tsioufis, C.; Liberopoulos, E.; Sfikakis, P.P.; Pitsavos, C.; et al. Mediterranean Diet Trajectories and 20-Year Incidence of Cardiovascular Disease: The ATTICA Cohort Study (2002–2022). Nutr. Metab. Cardiovasc. Dis. NMCD 2024, 34, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohn's Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Jin, L.-W.; Di Lucente, J.; Ruiz Mendiola, U.; Suthprasertporn, N.; Tomilov, A.; Cortopassi, G.; Kim, K.; Ramsey, J.J.; Maezawa, I. The Ketone Body β-Hydroxybutyrate Shifts Microglial Metabolism and Suppresses Amyloid-β Oligomer-Induced Inflammation in Human Microglia. FASEB J. 2023, 37, e23261. [Google Scholar] [CrossRef]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The Effect of a Ketogenic Diet on Inflammation-Related Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2024, nuad175. [Google Scholar] [CrossRef]
- Almodallal, Y.; Cook, K.; Lammert, L.M.; Lee, M.; Le-Rademacher, J.G.; Jatoi, A. Can Older Patients Adopt and Maintain a Ketogenic Diet? An Observational Study in Support of Clinical Trials in Older Patients. Medicine 2021, 100, e28033. [Google Scholar] [CrossRef]
- Butler, M.J.; Mackey-Alfonso, S.E.; Massa, N.; Baskin, K.K.; Barrientos, R.M. Dietary Fatty Acids Differentially Impact Phagocytosis, Inflammatory Gene Expression, and Mitochondrial Respiration in Microglial and Neuronal Cell Models. Front. Cell. Neurosci. 2023, 17, 1227241. [Google Scholar] [CrossRef]
- Aziz, T.; Khan, A.A.; Tzora, A.; Voidarou, C.; Skoufos, I. Dietary Implications of the Bidirectional Relationship between the Gut Microflora and Inflammatory Diseases with Special Emphasis on Irritable Bowel Disease: Current and Future Perspective. Nutrients 2023, 15, 2956. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Savastano, S.; Colao, A.; Muscogiuri, G. Adherence to Mediterranean Diet: Any Association with NAFLD? Antioxidants 2023, 12, 1318. [Google Scholar] [CrossRef]
- Conway, J.; Acharjee, A.; Duggal, N.A. Integrated Analysis Revealing Novel Associations between Dietary Patterns and the Immune System in Older Adults. Integr. Biol. 2024, 16, zyae010. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Morawin, B.; Tylutka, A.; Bielewicz, F.; Zembron-Lacny, A. Diagnostics of Inflammaging in Relation to Sarcopenia. Front. Public Health 2023, 11, 1162385. [Google Scholar] [CrossRef]
- Zeidan, R.S.; McElroy, T.; Rathor, L.; Martenson, M.S.; Lin, Y.; Mankowski, R.T. Sex Differences in Frailty among Older Adults. Exp. Gerontol. 2023, 184, 112333. [Google Scholar] [CrossRef]
- Wu, B.N.; O’Sullivan, A.J. Sex Differences in Energy Metabolism Need to Be Considered with Lifestyle Modifications in Humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; Hyatt, H.W.; Alvarez Jerez, P.; Mikkelsen, M.; Hass, C.J.; Edden, R.A.E.; Seidler, R.D.; Porges, E.C. In Vivo Brain Glutathione Is Higher in Older Age and Correlates with Mobility. Cereb. Cortex 2021, 31, 4576–4594. [Google Scholar] [CrossRef]
- Rio, P.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Pollutants, Microbiota and Immune System: Frenemies within the Gut. Front. Public Health 2024, 12, 1285186. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A.; et al. Brain Tyrosinase Overexpression Implicates Age-Dependent Neuromelanin Production in Parkinson’s Disease Pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Candore, G. Sex and Gender Affect Immune Aging. Front. Aging 2023, 4, 1272118. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Wang, J.; Zhao, Y.; Li, K.; Jing, Y.; Zhang, X.; Liu, Q.; Geng, X.; Li, G.; et al. Long-Term Persistent Organic Pollutants Exposure Induced Telomere Dysfunction and Senescence-Associated Secretary Phenotype. J. Gerontol. Ser. A 2018, 73, 1027–1035. [Google Scholar] [CrossRef]
- Bind, M.-A.; Lepeule, J.; Zanobetti, A.; Gasparrini, A.; Baccarelli, A.A.; Coull, B.A.; Tarantini, L.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J. Air Pollution and Gene-Specific Methylation in the Normative Aging Study: Association, Effect Modification, and Mediation Analysis. Epigenetics 2014, 9, 448–458. [Google Scholar] [CrossRef]
- Pan, R.; Wang, J.; Chang, W.; Song, J.; Yi, W.; Zhao, F.; Zhang, Y.; Fang, J.; Du, P.; Cheng, J.; et al. Association of PM 2.5 Components with Acceleration of Aging: Moderating Role of Sex Hormones. Environ. Sci. Technol. 2023, 57, 3772–3782. [Google Scholar] [CrossRef]
- Chaney, C.; Wiley, K.S. The Variable Associations between PFASs and Biological Aging by Sex and Reproductive Stage in NHANES 1999–2018. Environ. Res. 2023, 227, 115714. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Sauce, D.; Larsen, M.; Fastenackels, S.; Duperrier, A.; Keller, M.; Grubeck-Loebenstein, B.; Ferrand, C.; Debré, P.; Sidi, D.; Appay, V. Evidence of Premature Immune Aging in Patients Thymectomized during Early Childhood. J. Clin. Investig. 2009, 119, 3070–3078. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A Systems-Level Overview of Immune Cell Biology and Strategies for Improving Vaccine Responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef]
- Scurr, M.; Pembroke, T.; Bloom, A.; Roberts, D.; Thomson, A.; Smart, K.; Bridgeman, H.; Adams, R.; Brewster, A.; Jones, R.; et al. Low-Dose Cyclophosphamide Induces Antitumor T-Cell Responses, Which Associate with Survival in Metastatic Colorectal Cancer. Clin. Cancer Res. 2017, 23, 6771–6780. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.-J. Regulation of the Intestinal Tight Junction by Natural Polyphenols: A Mechanistic Perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef]
- Gargari, G.; Taverniti, V.; Del Bo’, C.; Bernardi, S.; Hidalgo-Liberona, N.; Meroño, T.; Andres-Lacueva, C.; Kroon, P.A.; Cherubini, A.; Riso, P.; et al. Higher Bacterial DNAemia Can Affect the Impact of a Polyphenol-Rich Dietary Pattern on Biomarkers of Intestinal Permeability and Cardiovascular Risk in Older Subjects. Eur. J. Nutr. 2022, 61, 1209–1220. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Elderman, M.E.; El Aidy, S.; Burgerhof, J.G.M.; Visser, H.; Vaughan, E.E.; Faas, M.M.; de Vos, P. Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long-Chain Inulin Intake. Mol. Nutr. Food Res. 2021, 65, 2000390. [Google Scholar] [CrossRef]
- Ren, M.; Li, M.-Y.; Lu, L.-Q.; Liu, Y.-S.; An, F.-K.; Huang, K.; Fu, Z. Arenga Pinnata Resistant Starch Modulate Gut Microbiota and Ameliorate Intestinal Inflammation in Aged Mice. Nutrients 2022, 14, 3931. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Borody, T.J.; Zhang, F. Encyclopedia of Fecal Microbiota Transplantation: A Review of Effectiveness in the Treatment of 85 Diseases. Chin. Med. J. 2022, 135, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Lanza, I.R. Age-Associated Inflammation and Implications for Skeletal Muscle Responses to Exercise. Exp. Gerontol. 2023, 177, 112177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, X.; He, F.; Huang, X. Inflammatory Biomarkers of Frailty: A Review. Exp. Gerontol. 2023, 179, 112253. [Google Scholar] [CrossRef]
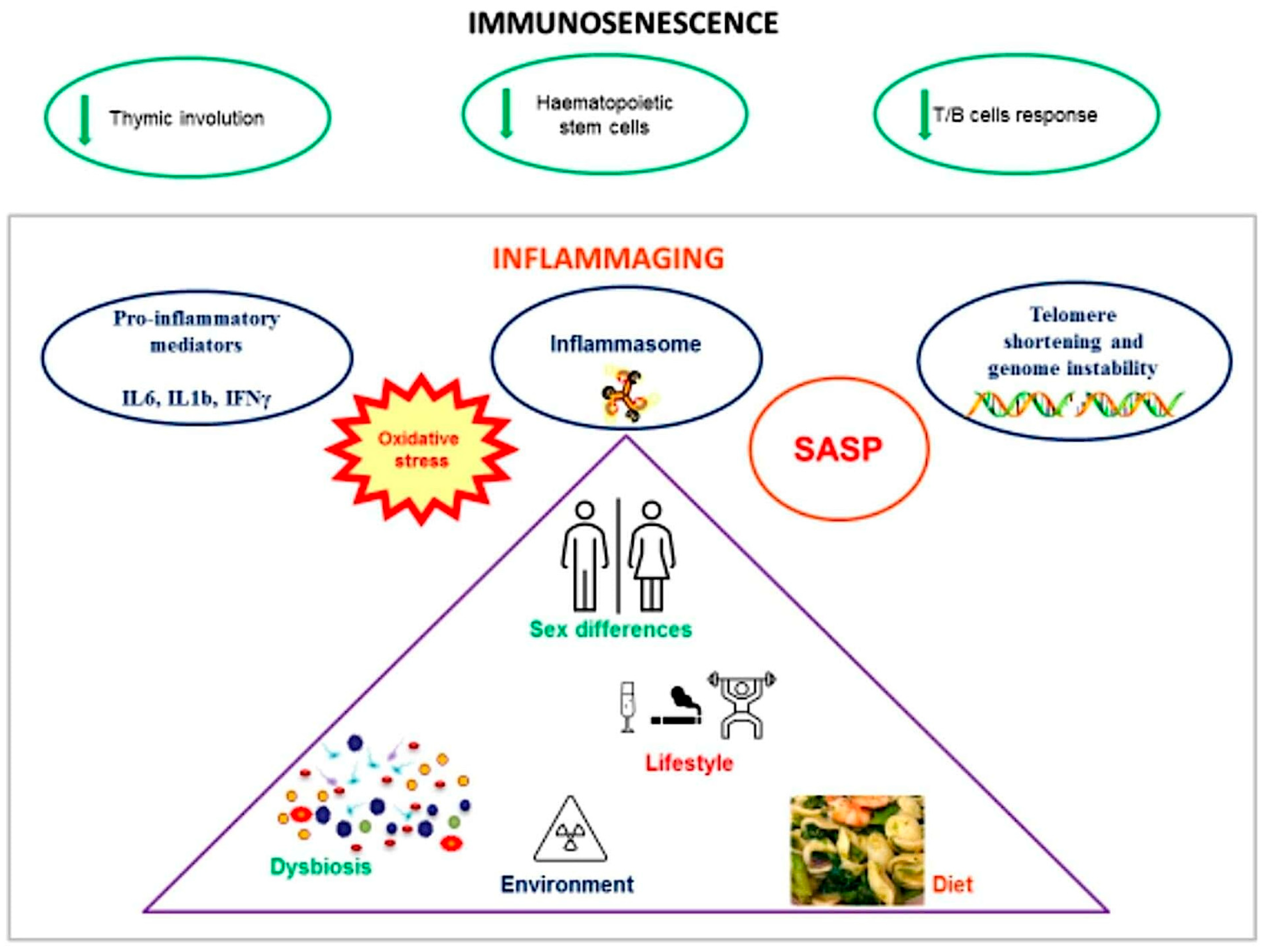
| Mechanisms of Immunosenescence and Inflammaging | References |
|---|---|
| Involution of the thymus | [7] |
| Dysfunction of hematopoietic stem cells | [7] |
| Development of SASP by senescent immune cells | [7] |
| Production of proinflammatory mediators | [8,9] |
| Decline in T and B cell responses | [15] |
| Metabolic changes (increased glycolysis, mitochondrial dysfunction, ROS) | [7,18] |
| Telomere shortening and genome instability | [21,22] |
| Immune phenotypic changes in senescent immune cells | [26,27] |
| Mechanism | Elderly GM | Effect | References |
|---|---|---|---|
| Lipopolysaccharides | Higher levels | Promotes TNF-α, IFN-β, IL-12, IL-1β, and IL-6 | [37] |
| Bile acid metabolism | Decrease in the bacterial enzyme bile salt hydrolase (BSH), expressed by Firmicutes, Bacteroidetes, and Actinobacteria | Failure to activate genes involved in intestinal conservation, suppression of bacterial proliferation, and maintenance of mucosal barrier integrity | [63] |
| Dysbiosis | Reduction in Bifidobacteria, Firmicutes, Clostridium cluster IV, and Clostridium cluster XIVa | Inhibits the IL-6/Stat3/IL-17 pathway, reducing the differentiation of CD4+ T cells into Th17 cells | [41,64] |
| Gut permeability | Increased | Disrupted tight junctions and reduced expression of E-cadherin and occludin proteins | [47] |
| Intestinal immune cells | Functionality deteriorates | Lowers the levels of immunoregulatory substances in LP CD4+ T cells and reduces the occurrence and efficacy of LP CD4+ Th17 cells | [56] |
| Gut cell autophagy | Reduced | Triggers the NF-κB pathway, initiating inflammasome activation | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldarelli, M.; Rio, P.; Marrone, A.; Giambra, V.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Inflammaging: The Next Challenge—Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences. Biomedicines 2024, 12, 1716. https://doi.org/10.3390/biomedicines12081716
Caldarelli M, Rio P, Marrone A, Giambra V, Gasbarrini A, Gambassi G, Cianci R. Inflammaging: The Next Challenge—Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences. Biomedicines. 2024; 12(8):1716. https://doi.org/10.3390/biomedicines12081716
Chicago/Turabian StyleCaldarelli, Mario, Pierluigi Rio, Andrea Marrone, Vincenzo Giambra, Antonio Gasbarrini, Giovanni Gambassi, and Rossella Cianci. 2024. "Inflammaging: The Next Challenge—Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences" Biomedicines 12, no. 8: 1716. https://doi.org/10.3390/biomedicines12081716






