Heart–Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia
Abstract
1. Introduction
2. The Interoceptive Pathway of Heart–Brain Interactions
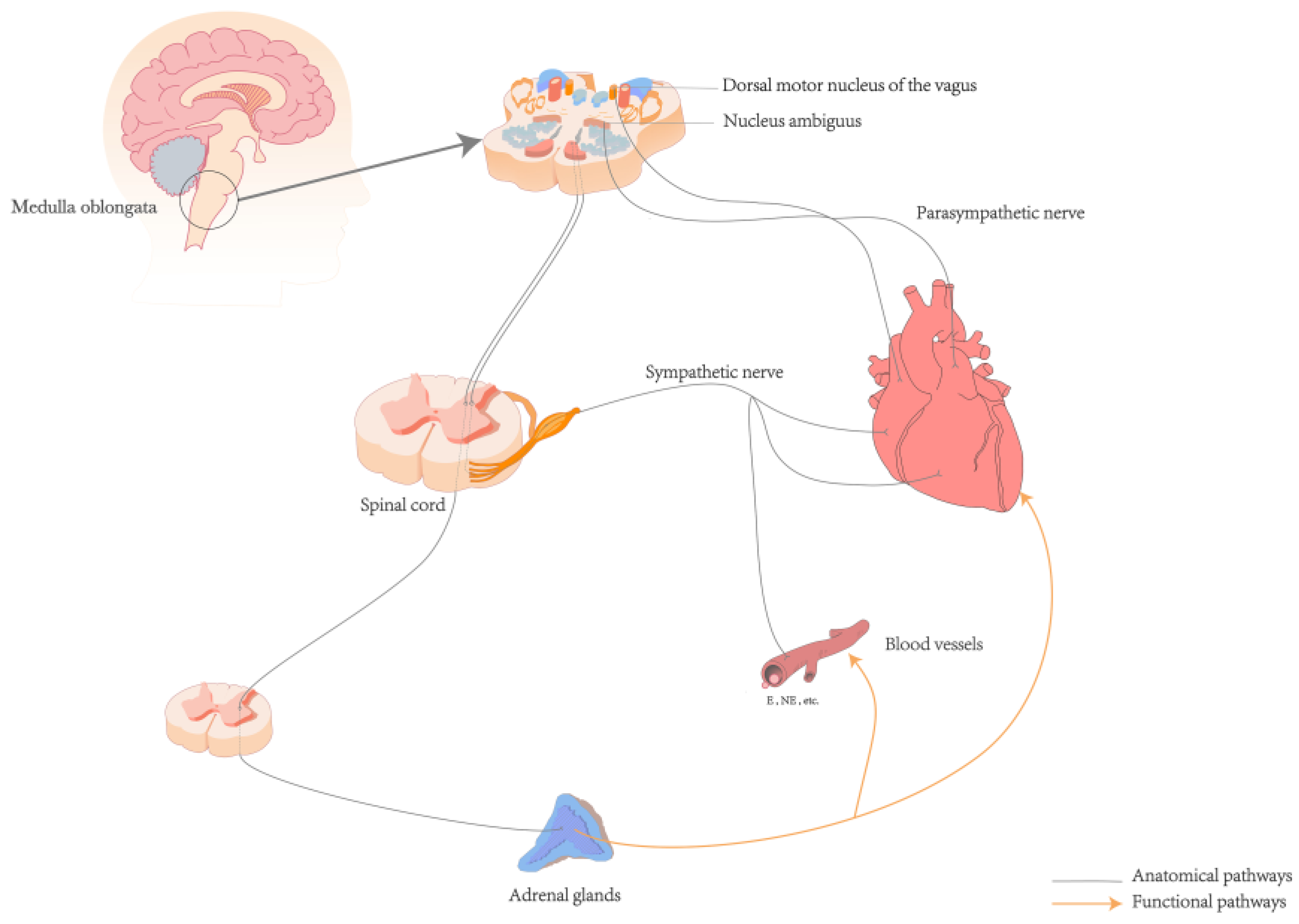
2.1. Neuroanatomical Basis
2.2. Ascending Pathway
3. The Involvement of the ANS in the Pathogenesis of Arrhythmias Triggered by Mental Stress
4. Relationship of Interactions
4.1. Genes
4.2. Personality Traits
4.3. Stress
4.4. Endocrine System
4.5. Inflammation
4.6. 5-Hydroxytryptamine
4.7. Behavioral Factors
5. Discussion
6. Current Clinical Status
7. Limitation
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Depression. Available online: https://www.who.int/health-topics/depression#tab=tab_1 (accessed on 17 April 2024).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska-Nowak, J.; Wachowska, K.; Nowak, A.; Orzechowska, A.; Szulc, A.; Płaza, O.; Gałecki, P. Exploring the Heart-Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines 2023, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.T.; Xu, Y.J.; Yang, Y.Q. Gender Differences in Arrhythmias: Focused on Atrial Fibrillation. J. Cardiovasc. Transl. Res. 2020, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef]
- Musselman, D.L.; Evans, D.L.; Nemeroff, C.B. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Arch. Gen. Psychiatry 1998, 55, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Müller-Tasch, T.; Peters-Klimm, F.; Schellberg, D.; Holzapfel, N.; Barth, A.; Jünger, J.; Szecsenyi, J.; Herzog, W. Depression Is a Major Determinant of Quality of Life in Patients with Chronic Systolic Heart Failure in General Practice. J. Card. Fail. 2007, 13, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Möller-Leimkühler, A.M. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin. Neurosci. 2007, 9, 71–83. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Shou, X.; Zhang, X.; Fan, S.; Chai, R.; Xue, W.; Hu, Y.; He, Q. Psycho-Cardiological Disease: A Bibliometric Review from 2001 to 2021. Front. Cardiovasc. Med. 2022, 9, 890329. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.G. Cardiac Arrhythmias: Diagnosis, Symptoms, and Treatments. Cell Biochem. Biophys. 2015, 73, 291–296. [Google Scholar] [CrossRef]
- Sepehri Shamloo, A.; Bunch, T.J.; Lin, Y.J.; de Oliveira Figueiredo, M.J.; Dagres, N.; Nielsen, J.C. Risk Assessment in Cardiac Arrhythmias. Eur. Heart J. 2020, 41, 4455–4457. [Google Scholar] [CrossRef]
- Brunckhorst, C.B.; Holzmeister, J.; Scharf, C.; Binggeli, C.; Duru, F. Stress, depression and cardiac arrhythmias. Ther. Umsch. 2003, 60, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.J.; Wang, B.B.; Hou, F.F.; Jiao, Y.; Li, H.W.; Lv, S.P.; Li, F.H. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace 2023, 25, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Marmot, M.; Hemingway, H. Systematic review of prospective cohort studies of psychosocial factors in the etiology and prognosis of coronary heart disease. Semin. Vasc. Med. 2002, 2, 267–314. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.J. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin. Neurosci. 2011, 13, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, P.D.; Garfinkel, S.N.; Taggart, P. Psychological stress, the central nervous system and arrhythmias. QJM 2023, 116, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ilter, A.; Aslan, M.; Caliskan Ilter, Z.; Besli, F.; Tusun, E. Major depressive disorder is associated with fragmented QRS. Acta Cardiol. 2017, 72, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Yilmaz, S. Major depressive disorder is an independent predictor of the electrocardiographic frontal QRS-T angle. Bratisl. Lek. Listy 2023, 124, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisey, A.M.; Parameswaran, R.; Bryant, C.; Anderson, R.D.; Hawson, J.; Chieng, D.; Segan, L.; Voskoboinik, A.; Sugumar, H.; Wong, G.R.; et al. Atrial Fibrillation Catheter Ablation vs. Medical Therapy and Psychological Distress: A Randomized Clinical Trial. JAMA 2023, 330, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Du, X.; Lu, S.; Yang, X.; Chang, S.; Liu, J.; Li, J.; Zhou, Y.; Macle, L.; Dong, J.; et al. Effect of Mental Health Status on Arrhythmia Recurrence after Catheter Ablation of Atrial Fibrillation. Can. J. Cardiol. 2019, 35, 831–839. [Google Scholar] [CrossRef]
- Zhuo, C.; Ji, F.; Lin, X.; Jiang, D.; Wang, L.; Tian, H.; Xu, Y.; Liu, S.; Chen, C. Depression and recurrence of atrial fibrillation after catheter ablation: A meta-analysis of cohort studies. J. Affect. Disord. 2020, 271, 27–32. [Google Scholar] [CrossRef]
- Thrall, G.; Lip, G.Y.; Carroll, D.; Lane, D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest 2007, 132, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Saljic, A.; Heijman, J. P2X7 receptors: Central drivers of the neurocardiac link between atrial fibrillation and depression? Europace 2024, 26, euae023. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Wang, M.; Yang, C.; Sun, G. Cardiovascular disease and depression: A narrative review. Front. Cardiovasc. Med. 2023, 10, 1274595. [Google Scholar] [CrossRef]
- Ai, Y.; Xing, Y.; Yan, L.; Ma, D.; Gao, A.; Xu, Q.; Zhang, S.; Mao, T.; Pan, Q.; Ma, X.; et al. Atrial Fibrillation and Depression: A Bibliometric Analysis from 2001 to 2021. Front. Cardiovasc. Med. 2022, 9, 775329. [Google Scholar] [CrossRef] [PubMed]
- Saljic, A.; Heijman, J.; Dobrev, D. Recent Advances in Antiarrhythmic Drug Therapy. Drugs 2023, 83, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Abdullah, A.; Nanna, M.G.; Soufer, R. The Brain-Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease. Curr. Cardiol. Rep. 2023, 25, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Harrison, N.A. Visceral influences on brain and behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef]
- Brandt, T.; Huppert, D. Brain beats heart: A cross-cultural reflection. Brain 2021, 144, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.D.; Paulesu, E.; Nihoyannopoulos, P.; Tousoulis, D.; Frackowiak, R.S.; Frith, C.D.; Jones, T.; Camici, P.G. Silent ischemia as a central problem: Regional brain activation compared in silent and painful myocardial ischemia. Ann. Intern. Med. 1996, 124, 939–949. [Google Scholar] [CrossRef]
- Gray, M.A.; Taggart, P.; Sutton, P.M.; Groves, D.; Holdright, D.R.; Bradbury, D.; Brull, D.; Critchley, H.D. A cortical potential reflecting cardiac function. Proc. Natl. Acad. Sci. USA 2007, 104, 6818–6823. [Google Scholar] [CrossRef]
- Jefferson, J.W. Psychocardiology: Meeting place of heart and mind. Psychosomatics 1985, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.J.; Bliss-Moreau, E.; Lindquist, K.A. The neurobiology of interoception and affect. Trends Cogn. Sci. 2024, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Engelen, T.; Solcà, M.; Tallon-Baudry, C. Interoceptive rhythms in the brain. Nat. Neurosci. 2023, 26, 1670–1684. [Google Scholar] [CrossRef]
- Berntson, G.G.; Khalsa, S.S. Neural Circuits of Interoception. Trends Neurosci. 2021, 44, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, D.; Rebollo, I.; Tallon-Baudry, C. Visceral Signals Shape Brain Dynamics and Cognition. Trends Cogn. Sci. 2019, 23, 488–509. [Google Scholar] [CrossRef]
- Florian, B.; Karin, M.; Karl-Jürgen, B.; Vitaly, N. The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. J. Neurosci. 2013, 33, 10503. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Hadaya, J.; Khalsa, S.S.; Yu, C.; Chang, R.; Shivkumar, K. The vagus nerve in cardiovascular physiology and pathophysiology: From evolutionary insights to clinical medicine. Semin. Cell Dev. Biol. 2024, 156, 190–200. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Chadda, K.R.; Ajijola, O.A.; Vaseghi, M.; Shivkumar, K.; Huang, C.L.; Jeevaratnam, K. Ageing, the autonomic nervous system and arrhythmia: From brain to heart. Ageing Res. Rev. 2018, 48, 40–50. [Google Scholar] [CrossRef]
- Armour, J.A. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm 2010, 7, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ladenbauer, J.; Babo-Rebelo, M.; Buot, A.; Lehongre, K.; Adam, C.; Hasboun, D.; Lambrecq, V.; Navarro, V.; Ostojic, S.; et al. Resting-State Neural Firing Rate Is Linked to Cardiac-Cycle Duration in the Human Cingulate and Parahippocampal Cortices. J. Neurosci. 2019, 39, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Décarie-Spain, L.; Hayes, A.M.R.; Lauer, L.T.; Kanoski, S.E. The gut-brain axis and cognitive control: A role for the vagus nerve. Semin. Cell Dev. Biol. 2024, 156, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Goldston, K.; Baillie, A.J. Depression and coronary heart disease: A review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin. Psychol. Rev. 2008, 28, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Breakspear, M.; Hu, X.; Guo, C.C. The integration of the internal and external milieu in the insula during dynamic emotional experiences. Neuroimage 2016, 124, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Hasnul, M.A.; Aziz, N.A.A.; Alelyani, S.; Mohana, M.; Aziz, A.A. Electrocardiogram-Based Emotion Recognition Systems and Their Applications in Healthcare-A Review. Sensors 2021, 21, 5015. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Elman, I.; Freeman, R.; Borsook, D. The omnipresence of autonomic modulation in health and disease. Prog. Neurobiol. 2022, 210, 102218. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Steinhauser, M.; Maier, M.E.; Thayer, J.F.; Battaglia, S. Error-related cardiac deceleration: Functional interplay between error-related brain activity and autonomic nervous system in performance monitoring. Neurosci. Biobehav. Rev. 2024, 157, 105542. [Google Scholar] [CrossRef]
- Schwartz, P.J.; La Rovere, M.T.; Vanoli, E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation 1992, 85, I77–I91. [Google Scholar] [PubMed]
- Cauti, F.M.; Rossi, P.; Sommer, P. The sympathetic nervous system and ventricular arrhythmias: An inseparable union. Eur. Heart J. 2021, 42, 3588–3590. [Google Scholar] [CrossRef] [PubMed]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D. A literature review of heart rate variability in depressive and bipolar disorders. Aust. New Zealand J. Psychiatry 2015, 50, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, L.; Liu, C. Heart rate variability monitoring for emotion and disorders of emotion. Physiol. Meas. 2019, 40, 064004. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.; Kemp, A.H. Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.A.; Chang, C.C.; Chen, C.L.; Kuo, T.B.; Lu, R.B.; Huang, S.Y. Major depression is associated with cardiac autonomic dysregulation. Acta Neuropsychiatr. 2012, 24, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Jamner, L.; Burg, M.; Dziura, J.; Brandt, C.; Liu, H.; Li, F.; Donovan, T.; Soufer, R. Triggering of symptomatic atrial fibrillation by negative emotion. J. Am. Coll. Cardiol. 2014, 64, 1533–1534. [Google Scholar] [CrossRef]
- Rugulies, R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am. J. Prev. Med. 2002, 23, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Synowski, S.J.; Newell, M.E.; Schmidt, L.A.; Waldstein, S.R.; Fox, N.A. Autonomic nervous system reactivity to positive and negative mood induction: The role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 2011, 86, 230–238. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Atkinson, M.; Tiller, W.A.; Rein, G.; Watkins, A.D. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am. J. Cardiol. 1995, 76, 1089–1093. [Google Scholar] [CrossRef]
- Gillie, B.L.; Thayer, J.F. Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Front. Psychol. 2014, 5, 758. [Google Scholar] [CrossRef] [PubMed]
- Perna, G.; Riva, A.; Defillo, A.; Sangiorgio, E.; Nobile, M.; Caldirola, D. Heart rate variability: Can it serve as a marker of mental health resilience?: Special Section on “Translational and Neuroscience Studies in Affective Disorders” Section Editor, Maria Nobile MD, PhD. J. Affect. Disord. 2020, 263, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Zeitler, E.P.; Poole, J.E.; Albert, C.M.; Al-Khatib, S.M.; Ali-Ahmed, F.; Birgersdotter-Green, U.; Cha, Y.M.; Chung, M.K.; Curtis, A.B.; Hurwitz, J.L.; et al. Arrhythmias in Female Patients: Incidence, Presentation and Management. Circ. Res. 2022, 130, 474–495. [Google Scholar] [CrossRef]
- Bernal, O.; Moro, C. Cardiac arrhythmias in women. Rev. Esp. Cardiol. 2006, 59, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.H.; Goldberger, J.J.; Ehlert, F.A.; Kruse, J.T.; Parker, M.A.; Kadish, A.H. Gender differences in heart rate before and after autonomic blockade: Evidence against an intrinsic gender effect. Am. J. Med. 1996, 100, 537–543. [Google Scholar] [CrossRef]
- Kittnar, O. Sex Related Differences in Electrocardiography. Physiol. Res. 2023, 72, S127–S135. [Google Scholar] [CrossRef]
- Abi-Gerges, N.; Philp, K.; Pollard, C.; Wakefield, I.; Hammond, T.G.; Valentin, J.P. Sex differences in ventricular repolarization: From cardiac electrophysiology to Torsades de Pointes. Fundam. Clin. Pharmacol. 2004, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.C.; Kurokawa, J.; Furukawa, T.; Clancy, C.E. Acute effects of sex steroid hormones on susceptibility to cardiac arrhythmias: A simulation study. PLoS Comput. Biol. 2010, 6, e1000658. [Google Scholar] [CrossRef]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Sarvaiya, N.; Neill Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef]
- Steptoe, A.; Ronaldson, A.; Kostich, K.; Lazzarino, A.I.; Urbanova, L.; Carvalho, L.A. The effect of beta-adrenergic blockade on inflammatory and cardiovascular responses to acute mental stress. Brain Behav. Immun. 2018, 70, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Colclough, G.L.; Smith, S.M.; Nichols, T.E.; Winkler, A.M.; Sotiropoulos, S.N.; Glasser, M.F.; Van Essen, D.C.; Woolrich, M.W. The heritability of multi-modal connectivity in human brain activity. Elife 2017, 6, e20178. [Google Scholar] [CrossRef]
- Busjahn, C.A.; Schulz-Menger, J.; Abdel-Aty, H.; Rudolph, A.; Jordan, J.; Luft, F.C.; Busjahn, A. Heritability of left ventricular and papillary muscle heart size: A twin study with cardiac magnetic resonance imaging. Eur. Heart J. 2009, 30, 1643–1647. [Google Scholar] [CrossRef]
- Chien, K.L.; Hsu, H.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Heritability and major gene effects on left ventricular mass in the Chinese population: A family study. BMC Cardiovasc. Disord. 2006, 6, 37. [Google Scholar] [CrossRef]
- Noh, H.M.; Lee, S.C.; Park, S.W.; Sung, J.; Song, Y.M. Genetic influence on left ventricular structure and function: A Korean twin and family study. Twin Res. Hum. Genet. 2015, 18, 281–289. [Google Scholar] [CrossRef][Green Version]
- Gronemann, F.H.; Jacobsen, R.K.; Wium-Andersen, M.K.; Jørgensen, M.B.; Osler, M.; Jørgensen, T.S.H. Association of Familial Aggregation of Major Depression with Risk of Major Depression. JAMA Psychiatry 2023, 80, 350–359. [Google Scholar] [CrossRef]
- Musliner, K.L.; Trabjerg, B.B.; Waltoft, B.L.; Laursen, T.M.; Mortensen, P.B.; Zandi, P.P.; Munk-Olsen, T. Parental history of psychiatric diagnoses and unipolar depression: A Danish National Register-based cohort study. Psychol. Med. 2015, 45, 2781–2791. [Google Scholar] [CrossRef]
- Klein, D.N.; Lewinsohn, P.M.; Rohde, P.; Seeley, J.R.; Olino, T.M. Psychopathology in the adolescent and young adult offspring of a community sample of mothers and fathers with major depression. Psychol. Med. 2005, 35, 353–365. [Google Scholar] [CrossRef]
- Zhao, B.; Li, T.; Fan, Z.; Yang, Y.; Shu, J.; Yang, X.; Wang, X.; Luo, T.; Tang, J.; Xiong, D.; et al. Heart-brain connections: Phenotypic and genetic insights from magnetic resonance images. Science 2023, 380, abn6598. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Genetic differences associated with dopamine and serotonin release mediate fear-induced bradycardia in the human brain. Transl. Psychiatry 2024, 14, 24. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Cheng, E.P.; Yuan, C.; Navedo, M.F.; Dixon, R.E.; Nieves-Cintrón, M.; Scott, J.D.; Santana, L.F. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ. Res. 2011, 109, 255–261. [Google Scholar] [CrossRef]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768–821. [Google Scholar] [CrossRef]
- Klein, D.N.; Kotov, R.; Bufferd, S.J. Personality and depression: Explanatory models and review of the evidence. Annu. Rev. Clin. Psychol. 2011, 7, 269–295. [Google Scholar] [CrossRef]
- Brosschot, J.F.; Gerin, W.; Thayer, J.F. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J. Psychosom. Res. 2006, 60, 113–124. [Google Scholar] [CrossRef]
- Versteeg, H.; Spek, V.; Pedersen, S.S.; Denollet, J. Type D personality and health status in cardiovascular disease populations: A meta-analysis of prospective studies. Eur. J. Prev. Cardiol. 2012, 19, 1373–1380. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Gerin, W.; Davidson, K.W.; Pickering, T.G.; Brosschot, J.F.; Thayer, J.F.; Christenfeld, N.; Linden, W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom. Med. 2003, 65, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Nestler, E.J. The neurobiology of stress: Vulnerability, resilience, and major depression. Proc. Natl. Acad. Sci. USA 2023, 120, e2312662120. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin. Neurosci. 2006, 8, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Pope, B.S.; Wood, S.K. Advances in understanding mechanisms and therapeutic targets to treat comorbid depression and cardiovascular disease. Neurosci. Biobehav. Rev. 2020, 116, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Heim, C.; Newport, D.J.; Heit, S.; Graham, Y.P.; Wilcox, M.; Bonsall, R.; Miller, A.H.; Nemeroff, C.B. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000, 284, 592–597. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and depression. Psychosom. Med. 2005, 67 (Suppl. S1), S26–S28. [Google Scholar] [CrossRef] [PubMed]
- Loosen, P.T. Hormones of the hypothalamic-pituitary-thyroid axis: A psychoneuroendocrine perspective. Pharmacopsychiatry 1986, 19, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, C.Y.; Michalani, M.L.E.; Moreira, R.J.; Machado, U.F. Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia. Int. J. Mol. Sci. 2023, 24, 16480. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Baldwin, M.W.; Dedovic, K.; Renwick, R.; Mahani, N.K.; Lord, C.; Meaney, M.; Lupien, S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage 2005, 28, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; McGonagle, K.A.; Swartz, M.; Blazer, D.G.; Nelson, C.B. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- de Vries, G.J.; Södersten, P. Sex differences in the brain: The relation between structure and function. Horm. Behav. 2009, 55, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, A.; Cingolani, E.; Shehata, M.; Wang, X.; Curtis, A.B.; Chugh, S.S. Sex Differences in Cardiac Arrhythmias: Clinical and Research Implications. Circ. Arrhythm. Electrophysiol. 2018, 11, e005680. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Beurel, E.; Loewenstein, D.A.; Lowell, J.A.; Craighead, W.E.; Dunlop, B.W.; Mayberg, H.S.; Dhabhar, F.; Dietrich, W.D.; Keane, R.W.; et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 2018, 99, 914–924.e913. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Petruso, F.; Giff, A.E.; Milano, B.A.; De Rossi, M.M.; Saccaro, L.F. Inflammation and emotion regulation: A narrative review of evidence and mechanisms in emotion dysregulation disorders. Neuronal Signal 2023, 7, Ns20220077. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders?: Where the Rubber Meets the Road. JAMA Psychiatry 2015, 72, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Dunjic-Kostic, B.; Ivkovic, M.; Radonjic, N.V.; Petronijevic, N.D.; Pantovic, M.; Damjanovic, A.; Poznanovic, S.T.; Jovanovic, A.; Nikolic, T.; Jasovic-Gasic, M. Melancholic and atypical major depression—Connection between cytokines, psychopathology and treatment. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Xiao, H.; Zhang, Y.; Gao, W.; Qi, H.; Zhang, J. Association between neutrophil-lymphocyte ratio and perinatal depressive symptoms among Chinese women. J. Psychosom. Res. 2023, 166, 111101. [Google Scholar] [CrossRef] [PubMed]
- La Verde, M.; Luciano, M.; Fordellone, M.; Sampogna, G.; Lettieri, D.; Palma, M.; Torella, D.; Marrapodi, M.M.; Di Vincenzo, M.; Torella, M. Postpartum Depression and Inflammatory Biomarkers of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Monocyte-Lymphocyte Ratio: A Prospective Observational Study. Gynecol. Obstet. Investig. 2024, 89, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocrinol. 2019, 54, 100746. [Google Scholar] [CrossRef]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4765. [Google Scholar] [CrossRef]
- Elsheikh, S.; Hill, A.; Irving, G.; Lip, G.Y.H.; Abdul-Rahim, A.H. Atrial fibrillation and stroke: State-of-the-art and future directions. Curr. Probl. Cardiol. 2024, 49, 102181. [Google Scholar] [CrossRef]
- Koppikar, S.; Baranchuk, A.; Guzmán, J.C.; Morillo, C.A. Stroke and ventricular arrhythmias. Int. J. Cardiol. 2013, 168, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P. Stroke, depression, and self-harm in later life. Curr. Opin. Psychiatry 2023, 36, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Saccaro, L.F.; Pico, F.; Chadenat, M.L.; Richard, O.; Launay, J.M.; Bastenaire, B.; Jullien, P.; Lambert, J.; Feuga, V.; Macquet, M.; et al. Platelet, Plasma, Urinary Tryptophan-Serotonin-Kynurenine Axis Markers in Hyperacute Brain Ischemia Patients: A Prospective Study. Front. Neurol. 2021, 12, 782317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.C.; Gluck, N.; Arnulf, I.; Quintin, P.; Leboyer, M.; Pecquery, R.; Launay, J.M.; Perez-Diaz, F.; Spreux-Varoquaux, O. Decreased platelet serotonin transporter sites and increased platelet inositol triphosphate levels in patients with unipolar depression: Effects of clomipramine and fluoxetine. Clin. Pharmacol. Ther. 1999, 66, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Dotson, V.M.; McClintock, S.M.; Verhaeghen, P.; Kim, J.U.; Draheim, A.A.; Syzmkowicz, S.M.; Gradone, A.M.; Bogoian, H.R.; Wit, L. Depression and Cognitive Control across the Lifespan: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2020, 30, 461–476. [Google Scholar] [CrossRef]
- Numbers, K.; Jang, S.; Brodaty, H.; Sachdev, P.S.; Draper, B.; Reppermund, S. Instrumental Activities of Daily Living by Subjective and Objective Measures: The Impact of Depression and Personality. Front. Aging Neurosci. 2022, 14, 829544. [Google Scholar] [CrossRef] [PubMed]
- Pearson, O.; Uglik-Marucha, N.; Miskowiak, K.W.; Cairney, S.A.; Rosenzweig, I.; Young, A.H.; Stokes, P.R.A. The relationship between sleep disturbance and cognitive impairment in mood disorders: A systematic review. J. Affect. Disord. 2023, 327, 207–216. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. In the Hands of Hypnos: Associations between Sleep, Cognitive Performance and Financial Capacity in aMCI and Mild AD. Sleep Sci. 2023, 16, 231–236. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Copinschi, G.; Buxton, O.; Van Cauter, E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997, 20, 865–870. [Google Scholar] [PubMed]
- Gangwisch, J.E. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes. Rev. 2009, 10 (Suppl. S2), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Watson, K.T.; Simard, J.F.; Henderson, V.W.; Nutkiewicz, L.; Lamers, F.; Nasca, C.; Rasgon, N.; Penninx, B. Incident Major Depressive Disorder Predicted by Three Measures of Insulin Resistance: A Dutch Cohort Study. Am. J. Psychiatry 2021, 178, 914–920. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Charlson, F.J.; Stapelberg, N.J.; Baxter, A.J.; Whiteford, H.A. Should global burden of disease estimates include depression as a risk factor for coronary heart disease? BMC Med. 2011, 9, 47. [Google Scholar] [CrossRef]
- Heissel, A.; Heinen, D.; Brokmeier, L.L.; Skarabis, N.; Kangas, M.; Vancampfort, D.; Stubbs, B.; Firth, J.; Ward, P.B.; Rosenbaum, S.; et al. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. Br. J. Sports Med. 2023, 57, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- La Verde, M.; Luciano, M.; Fordellone, M.; Brandi, C.; Carbone, M.; Di Vincenzo, M.; Lettieri, D.; Palma, M.; Marrapodi, M.M.; Scalzone, G.; et al. Is there a correlation between prepartum anaemia and an increased likelihood of developing postpartum depression? A prospective observational study. Arch. Gynecol. Obstet. 2024, 310, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Badfar, G.; Khalighi, Z.; Qasemi, P.; Shohani, M.; Soleymani, A.; Abbasalizadeh, S. The association between anemia and postpartum depression: A systematic review and meta-analysis. Casp. J. Intern. Med. 2019, 10, 115–124. [Google Scholar] [CrossRef]
- Maeda, Y.; Ogawa, K.; Morisaki, N.; Tachibana, Y.; Horikawa, R.; Sago, H. Association between perinatal anemia and postpartum depression: A prospective cohort study of Japanese women. Int. J. Gynaecol. Obstet. 2020, 148, 48–52. [Google Scholar] [CrossRef]
- Feiner, J.R.; Finlay-Morreale, H.E.; Toy, P.; Lieberman, J.A.; Viele, M.K.; Hopf, H.W.; Weiskopf, R.B. High oxygen partial pressure decreases anemia-induced heart rate increase equivalent to transfusion. Anesthesiology 2011, 115, 492–498. [Google Scholar] [CrossRef]
- MacCormack, J.K.; Armstrong-Carter, E.L.; Gaudier-Diaz, M.M.; Meltzer-Brody, S.; Sloan, E.K.; Lindquist, K.A.; Muscatell, K.A. β-Adrenergic Contributions to Emotion and Physiology During an Acute Psychosocial Stressor. Psychosom. Med. 2021, 83, 959–968. [Google Scholar] [CrossRef]
- Pistoia, F.; Carolei, A.; Sacco, S.; Conson, M.; Pistarini, C.; Cazzulani, B.; Stewart, J.; Franceschini, M.; Sarà, M. Contribution of Interoceptive Information to Emotional Processing: Evidence from Individuals with Spinal Cord Injury. J. Neurotrauma 2015, 32, 1981–1986. [Google Scholar] [CrossRef]
- Kingma, J.; Simard, C.; Drolet, B. Overview of Cardiac Arrhythmias and Treatment Strategies. Pharmaceuticals 2023, 16, 844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, K.N.; Han, K.D.; Han, K.M.; Min, K.; Choi, H.Y.; Choi, Y.Y.; Shim, J.; Choi, J.I.; Kim, Y.H. Association of Depression With Atrial Fibrillation in South Korean Adults. JAMA Netw. Open 2022, 5, e2141772. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Bigger, J.T.; Blumenthal, J.A.; Frasure-Smith, N.; Kaufmann, P.G.; Lespérance, F.; Mark, D.B.; Sheps, D.S.; Taylor, C.B.; Froelicher, E.S. Depression and Coronary Heart Disease. Circulation 2008, 118, 1768–1775. [Google Scholar] [CrossRef]
- Hu, M.X.; Milaneschi, Y.; Lamers, F.; Nolte, I.M.; Snieder, H.; Dolan, C.V.; Penninx, B.; de Geus, E.J.C. The association of depression and anxiety with cardiac autonomic activity: The role of confounding effects of antidepressants. Depress. Anxiety 2019, 36, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.; de Geus, E.J.; Zitman, F.G.; Hoogendijk, W.J.; van Dyck, R.; Penninx, B.W. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch. Gen. Psychiatry 2008, 65, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, L.T.; Hasegawa, T.; Nagata, K. Effects of antidepressant treatment on heart rate variability in major depression: A quantitative review. Biopsychosoc. Med. 2008, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.; de Geus, E.J.; van Dyck, R.; Penninx, B.W. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosom. Med. 2009, 71, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Lederbogen, F.; Gernoth, C.; Weber, B.; Colla, M.; Kniest, A.; Heuser, I.; Deuschle, M. Antidepressive treatment with amitriptyline and paroxetine: Comparable effects on heart rate variability. J. Clin. Psychopharmacol. 2001, 21, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Tulen, J.H.; Bruijn, J.A.; de Man, K.J.; Pepplinkhuizen, L.; van den Meiracker, A.H.; Man’t Veld, A.J. Cardiovascular variability in major depressive disorder and effects of imipramine or mirtazapine (Org 3770). J. Clin. Psychopharmacol. 1996, 16, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Volkers, A.C.; Tulen, J.H.; van den Broek, W.W.; Bruyn, J.A.; Passchier, J.; Pepplinkhuizen, L. Effects of imipramine, fluvoxamine and depressive mood on autonomic cardiac functioning in major depressive disorder. Pharmacopsychiatry 2004, 37, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.; de Geus, E.J.; van Dyck, R.; Penninx, B.W. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol. Psychiatry 2010, 68, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef]
- Imboden, C.; Gerber, M.; Beck, J.; Holsboer-Trachsler, E.; Pühse, U.; Hatzinger, M. Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. J. Affect. Disord. 2020, 276, 866–876. [Google Scholar] [CrossRef]
- Wise, T.; Robinson, O.J.; Gillan, C.M. Identifying Transdiagnostic Mechanisms in Mental Health Using Computational Factor Modeling. Biol. Psychiatry 2023, 93, 690–703. [Google Scholar] [CrossRef]
- Jenkinson, P.M.; Fotopoulou, A.; Ibañez, A.; Rossell, S. Interoception in anxiety, depression, and psychosis: A review. EClinicalMedicine 2024, 73, 102673. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Manassei, M.F.; Hamilton-Fletcher, G.; In den Bosch, Y.; Critchley, H.D.; Engels, M. Interoceptive dimensions across cardiac and respiratory axes. Phil. Trans. R. Soc. B 2016, 371, 20160014. [Google Scholar] [CrossRef]
- Hsueh, B.; Chen, R.; Jo, Y.; Tang, D.; Raffiee, M.; Kim, Y.S.; Inoue, M.; Randles, S.; Ramakrishnan, C.; Patel, S.; et al. Cardiogenic control of affective behavioural state. Nature 2023, 615, 292–299. [Google Scholar] [CrossRef]
- Ceunen, E.; Vlaeyen, J.W.; Van Diest, I. On the Origin of Interoception. Front. Psychol. 2016, 7, 743. [Google Scholar] [CrossRef]
- Candia-Rivera, D.; Catrambone, V.; Thayer, J.F.; Gentili, C.; Valenza, G. Cardiac sympathetic-vagal activity initiates a functional brain-body response to emotional arousal. Proc. Natl. Acad. Sci. USA 2022, 119, e2119599119. [Google Scholar] [CrossRef]
- Michael, J.A.; Kaur, M. The Heart-Brain Connection in Depression: Can it inform a personalised approach for repetitive transcranial magnetic stimulation (rTMS) treatment? Neurosci. Biobehav. Rev. 2021, 127, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Hawken, S.; Ounpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthi-amorn, C.; Sato, H.; Yusuf, S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Kuper, H.; Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef]
- Du, H.; Yang, L.; Hu, Z.; Zhang, H. Anxiety is associated with higher recurrence of atrial fibrillation after catheter ablation: A meta-analysis. Clin. Cardiol. 2022, 45, 243–250. [Google Scholar] [CrossRef]
- Chen, L.Y.; Ribeiro, A.L.P.; Platonov, P.G.; Cygankiewicz, I.; Soliman, E.Z.; Gorenek, B.; Ikeda, T.; Vassilikos, V.P.; Steinberg, J.S.; Varma, N.; et al. P Wave Parameters and Indices: A Critical Appraisal of Clinical Utility, Challenges, and Future Research-A Consensus Document Endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ. Arrhythm. Electrophysiol. 2022, 15, e010435. [Google Scholar] [CrossRef]
- Patel, D.; Mohanty, P.; Di Biase, L.; Sanchez, J.E.; Shaheen, M.H.; Burkhardt, J.D.; Bassouni, M.; Cummings, J.; Wang, Y.; Lewis, W.R.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm 2010, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Guillory, S.A.; Bujarski, K.A. Exploring emotions using invasive methods: Review of 60 years of human intracranial electrophysiology. Soc. Cogn. Affect. Neurosci. 2014, 9, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.P.; Wright, B.J.; Beaton, R.; Monger, K.; Raison, C.L.; Lowry, C.A.; Evans, L.; Hale, M.W. Severity of depressive symptoms moderates the sympathoinhibitory effect of local skin warming following exposure to a social stressor. Psychoneuroendocrinology 2024, 159, 106420. [Google Scholar] [CrossRef] [PubMed]
- Citrenbaum, C.; Corlier, J.; Ngo, D.; Vince-Cruz, N.; Wilson, A.; Wilke, S.; Krantz, D.; Tadayonnejad, R.; Ginder, N.; Levitt, J.; et al. Pretreatment pupillary reactivity is associated with outcome of Repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder (MDD). J. Affect. Disord. 2023, 339, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Candia-Rivera, D.; Norouzi, K.; Ramsøy, T.Z.; Valenza, G. Dynamic fluctuations in ascending heart-to-brain communication under mental stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R513–R525. [Google Scholar] [CrossRef]
- Cox, O.D.; Munjal, A.; McCall, W.V.; Miller, B.J.; Baeken, C.; Rosenquist, P.B. A review of clinical studies of electrodermal activity and transcranial magnetic stimulation. Psychiatry Res. 2023, 329, 115535. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Zhang, W. Heart–Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia. Biomedicines 2024, 12, 1719. https://doi.org/10.3390/biomedicines12081719
Fang S, Zhang W. Heart–Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia. Biomedicines. 2024; 12(8):1719. https://doi.org/10.3390/biomedicines12081719
Chicago/Turabian StyleFang, Shuping, and Wei Zhang. 2024. "Heart–Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia" Biomedicines 12, no. 8: 1719. https://doi.org/10.3390/biomedicines12081719
APA StyleFang, S., & Zhang, W. (2024). Heart–Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia. Biomedicines, 12(8), 1719. https://doi.org/10.3390/biomedicines12081719








