Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biomaterials
2.2. Ethics Statement
2.3. Clinical Evaluation
2.4. Anesthesia
2.5. Study Design
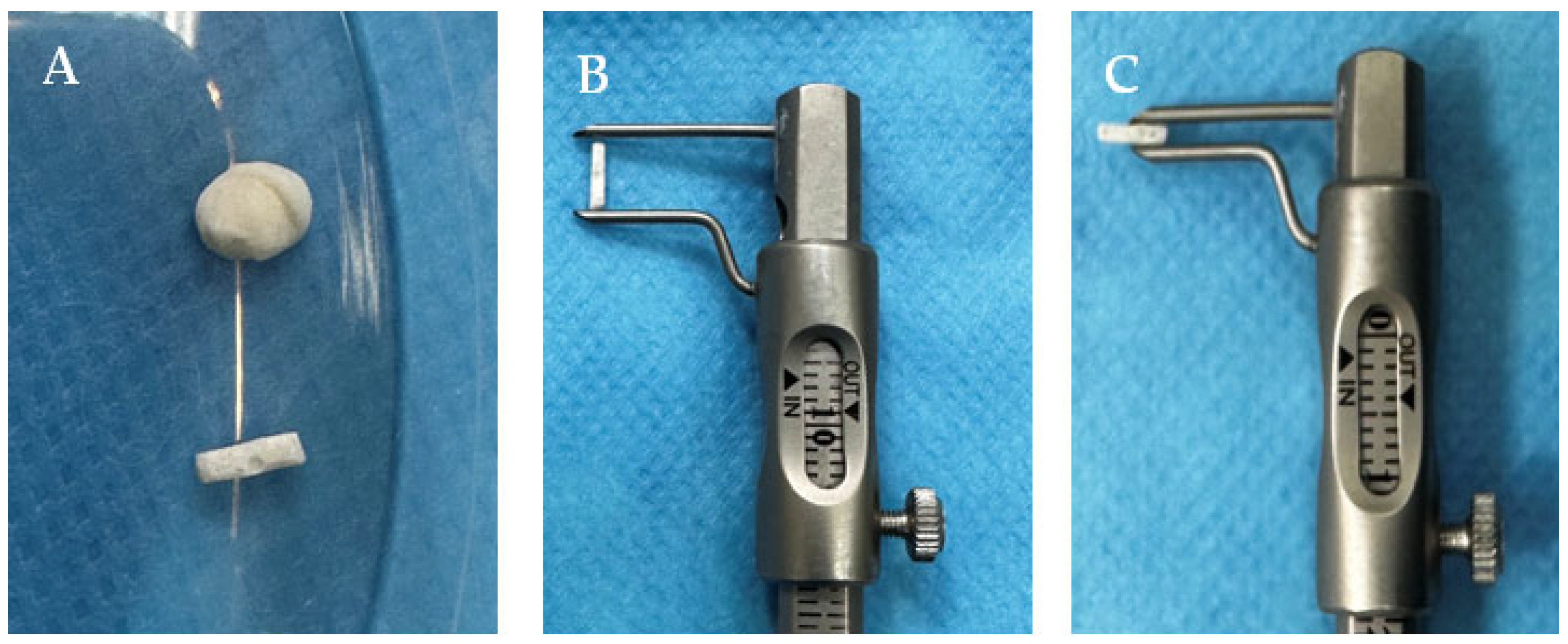
2.6. Biomaterial Implantation
2.6.1. Subcutaneous
2.6.2. Intramuscular/Perimuscular
2.7. Computed Tomography Scans
2.8. Histopathological Examination
3. Results
3.1. Clinical Evaluation
3.2. Postoperative Care
3.2.1. Subcutaneous
3.2.2. Intramuscular/Perimuscular
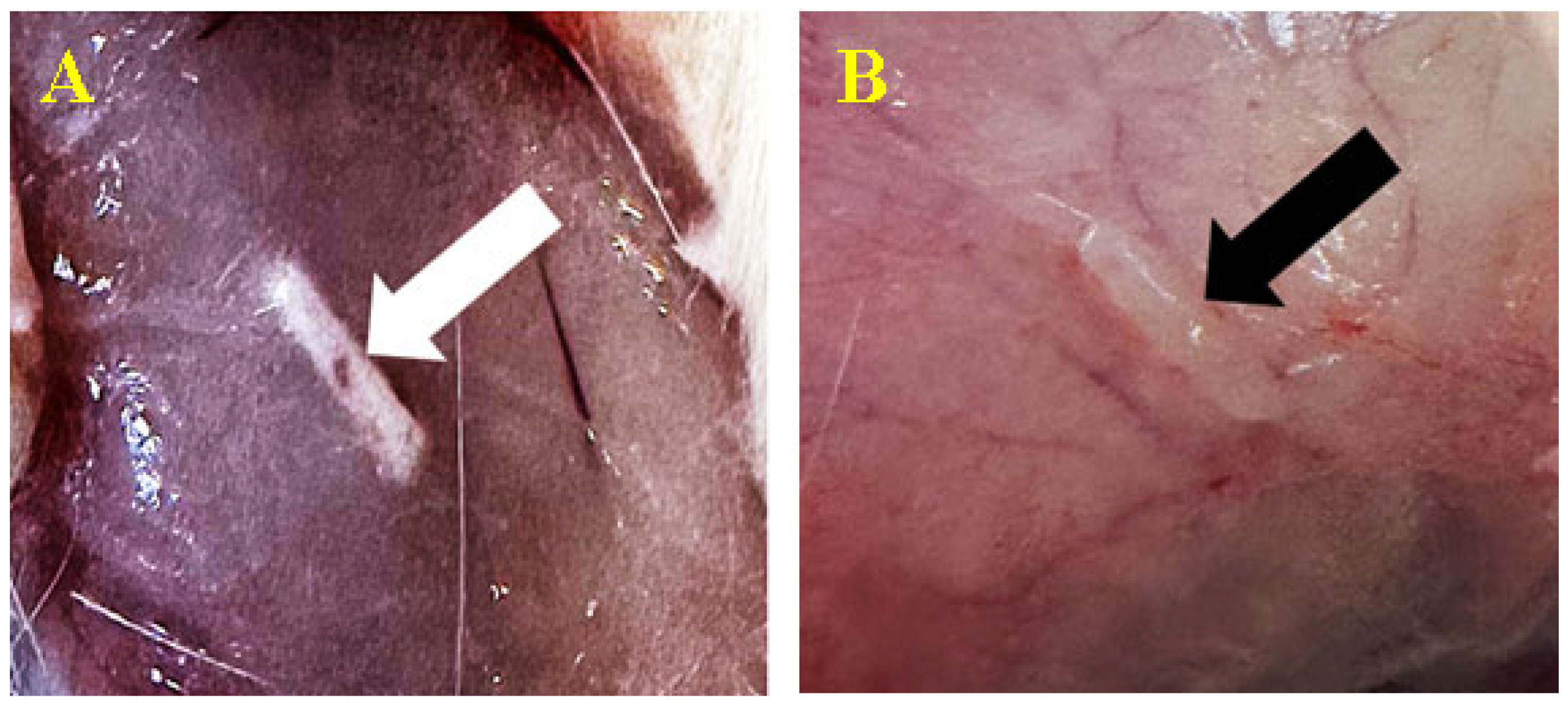
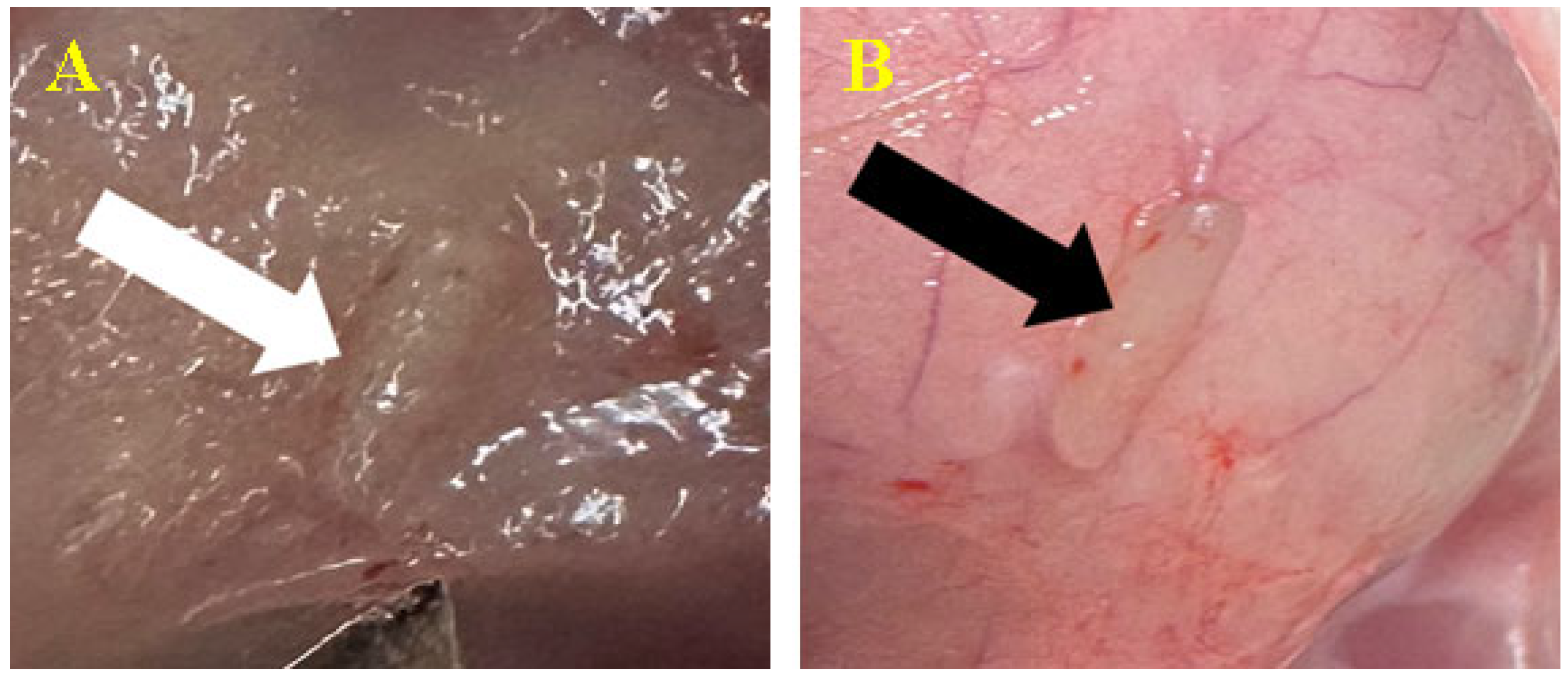
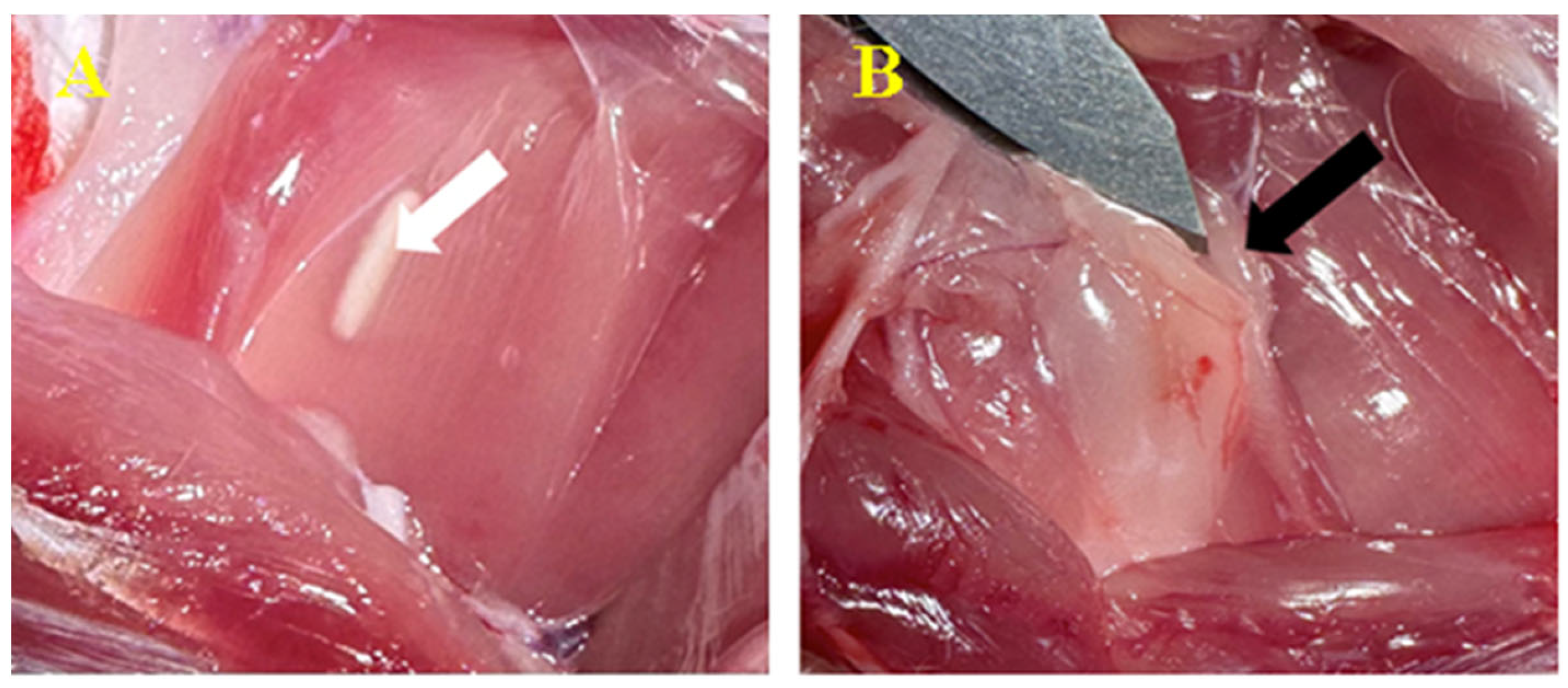
3.3. Biomaterial Implantation
3.3.1. Subcutaneous
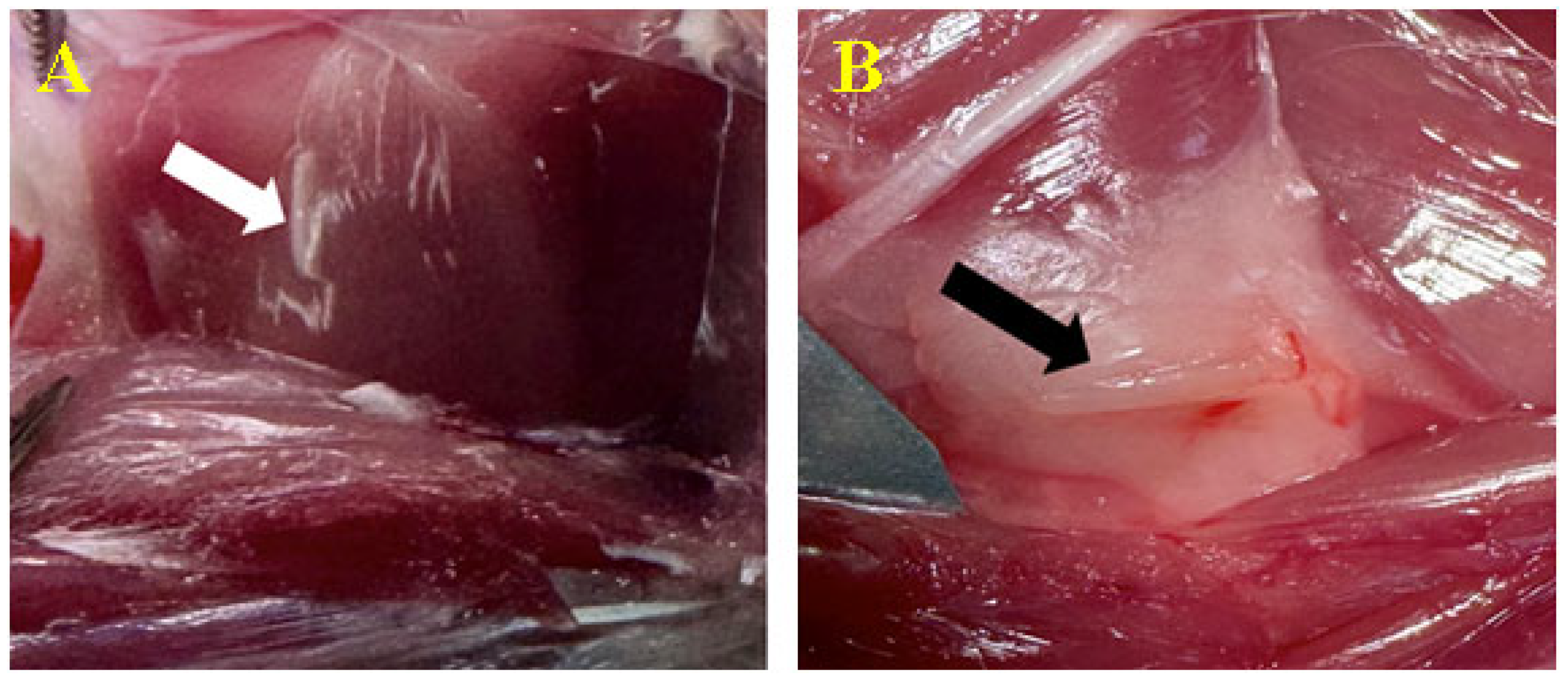
3.3.2. Peri/Intramuscular
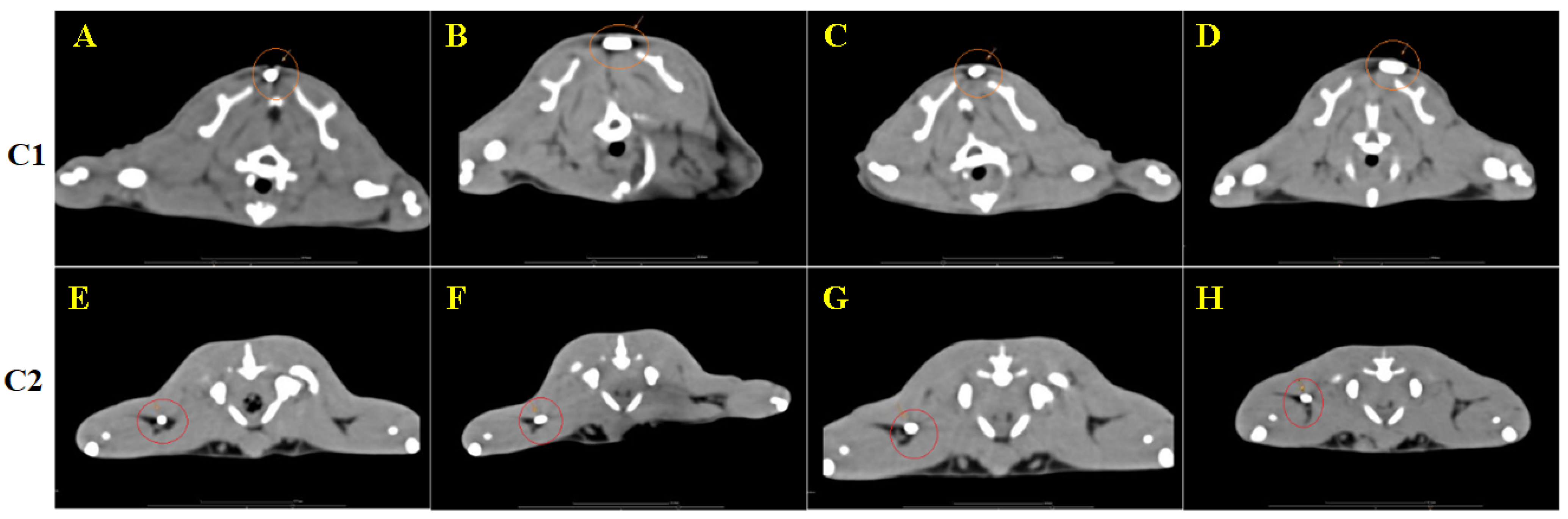
3.4. CT Image Findings
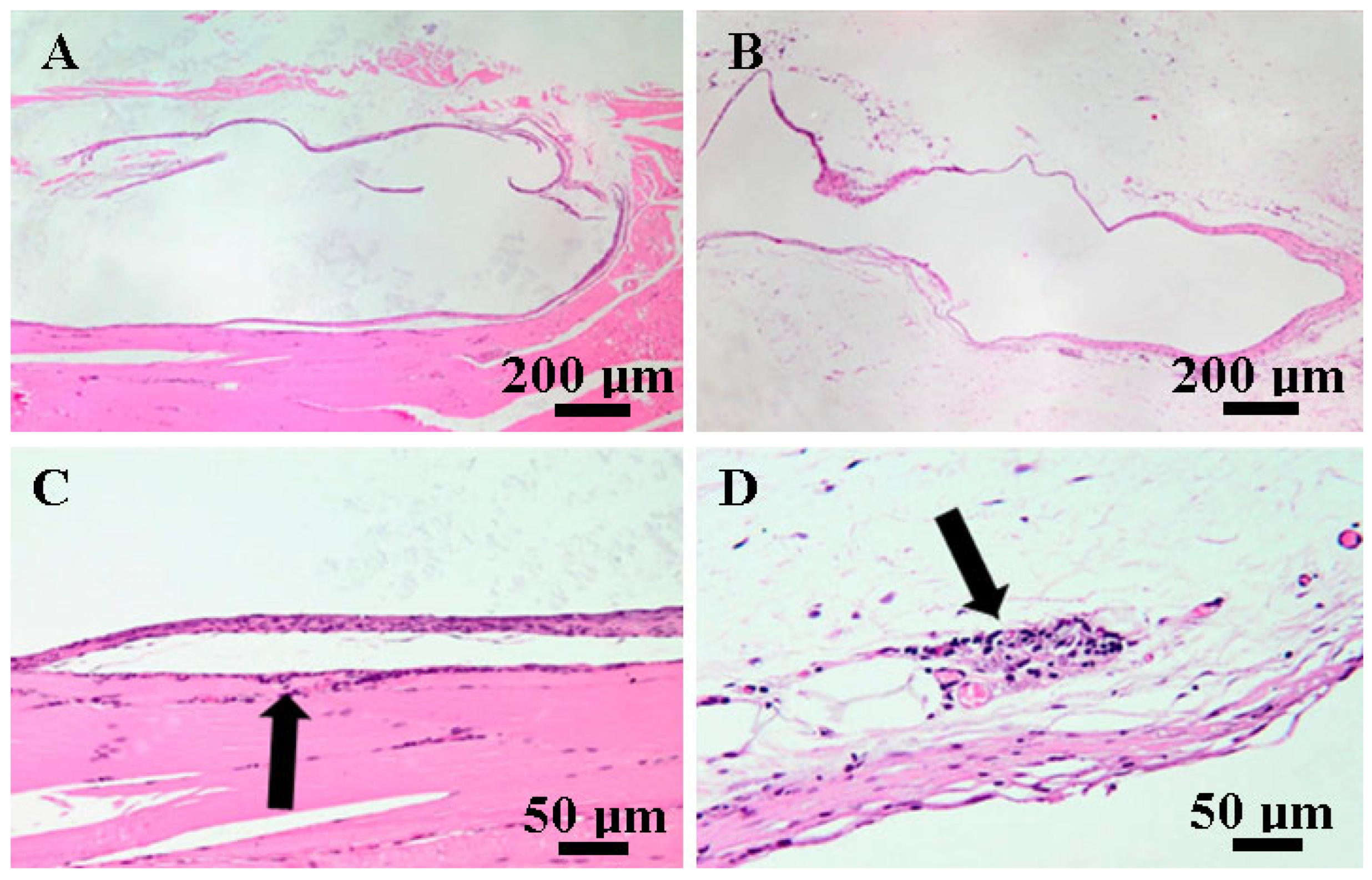
3.5. Histopathological Findings
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conte, R.; Di Salle, A.; Riccitiello, F.; Petillo, O.; Peluso, G.; Calarco, A. Biodegradable polymers in dental tissue engineering and regeneration. AIMS Mater. Sci. 2018, 5, 1073–1101. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P.V. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid. Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.-M.; Lesieur, J.; Vacher, C.; Chaussain, C.; Rochefort, G.Y. Strategies Developed to Induce, Direct, and Potentiate Bone Healing. Front. Physiol. 2017, 8, 927. [Google Scholar] [CrossRef]
- Chisnoiu, R.; Moldovan, M.; Păstrav, O.; Delean, A.; Chisnoiu, A. The influence of three endodontic sealers on bone healing: An experimental study. Folia Morphol. 2016, 75, 14–20. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Wu, S.-C.; Chen, H.; Tsai, L.-L.; Tzeng, J.-J.; Lin, C.-H.; Lin, Y.-M. Synthesis and Characterization of Polycaprolactone-Based Polyurethanes for the Fabrication of Elastic Guided Bone Regeneration Membrane. BioMed Res. Int. 2018, 2018, 3240571. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2004; pp. 209–222. ISBN 9780080470368. [Google Scholar]
- ISO10993-5; BeomdP. International Organization for Standardization: Geneva, Switzerland, 1999.
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peștean, C.; Nagy, A.; Gal, A.F.; Tăbăran, F.; Purdoiu, R.C.; Licărete, E. The Impact of Composites with Silicate-Based Glasses and Gold Nanoparticles on Skin Wound Regeneration. Molecules 2021, 26, 620. [Google Scholar] [CrossRef]
- Mirea, R.; Biris, I.M.; Ceatra, L.C.; Ene, R.; Paraschiv, A.; Cucuruz, A.T.; Sbarcea, G.; Popescu, E.; Badea, T. In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals 2021, 11, 857. [Google Scholar] [CrossRef]
- Wataha, J.C. Principles of biocompatibility for dental practitioners. J. Prosthet. Dent. 2001, 86, 203–209. [Google Scholar] [CrossRef]
- Ardelean, A.I.; Dragomir, M.F.; Moldovan, M.; Sarosi, C.; Paltinean, G.A.; Pall, E.; Tudoran, L.B.; Petean, I.; Oana, L. In Vitro Study of Composite Cements on Mesenchymal Stem Cells of Palatal Origin. Int. J. Mol. Sci. 2023, 24, 10911. [Google Scholar] [CrossRef] [PubMed]
- Mirea, R.; Cucuruz, A.T.; Ceatra, L.C.; Badea, T.; Biris, I.; Popescu, E.; Paraschiv, A.; Ene, R.; Sbarcea, G.; Cretu, M. In-Depth Comparative Assessment of Different Metallic Biomaterials in Simulated Body Fluid. Materials 2021, 14, 2774. [Google Scholar] [CrossRef] [PubMed]
- Pătroi, D.; Gociu, M.; Prejmerean, C.; Colceriu, L.; Silaghi-Dumitrescu, L.; Moldovan, M.; Naicu, V. Assessing the biocompatibility of a dental composite product. Rom. J. Morphol. Embryol. 2013, 54, 321–326. [Google Scholar] [PubMed]
- Páll, E.; Florea, A.; Soriţău, O.; Cenariu, M.; Petruţiu, A.S.; Roman, A. Comparative Assessment of Oral Mesenchymal Stem Cells Isolated from Healthy and Diseased Tissues. Microsc. Microanal. 2015, 21, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Mäkelä, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017, 7, Cd001830. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Marovic, D.; Par, M.; Khai, L.; Thieu, M.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Yoshihara, K.; Yoshida, Y. Development of new diacrylate monomers as substitutes for Bis-GMA and UDMA. Dent. Mater. 2021, 37, e391–e398. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Moszner, N.; Fischer, U.K.; Angermann, J.; Rheinberger, V. A partially aromatic urethane dimethacrylate as a new substitute for Bis-GMA in restorative composites. Dent. Mater. 2008, 24, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Hollander, D.; Krugliak, P.; Katz, K. PEG 400, a hydrophilic molecular probe for measuring intestinal permeability. Gastroenterology 1990, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mohl, S.; Winter, G. Continuous release of rh-interferon α-2a from triglyceride matrices. J. Control. Release 2004, 97, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The future of bioactive ceramics. J. Mater. Sci. Mater. Med. 2015, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 643781. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Erich Serfözö, N.; Prodan, D.; Diegelmann, J.; Moldovan, M. Synthesis and performance of experimental resin-based dental adhesives reinforced with functionalized graphene and hydroxyapatite fillers. Mater. Des. 2022, 221, 110985. [Google Scholar] [CrossRef]
- Al-Husinat, L.; Jouryyeh, B.; Al Sharie, S.; Al Modanat, Z.; Jurieh, A.; Al Hseinat, L.; Varrassi, G. Bone Cement and Its Anesthetic Complications: A Narrative Review. J. Clin. Med. 2023, 12, 2105. [Google Scholar] [CrossRef]
- Lupescu, O.; Nagea, M.; Scurtu, R.; Ciurea, N.M.; Dimitriu, A.L.; Marcov, N.; Popescu, G.I.; Bondari, S. Acute cellulitis as local reaction to orthopedic implant—Case presentation. Rom. J. Morphol. Embryol. 2016, 57, 1137–1143. [Google Scholar]
- Tisler, C.E.; Moldovan, M.; Petean, I.; Buduru, S.D.; Prodan, D.; Sarosi, C.; Leucuţa, D.-C.; Chifor, R.; Badea, M.E.; Ene, R. Human Enamel Fluorination Enhancement by Photodynamic Laser Treatment. Polymers 2022, 14, 2969. [Google Scholar] [CrossRef]
- Mousavinasab, S.M. Biocompatibility of composite resins. Dent. Res. J. 2011, 8 (Suppl. S36), S21–S29. [Google Scholar]
- Schmid-Schwap, M.; Franz, A.; König, F.; Bristela, M.; Lucas, T.; Piehslinger, E.; Watts, D.C.; Schedle, A. Cytotoxicity of four categories of dental cements. Dent. Mater. 2009, 25, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Darmani, H.; Al-Hiyasat, A.S.; Milhem, M.M. Cytotoxicity of dental composites and their leached components. Quintessence Int. 2007, 38, 789–795. [Google Scholar] [PubMed]
- Moldovan, M.; Dudea, D.; Cuc, S.; Sarosi, C.; Prodan, D.; Petean, I.; Furtos, G.; Ionescu, A.; Ilie, N. Chemical and structural assessment of new dental composites with graphene exposed to staining agents. J. Funct. Biomater. 2023, 14, 163. [Google Scholar] [CrossRef]
- Mârza, S.M.; Dăescu, A.M.; Purdoiu, R.C.; Dragomir, M.; Tătaru, M.; Melega, I.; Nagy, A.-L.; Gal, A.; Tăbăran, F.; Bogdan, S.; et al. Healing of Skin Wounds in Rats Using Creams Based on Symphytum Officinale Extract. Int. J. Mol. Sci. 2024, 25, 3099. [Google Scholar] [CrossRef]
- Gociu, M.; Pătroi, D.; Prejmerean, C.; Păstrăv, O.; Boboia, S.; Prodan, D.; Moldovan, M. Biology and cytotoxicity of dental materials: An in vitro study. Rom. J. Morphol. Embryol. 2013, 54, 261–265. [Google Scholar]
- Kirkpatrick, C.; Bittinger, F.; Wagner, M.; Köhler, H.; Van Kooten, T.; Klein, C.; Otto, M. Current trends in biocompatibility testing. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, B.C.; Ivanov, D. Natural and synthetic polymers for designing composite materials. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 233–286. [Google Scholar]
- Whishaw, I.Q.; Kolb, B. Analysis of behavior in laboratory rats. In The Laboratory Rat; Elsevier: Amsterdam, The Netherlands, 2020; pp. 215–242. [Google Scholar]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]
- ISO/DIS 7405; Dentistry-Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 14155; Clinical Investigations of Medical Devices for Human Subjects- Good Practice. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO. Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements neIG-v; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Nagatomo, F.; Fujino, H.; Kondo, H.; Ishihara, A. Oxygen concentration-dependent oxidative stress levels in rats. Oxidative Med. Cell. Longev. 2012, 2012, 381763. [Google Scholar] [CrossRef]
- Didion, J.P.; de Villena, F.P. Deconstructing Mus gemischus: Advances in understanding ancestry, structure, and variation in the genome of the laboratory mouse. Mamm. Genome 2013, 24, 1–20. [Google Scholar] [CrossRef]
- Hutt, S.J.; Hutt, C. Direct Observation and Measurement of Behavior; Springfield: Geneseo, IL, USA, 1970; p. 224. [Google Scholar]
- Greenway, K.S.R.; Vargas Carvajal, D.; Nair, R.; Lunt, J.; Tan, T.; Smith, A.J.; Johnson, M.; Ross, K.A. Hounsfield Unit. Reference Article. Available online: https://radiopaedia.org/ (accessed on 14 April 2024).
- Schultze-Mosgau, S.; Keilholz, L.; Rödel, F.; Labahn, D.; Neukam, F.W. Experimental model for transplantation of a modified free myocutaneous gracilis flap to an irradiated neck region in rats. Int. J. Oral Maxillofac. Surg. 2001, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.N.; Saveleva, M.S.; Kozadaev, M.N.; Matveeva, O.V.; Sal’kovskiy, Y.E.; Lyubun, G.P.; Gorin, D.A.; Norkin, I.A. New Approaches to Scaffold Biocompatibility Assessment. BioNanoScience 2019, 9, 395–405. [Google Scholar] [CrossRef]
- Abou ElReash, A.; Hamama, H.; Abdo, W.; Wu, Q.; Zaen El-Din, A.; Xiaoli, X. Biocompatibility of new bioactive resin composite versus calcium silicate cements: An animal study. BMC Oral Health 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Cota, J.; Arras, M.; Dobbins, T.; Geller, E.; Ilgen, J.; O’Neill, R.; Smith, J.; Williams, S.; Yates, J. An evaluation of the effect of post-surgical analgesia on weight gain and food and water consumption in laboratory rats. Lab. Anim. 2005, 34, 1554. [Google Scholar]
- Frazão, L.P.; Vieira de Castro, J.; Neves, N.M. In Vivo Evaluation of the Biocompatibility of Biomaterial Device. Adv. Exp. Med. Biol. 2020, 1250, 109–124. [Google Scholar] [PubMed]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Halloysite Nanotubes and Sepiolite for Health Applications. Int. J. Mol. Sci. 2023, 24, 4801. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Hern, H.G. Wound management principles. In Rosen’s Emergency Medicine, 8th ed.; Marx, J., Hockbergr, R., Walls, R., Eds.; Saunders: Philadelphia, PA, USA, 2014; pp. 751–766. [Google Scholar]
- Kyriakides, T.R.; Kim, H.J.; Zheng, C.; Harkins, L.; Tao, W.; Deschenes, E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed. Mater. 2022, 17, 022007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Bercker, S.; Bert, B.; Bittigau, P.; Felderhoff-Müser, U.; Bührer, C.; Ikonomidou, C.; Weise, M.; Kaisers, U.X.; Kerner, T. Neurodegeneration in Newborn Rats Following Propofol and Sevoflurane Anesthesia. Neurotox. Res. 2009, 16, 140–147. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Meng, S.; Sun, M.; Chen, Y.; Hong, Y. Isoflurane-induced neuroinflammation and NKCC1/KCC2 dysregulation result in long-term cognitive disorder in neonatal mice. BMC Anesthesiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.Y.; Anderson, D.E. Bone and Cartilage Interferences with Orthopedic Implans: A Literature Review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release?A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Donath, K. The diagnostic value of the new method for the study of undecalcified bones and teeth with attached soft tissue, (Säge-Schliff, (Sawing and Grinding) Technique). Pathol. Res. Pract. 1985, 179, 631–633. [Google Scholar] [CrossRef]
- Schmalz, G.; Arenholt-Bindslev, D. Determination of Biocompatibility. In Biocompatibility of Dental Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–43. [Google Scholar]
- Girão, L.B.; de Lima Martins, J.O.; Mota Lemos, J.V.; Pinto, M.R.; Marques Lima Rolim, J.P.; Fernandes Alves e Silva, F.C.; de Paulo Aragão Saboia, V.; Bitu Sousa, F.; de Barros Silva, P.G. Influence of the degree of conversion and Bis-GMA residues of bulk fill resins on tissue toxicity in an subcutaneous model in rats. J. Appl. Biomater. Funct. Mater. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characteriza-tion and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- de Carvalho, D.K.; de Sousa, D.L.; Barbugli, P.A.; Cerri, P.S.; Salih, V.M.; Vergani, C.E. Development and characterization of a 3D oral mucosa model as a tool for host-pathogen interactions. J. Microbiol. Methods 2018, 152, 52–60. [Google Scholar] [CrossRef]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro threedimensional organotypic culture models of the oral mucosa. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Threedimensional in vitro oral mucosa models of fungal and bacterial infections. Tissue Eng. Part B Rev. 2020, 26, 443–460. [Google Scholar] [CrossRef]




| Materials | Manufacturer | Matrix Monomers | Total Content Filler |
|---|---|---|---|
| Light-curing hybrid cement composite (C1) | UBB-ICCRR, Cluj-Napoca, Romania | Bis-GMA 1; UDMA; HEMA TEGDMA 3; | 65 weight%,—HA 2 (particle size 0.01–0.06 μm and 5–8 nm); silica, barium glass (BaO) (particle size 0.01–0.035 μm and 2–6 nm); quartz |
| Light-curing hybrid cement composite (C2) | UBB-ICCRR, Cluj-Napoca, Romania | Bis-GMA 1; UDMA; TEGDMA 3; | 65 weight%, HA 2 (particle size 0.01–60 μm and 5–8 nm); silica, glass filler (with BaF2) (particle size 2–6 nm); fluoroaluminosilicate glass (0.04–0.50 μm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardelean, A.I.; Marza, S.M.; Negoescu, A.; Dragomir, M.F.; Sarosi, C.; Moldovan, M.; Ene, R.; Oana, L. Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines 2024, 12, 1718. https://doi.org/10.3390/biomedicines12081718
Ardelean AI, Marza SM, Negoescu A, Dragomir MF, Sarosi C, Moldovan M, Ene R, Oana L. Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines. 2024; 12(8):1718. https://doi.org/10.3390/biomedicines12081718
Chicago/Turabian StyleArdelean, Alina Ioana, Sorin Marian Marza, Andrada Negoescu, Madalina Florina Dragomir, Codruta Sarosi, Marioara Moldovan, Razvan Ene, and Liviu Oana. 2024. "Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats" Biomedicines 12, no. 8: 1718. https://doi.org/10.3390/biomedicines12081718
APA StyleArdelean, A. I., Marza, S. M., Negoescu, A., Dragomir, M. F., Sarosi, C., Moldovan, M., Ene, R., & Oana, L. (2024). Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines, 12(8), 1718. https://doi.org/10.3390/biomedicines12081718







