The Role of Bone Edema in Plantar Fasciitis Treated with Temperature-Controlled High-Energy Adjustable Multi-Mode Emission Laser (THEAL) and Exercise: A Prospective Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
- -
- The control group: treatment with stretching exercises.
- -
- The laser group: THEAL treatment (temperature-controlled high-energy adjustable multi-mode emission laser) and stretching exercises. The two treatments began together; on the same day, each patient first carried out the laser session and then the exercises.
2.1. Control Group
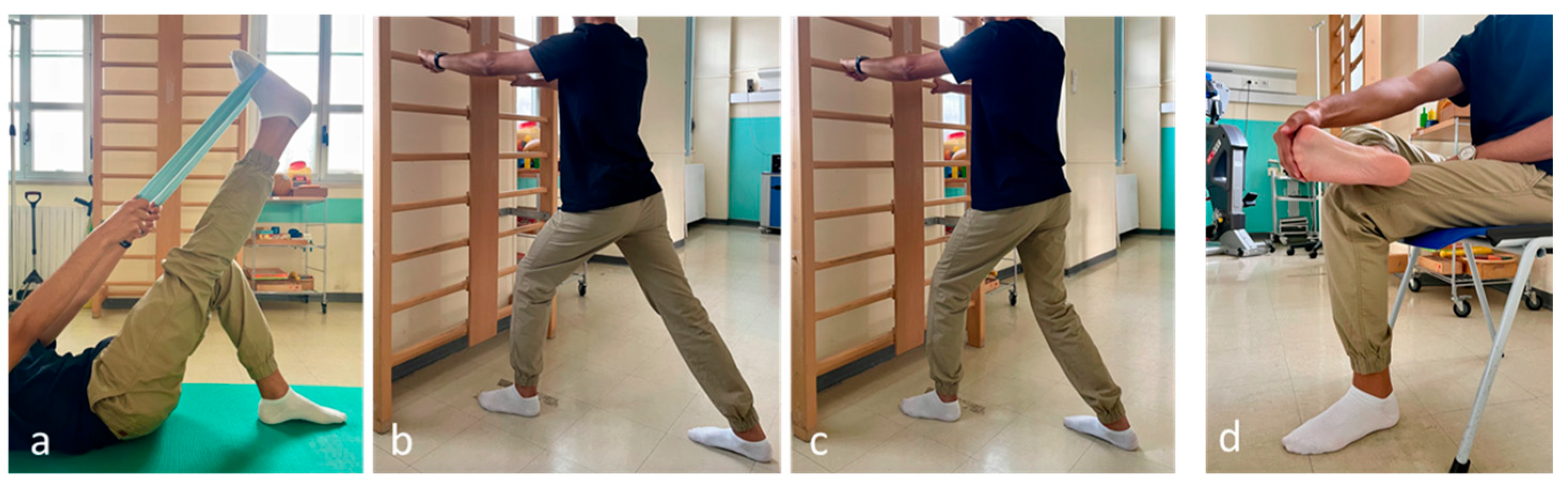
- Stretching the hamstrings and ankle plantar flexors (supine straight leg raise).
- Self-stretching of the leg muscles: the patient bends forward in a standing position with the affected foot furthest from the wall, keeping the heel on the floor.
- The soleus muscle is stretched with the knee flexed and the gastrocnemius muscle with the knee extended.
- Self-stretching of the plantar fascia: in a sitting position, the patient crosses the affected foot over the contralateral thigh and performs a passive extension of the metatarsophalangeal joints (Figure 1).
2.2. Laser Group
2.3. Sample Size Calculation
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
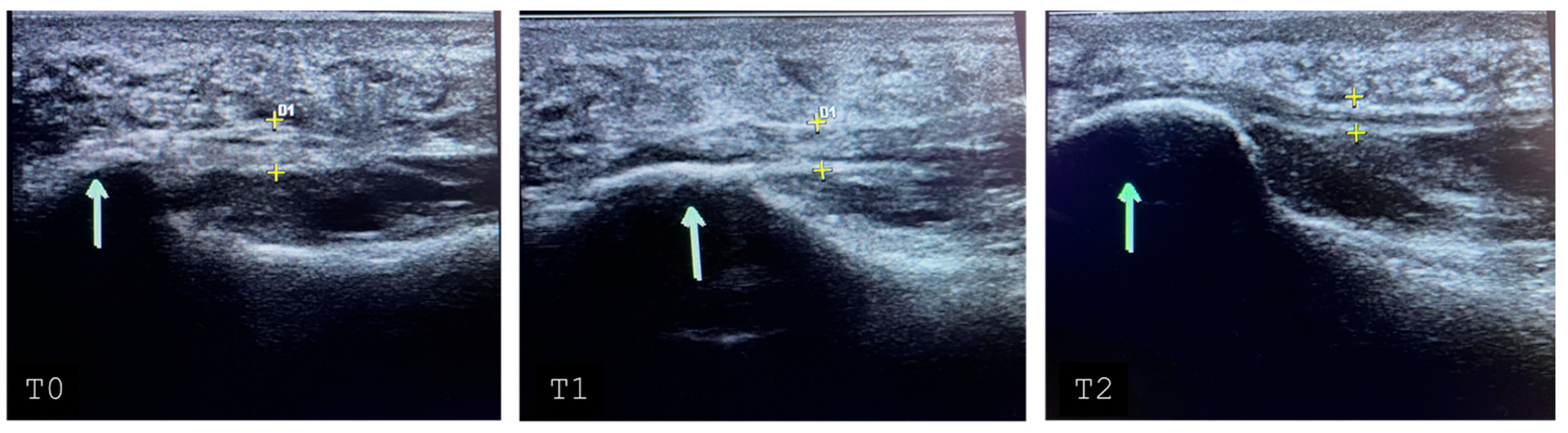
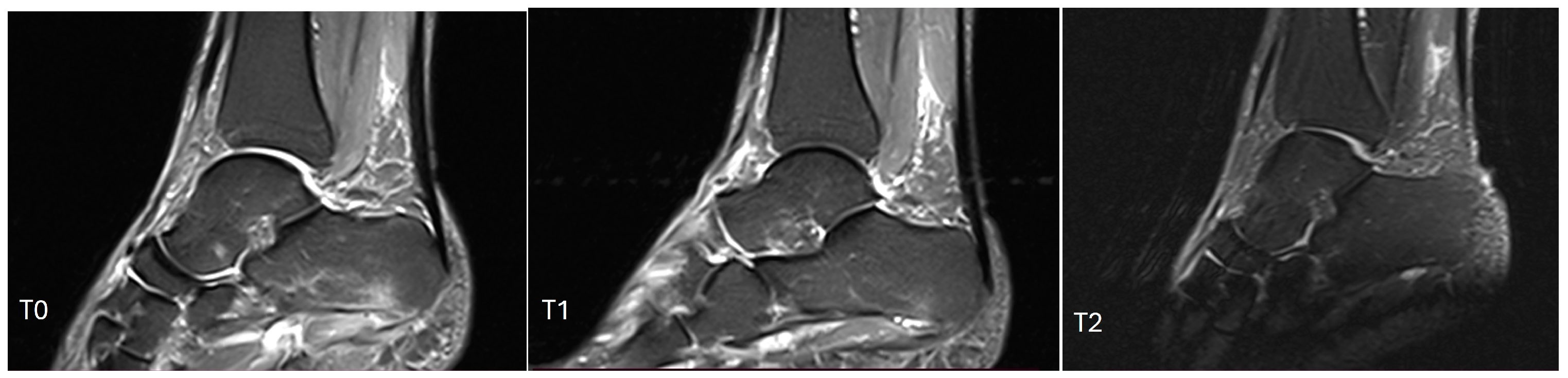
3.2. Endpoints
3.3. Inferential Statistics
3.4. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, S.E.; Dale, A.M.; Hayes, M.H.; Johnson, J.E.; McCormick, J.J.; Racette, B.A. Clinical presentation and self-reported patterns of pain and function in patients with plantar heel pain. Foot Ankle Int. 2012, 33, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Gill, T.K.; Menz, H.B.; Taylor, A.W. Prevalence and correlates of foot pain in a population-based study: The North West Adelaide health study. J. Foot Ankle Res. 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Grasel, R.P.; Schweitzer, M.E.; Kovalovich, A.M.; Karasick, D.; Wapner, K.; Hecht, P.; Wander, D. MR imaging of plantar fasciitis: Edema, tears, and occult marrow abnormalities correlated with outcome. AJR Am. J. Roentgenol. 1999, 173, 699–701. [Google Scholar] [CrossRef]
- Herber, S.; Kalden, P.; Kreitner, K.F.; Riedel, C.; Rompe, J.D.; Thelen, M. MRI in chronic epicondylitis humeri radialis using 1.0 T equipment--contrast medium administration necessary? Rofo 2001, 173, 454–459. [Google Scholar] [CrossRef]
- Berkowitz, J.F.; Kier, R.; Rudicel, S. Plantar fasciitis: MR imaging. Radiology 1991, 179, 665–667. [Google Scholar] [CrossRef]
- Sutera, R.; Iovane, A.; Sorrentino, F.; Candela, F.; Mularo, V.; La Tona, G.; Midiri, M. Plantar fascia evaluation with a dedicated magnetic resonance scanner in weight-bearing position: Our experience in patients with plantar fasciitis and in healthy volunteers. Radiol. Med. 2010, 115, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Steinborn, M.; Schmitz, C.; Stäbler, A.; Köhler, S.; Pfahler, M.; Dürr, H.R.; Refior, H.J. Extracorporeal Shock Wave Application for Chronic Plantar Fasciitis Associated with Heel Spurs: Prediction of Outcome by Magnetic Resonance Imaging. J. Rheumatol. 2000, 27, 2455–2462. [Google Scholar]
- Rhim, H.C.; Kwon, J.; Park, J.; Borg-Stein, J.; Tenforde, A.S. A Systematic Review of Systematic Reviews on the Epidemiology, Evaluation, and Treatment of Plantar Fasciitis. Life 2021, 11, 1287. [Google Scholar] [CrossRef]
- Tafur, J.; Mills, P.J. Low-intensity light therapy: Exploring the role of redox mechanisms. Photomed. Laser Surg. 2008, 26, 323–328. [Google Scholar] [CrossRef]
- Prindeze, N.J.; Moffatt, L.T.; Shupp, J.W. Mechanisms of action for light therapy: A review of molecular interactions. Exp. Biol. Med. 2012, 237, 1241–1248. [Google Scholar] [CrossRef]
- Notarnicola, A.; Covelli, I.; De Giorgi, S.; Moretti, B. High intensity laser therapy in the treatment of tendinopathy: A brief narrative review and update of current literature. Minerva Orthop. 2024, 75, 32–42. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Rifino, F.; Pesce, V.; Gallone, M.F.; Covelli, I.; Moretti, B. Short-term effect of shockwave therapy, temperature controlled high energy adjustable multi-mode emission laser or stretching in Dupuytren’s disease: A prospective randomized clinical trial. J. Biol. Regul. Homeost. Agents 2017, 31, 775–784. [Google Scholar] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Tafuri, S.; Forcignanò, M.I.; Panella, A.; Moretti, B. CHELT therapy in the treatment of chronic insertional Achilles tendinopathy. Lasers Med. Sci. 2014, 29, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- McPoil, T.G.; Martin, R.L.; Cornwall, M.W.; Wukich, D.K.; Irrgang, J.J.; Godges, J.J. Heel pain–plantar fasciitis: Clinical practice guildelines linked to the international classification of function, disability, and health from the orthopaedic section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A18. [Google Scholar] [CrossRef] [PubMed]
- Renan-Ordine, R.; Alburquerque-Sendín, F.; de Souza, D.P.; Cleland, J.A.; Fernández-de-Las-Peñas, C. Effectiveness of myofascial trigger point manual therapy combined with a self-stretching protocol for the management of plantar heel pain: A randomized controlled trial. J. Orthop. Sports Phys. Ther. 2011, 41, 43–50. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.M.; Landorf, K.B.; Cotchett, M.P.; Menz, H.B.; Gregg, J.M.; De Luca, J. Hyperemia in plantar fasciitis determined by power doppler ultrasound. J. Orthop. Sports Phys. Ther 2013, 43, 875–880. [Google Scholar] [CrossRef]
- Naruseviciute, D.; Kubilius, R. The effect of high-intensity versus low-level laser therapy in the management of plantar fasciitis: Randomized participant blind controlled trial. Clin. Rehabil. 2020, 34, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Tkocz, P.; Matusz, T.; Kosowski, Ł.; Walewicz, K.; Argier, Ł.; Kuszewski, M.; Hagner-Derengowska, M.; Ptaszkowski, K.; Dymarek, R.; Taradaj, J. A Randomised-Controlled Clinical Study Examining the Effect of High-Intensity Laser Therapy (HILT) on the Management of Painful Calcaneal Spur with Plantar Fasciitis. J. Clin. Med. 2021, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Van Nostran, W. Assessing pain intensity with the visual analog scale: A plea for uniformity. J. Clin. Pharmacol. 2014, 54, 241–244. [Google Scholar] [CrossRef]
- Budiman-Mak, E.; Conrad, K.J.; Roach, K.E. The Foot Function Index: A measure of foot pain and disability. J. Clin. Epidemiol. 1991, 44, 561–570. [Google Scholar] [CrossRef]
- Roles, N.C.; Maudsley, R.H. Radial tunnel syndrome: Resistant tennis elbow as a nerve entrapment. J. Bone Jt. Surg. Br. 1972, 54, 499–508. [Google Scholar] [CrossRef]
- Karabay, N.; Toros, T.; Hure, C. Ultrasonographic evaluation in plantar fasciitis Comparative Study. J. Foot Ankle Surg. 2007, 46, 442–446. [Google Scholar] [CrossRef]
- Ridge, S.T.; Johnson, A.W.; Mitchell, U.H.; Hunter, I.; Robinson, E.; Rich, B.S.; Brown, S.D. Foot bone marrow edema after a 10-wk transition to minimalist running shoes. Med. Sci. Sports Exerc. 2013, 45, 1363–1368. [Google Scholar] [CrossRef]
- Fernandez-Canton, G.; Casado, O.; Capelastegui, A.; Astigarraga, E.; Larena, J.A.; Merino, A. Bone marrow edema syndrome of the foot: One year follow-up with MR imaging. Skeletal Radiol. 2003, 32, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kamonseki, D.H.; Gonçalves, G.A.; Yi, L.C.; Lombardi, I.J. Effect of stretching with and without muscle strengthening exercises for the foot and hip in patients with plantar fasciitis: A randomized controlled single-blind clinical trial. Man. Ther. 2016, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ordahan, B.; Karahan, A.Y.; Kaydok, E. The effect of high-intensity versus low-level laser therapy in the management of plantar fasciitis: A randomized clinical trial. Lasers Med. Sci. 2018, 33, 1363–1369. [Google Scholar] [CrossRef]
- Yesil, H.; Dundar, U.; Toktas, H.; Eyvaz, N.; Yeşil, M. The effect of high intensity laser therapy in the management of painful calcaneal spur: A double blind, placebo-controlled study. Lasers Med. Sci. 2020, 35, 841–852. [Google Scholar] [CrossRef]
- Jarde, O.; Diebold, P.; Havet, E.; Boulu, G.; Vernois, J. Degenerative lesions of the plantar fascia: Surgical treatment by fasciectomy and excision of the heel spur. A report on 38 cases. Acta Orthop. Belg. 2003, 69, 267–274. [Google Scholar] [PubMed]
- Lemont, H.; Ammirati, K.M.; Usen, N. Plantar fasciitis: A degenerative process (fasciosis) without inflammation. J. Am. Podiatr. Med. Assoc. 2003, 93, 234–237. [Google Scholar] [CrossRef]
- Drake, C.; Whittaker, G.A.; Kaminski, M.R.; Chen, J.; Keenan, A.M.; Rathleff, M.S.; Robinson, P.; Landorf, K.B. Medical imaging for plantar heel pain: A systematic review and meta-analysis. J. Foot Ankle Res. 2022, 15, 4. [Google Scholar] [CrossRef]
- Cetin, A.; Kiratli, P.; Ceylan, E.; Sivri, A.; Dincer, F. Evaluation of chronic plantar fasciitis by scintigraphy and relation to clinical parameters. J. Musculoskelet. Pain 2001, 9, 55–61. [Google Scholar] [CrossRef]
- Sweeting, D.; Parish, B.; Hooper, L.; Chester, R. The effectiveness of manual stretching in the treatment of plantar heel pain: A systematic review. J. Foot Ankle Res. 2011, 25, 19. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J. Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef]
- Langberg, H.; Ellingsgaard, H.; Madsen, T.; Jansson, J.; Magnusson, S.P.; Aagaard, P.; Kjaer, M. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand. J. Med. Sci. Sports 2007, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Tafuri, S.; Gallone, M.F.; Moretti, L.; Moretti, B. High level laser therapy for the treatment of lower back pain: Clinical efficacy and comparison of different wavelengths. J. Biol. Regul. Homeost. Agents 2017, 30, 1157–1164. [Google Scholar]
- Hamilton, H.K.; Dover, J.S.; Arndt, K.A. Successful treatment of disfiguring hemosiderin-containing hyperpigmentation with the Q-switched 650-nm wavelength laser. JAMA Dermatol. 2014, 150, 1221–1222. [Google Scholar] [CrossRef][Green Version]
- Lopes-Martins, R.A.; Albertini, R.; Martins, P.S.; Bjordal, J.M.; FariaNeto, H.C. Spontaneous effects of low-level laser therapy (650nm) in acute inflammatory mouse pleurisy induced by Carrageenan. Photomed. Laser Surg. 2005, 23, 377–381. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Waynant, R.W.; Ilev, I.K.; Wu, X.; Barna, L.; Smith, K.; Heckert, R.; Gerst, H.; Anders, J.J. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005, 36, 171–185. [Google Scholar] [CrossRef]
- Anderson, P.R. Cutaneous Laser Surgery; Laser-Tissue Interactions; Mosby Inc.: St. Louis, MO, USA, 1999; pp. 13–18. [Google Scholar]
- Coombe, A.R.; Ho, C.T.; Darendeliler, M.A.; Hunter, N.; Philips, J.R.; Chapple, C.C.; Yum, L.W. The effects of low level laser irradiation on osteoblastic cells. Clin. Orthod. Res. 2001, 4, 3–14. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Matsumoto, M.A. Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J. Oral Rehabil. 2008, 35, 925–933. [Google Scholar] [CrossRef]
- Shibata, M.; Kodani, I.; Osaki, M.; Araki, K.; Adachi, H.; Ryoke, K.; Ito, H. Cyclo-oxygenase-1 and -2 expression in human oral mucosa, dysplasias and squamous cell carcinomas and their pathological significance. Oral Oncol. 2005, 41, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.Y.; Byun, I.H.; Yun, I.S.; Kim, J.Y.; Roh, T.S.; Lew, D.H.; Kim, Y.S. The effect of light-emitting diode (590/830 nm)-based low-level laser therapy on posttraumatic edema of facial bone fracture patients. J. Craniomaxillofac. Surg. 2017, 45, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Covelli, I.; Macchiarola, D.; Bianchi, F.P.; Cassano, G.D.; Moretti, B. The Efficacy of Temperature-Controlled High-Energy Polymodal Laser Therapy in Tendinopathy of the Shoulder. J. Clin. Med. 2023, 12, 2583. [Google Scholar] [CrossRef] [PubMed]

| Phase | Wavelength | Power | Modality of Emission | Source | Total Energy | Thermal Control |
|---|---|---|---|---|---|---|
| 1st | ||||||
| 650 nm | 1 W | CW | Small Infra-Red | 1800 J | 40–43 °C | |
| 810 nm | 2.5 W | CW | ||||
| 980 nm | 1.2 W | CW | ||||
| 1064 nm | 1.2 W | CW | ||||
| 2nd | ||||||
| 650 nm | 2 W | CW | Large Infra-Red | 1750 J | 38–42 °C | |
| 810 nm | 10 W | PBM | ||||
| 980 nm | 1 W | E2C | ||||
| 1064 nm | 1 W | E2C | ||||
| 3rd | ||||||
| 650 nm | 2 W | CW | Collimated Infra-Red | 120 J | 42 °C | |
| 810 nm | 2 W | CW | ||||
| 980 nm | 1 W | CW | ||||
| 1064 nm | 1 W | CW | ||||
| Total | - | - | - | - | 3670 J | - |
| Characteristics | n | % | |
|---|---|---|---|
| Sex | Male | 24 | 51.06 |
| Female | 23 | 48.94 | |
| Smoking habit | No | 42 | 89.36 |
| Yes | 5 | 10.64 | |
| Cardiovascular comorbidities | No | 30 | 63.83 |
| Yes | 17 | 36.17 | |
| Metabolic comorbidities | No | 31 | 65.96 |
| Yes | 16 | 34.04 | |
| Previous non-steroid anti-inflammatory therapy | No | 34 | 72.34 |
| Yes | 13 | 27.66 | |
| Previous physiotherapy | No | 31 | 65.96 |
| Yes | 16 | 34.04 | |
| Presence of bone edema | No | 23 | 48.94 |
| Yes | 24 | 51.06 | |
| Presence of spurs | No | 19 | 40.43 |
| Yes | 28 | 59.57 | |
| Laterality | Right foot | 17 | 36.17 |
| Left foot | 30 | 63.83 | |
| VAS | FFI | RM | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T1 | T2 | |
| Laser group | 7.35 ± 1.55 | 3.70 ± 2.20 | 2.78 ± 1.95 | 47.86 ± 17.22 | 25.83 ± 17.21 | 17.59 ± 15.44 | 1.83 ± 0.83 | 1.39 ± 0.66 |
| Control group | 7.33 ± 1.52 | 4.42 ± 1.77 | 3.21 ± 2.02 | 50.07 ± 16.74 | 27.69 ± 19.50 | 20.22 ± 19.21 | 2.08 ± 0.93 | 1.96 ± 1.00 |
| Overall | 7.34 ± 1.52 | 4.06 ± 2.00 | 3.00 ± 1.98 | 48.98 ± 16.83 | 26.78 ± 18.24 | 18.93 ± 17.33 | 1.96 ± 0.88 | 1.68 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covelli, I.; De Giorgi, S.; Di Lorenzo, A.; Moretti, B.; Solarino, G.; Notarnicola, A. The Role of Bone Edema in Plantar Fasciitis Treated with Temperature-Controlled High-Energy Adjustable Multi-Mode Emission Laser (THEAL) and Exercise: A Prospective Randomized Clinical Trial. Biomedicines 2024, 12, 1729. https://doi.org/10.3390/biomedicines12081729
Covelli I, De Giorgi S, Di Lorenzo A, Moretti B, Solarino G, Notarnicola A. The Role of Bone Edema in Plantar Fasciitis Treated with Temperature-Controlled High-Energy Adjustable Multi-Mode Emission Laser (THEAL) and Exercise: A Prospective Randomized Clinical Trial. Biomedicines. 2024; 12(8):1729. https://doi.org/10.3390/biomedicines12081729
Chicago/Turabian StyleCovelli, Ilaria, Silvana De Giorgi, Antonio Di Lorenzo, Biagio Moretti, Giuseppe Solarino, and Angela Notarnicola. 2024. "The Role of Bone Edema in Plantar Fasciitis Treated with Temperature-Controlled High-Energy Adjustable Multi-Mode Emission Laser (THEAL) and Exercise: A Prospective Randomized Clinical Trial" Biomedicines 12, no. 8: 1729. https://doi.org/10.3390/biomedicines12081729
APA StyleCovelli, I., De Giorgi, S., Di Lorenzo, A., Moretti, B., Solarino, G., & Notarnicola, A. (2024). The Role of Bone Edema in Plantar Fasciitis Treated with Temperature-Controlled High-Energy Adjustable Multi-Mode Emission Laser (THEAL) and Exercise: A Prospective Randomized Clinical Trial. Biomedicines, 12(8), 1729. https://doi.org/10.3390/biomedicines12081729







