Erythropoietin Effect on Complement Activation in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Flow Cytometric Analysis for CD55, CD46, and CD59 Protein Expression
2.3. C3a and C5b-9 Measurement
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
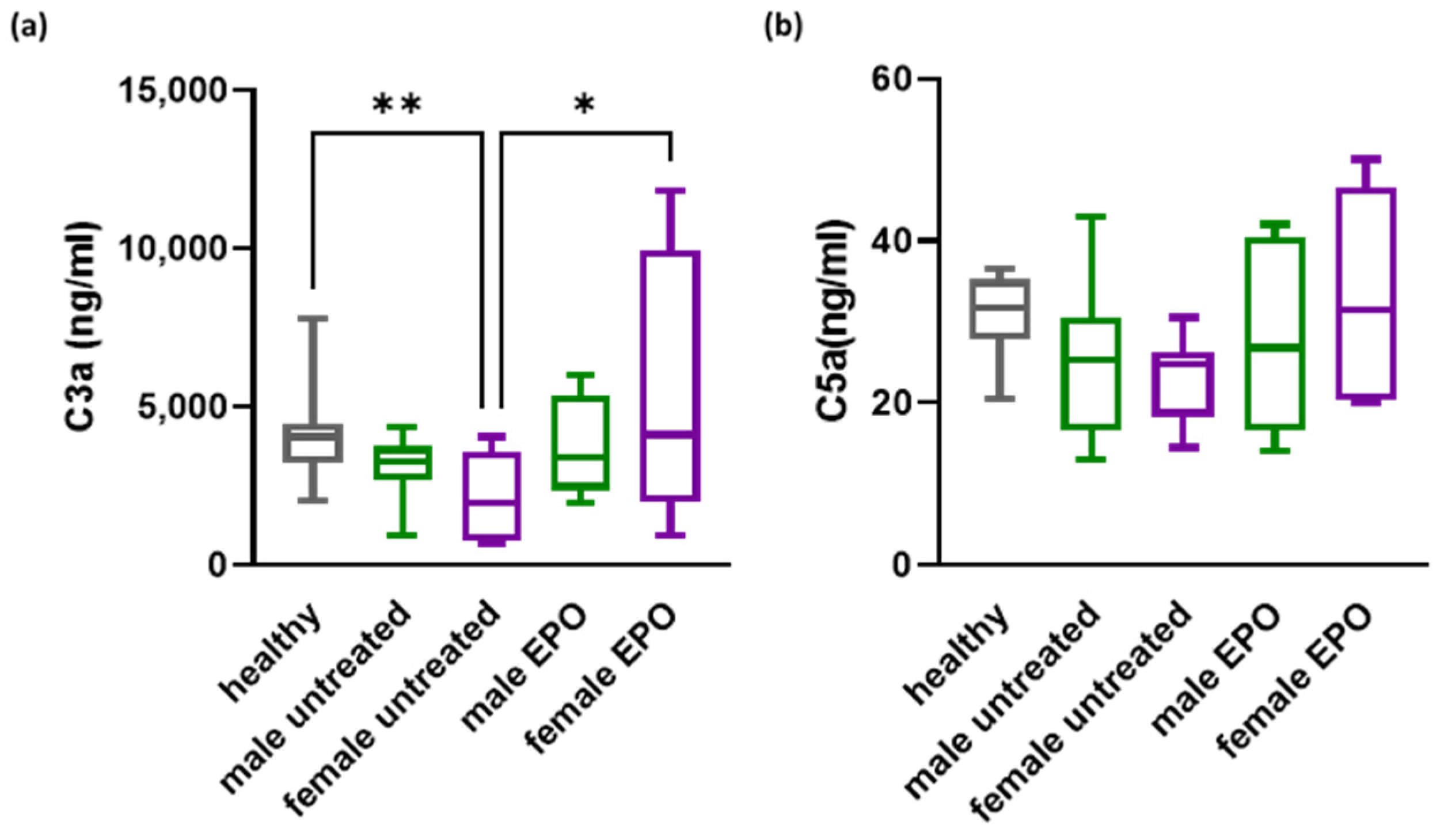
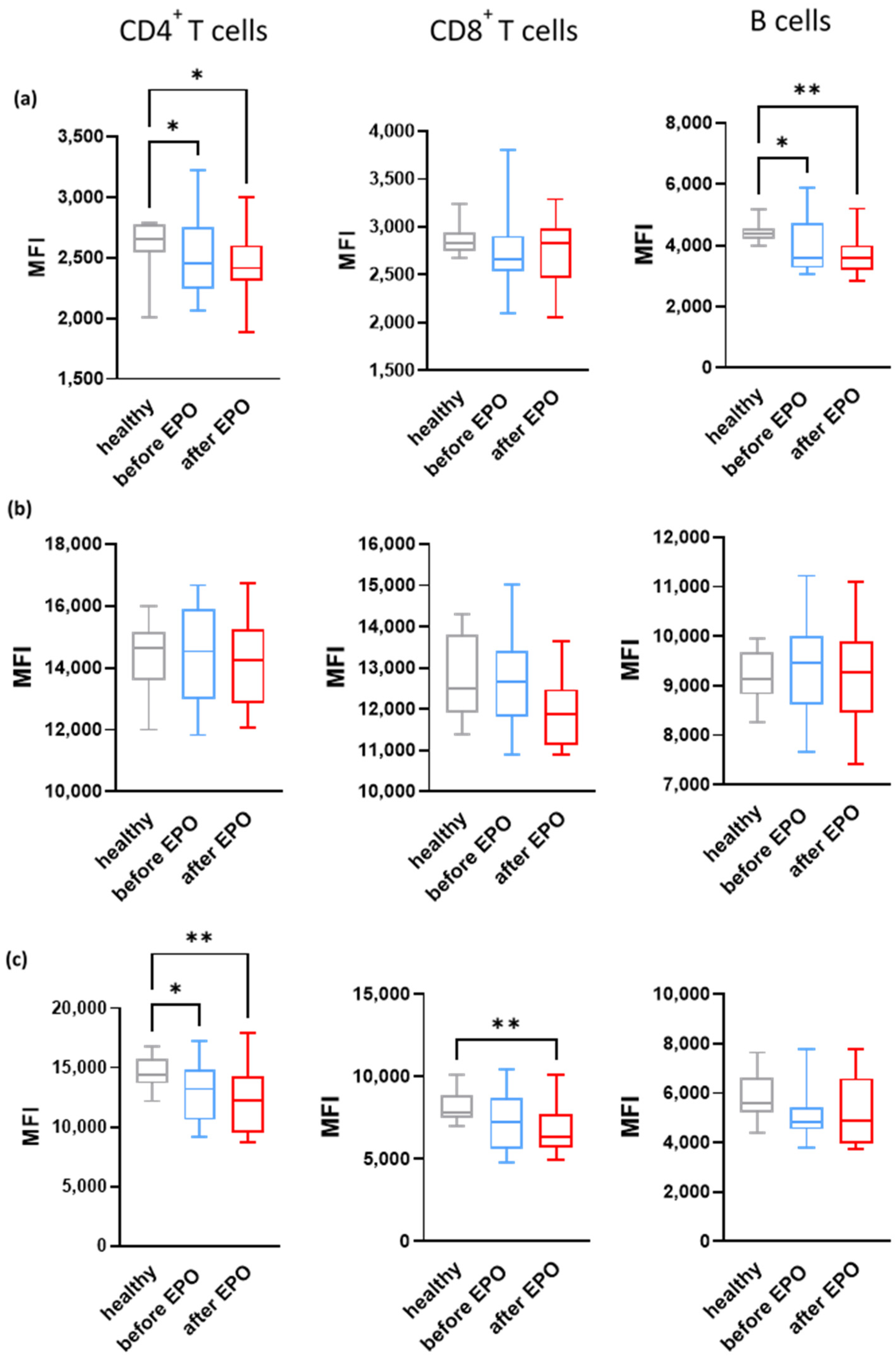
3.2. Effect of EPO on Complement in CKD Patients
3.3. Correlations of Complement Factors and Regulatory Proteins with Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Lianos, E.A.; Detsika, M.G. Immune-Related Functions of Heme Oxygenase-1. Antioxidants 2023, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Quigg, R.J.; Nicholson-Weller, A.; Cybulsky, A.V.; Badalamenti, J.; Salant, D.J. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J. Immunol. 1989, 142, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Rollins, S.A.; Sims, P.J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J. Immunol. 1990, 144, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Christmas, S.E.; de la Mata Espinosa, C.T.; Halliday, D.; Buxton, C.A.; Cummerson, J.A.; Johnson, P.M. Levels of expression of complement regulatory proteins CD46, CD55 and CD59 on resting and activated human peripheral blood leucocytes. Immunology 2006, 119, 522–528. [Google Scholar] [CrossRef]
- Nuutila, J.; Jalava-Karvinen, P.; Hohenthal, U.; Kotilainen, P.; Pelliniemi, T.T.; Nikoskelainen, J.; Lilius, E.M. Use of complement regulators, CD35, CD46, CD55, and CD59, on leukocytes as markers for diagnosis of viral and bacterial infections. Hum. Immunol. 2013, 74, 522–530. [Google Scholar] [CrossRef]
- Pickering, M.; Cook, H.T. Complement and glomerular disease: New insights. Curr. Opin. Nephrol. Hypertens. 2011, 20, 271–277. [Google Scholar] [CrossRef]
- Akiyama, S.; Imai, E.; Maruyama, S. Immunology of membranous nephropathy. F1000Research 2019, 8, 734. [Google Scholar] [CrossRef]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef]
- Provatopoulou, S.T.; Ziroyiannis, P.N. Clinical use of erythropoietin in chronic kidney disease: Outcomes and future prospects. Hippokratia 2011, 15, 109–115. [Google Scholar] [PubMed]
- Ohi, H.; Tamano, M.; Sudo, S.; Okada, N. Recombinant EPO therapy increases erythrocyte expression of complement regulatory proteins. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 41, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Eldewi, D.M.; Alhabibi, A.M.; El Sayed, H.M.E.; Mahmoud, S.A.K.; El Sadek, S.M.; Gouda, R.M.; Hassan, M.; Ibrahim, A.H.; Abd El Haliem, N.F. Expression levels of complement regulatory proteins (CD35, CD55 and CD59) on peripheral blood cells of patients with chronic kidney disease. Int. J. Gen. Med. 2019, 12, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Makrilakis, K.; Kalpourtzi, N.; Ioannidis, I.; Iraklianou, S.; Raptis, A.; Sotiropoulos, A.; Gavana, M.; Vantarakis, A.; Kantzanou, M.; Hadjichristodoulou, C.; et al. Prevalence of diabetes and pre-diabetes in Greece. Results of the First National Survey of Morbidity and Risk Factors (EMENO) study. Diabetes Res. Clin. Pract. 2021, 172, 108646. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Menti, A.; Kalpourtzi, N.; Gavana, M.; Vantarakis, A.; Chlouverakis, G.; Hajichristodoulou, C.; Trypsianis, G.; Voulgari, P.V.; Alamanos, Y.; et al. Prevalence, awareness, treatment and control of hypertension in Greece: EMENO national epidemiological study. J. Hypertens. 2021, 39, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Kidney Diseases Improving Global Outcome (KDIGO) Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 279–335. [Google Scholar]
- Detsika, M.G.; Diamanti, E.; Ampelakiotou, K.; Jahaj, E.; Tsipilis, S.; Athanasiou, N.; Dimopoulou, I.; Orfanos, S.E.; Tsirogianni, A.; Kotanidou, A. C3a and C5b-9 Differentially Predict COVID-19 Progression and Outcome. Life 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- de Seigneux, S.; Lundby, A.K.; Berchtold, L.; Berg, A.H.; Saudan, P.; Lundby, C. Increased Synthesis of Liver Erythropoietin with CKD. J. Am. Soc. Nephrol. JASN 2016, 27, 2265–2269. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, L.; Metzger, M.; Casadevall, N.; Haymann, J.P.; Karras, A.; Boffa, J.J.; Flamant, M.; Vrtovsnik, F.; Stengel, B.; Froissart, M.; et al. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2012, 7, 35–42. [Google Scholar] [CrossRef]
- Di Iorio, B.; De Nicola, L.; Bellizzi, V.; Minutolo, R.; Zamboli, P.; Rubino, R.; Fuiano, G.; Conte, G. Efficacy of erythropoietin on dialysis in patients with beta thalassemia minor. Blood Purif. 2004, 22, 453–460. [Google Scholar] [CrossRef]
- Nabil, H.; Mazighi, S.; Chettati, M.; Fadili, W.; Lisri, M.; Laouad, I. MO795: Management of Anemia in Hemodialysis Patients with Thalassaemia Minor. Nephrol. Dial. Transplant. 2022, 37, gfac081-001. [Google Scholar] [CrossRef]
- Gaya da Costa, M.; Poppelaars, F.; van Kooten, C.; Mollnes, T.E.; Tedesco, F.; Wurzner, R.; Trouw, L.A.; Truedsson, L.; Daha, M.R.; Roos, A.; et al. Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population. Front. Immunol. 2018, 9, 2664. [Google Scholar] [CrossRef]
- Sodhi, E.U.; Philpott, H.T.; Carter, M.M.; Birmingham, T.B.; Appleton, C.T. Sex-Differences and Associations Between Complement Activation and Synovial Vascularization in Patients with Late-Stage Knee Osteoarthritis. Front. Immunol. 2022, 13, 890094. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Rojo, V.M.a.A. Hypoxia-Driven Responses in Chronic Kidney Disease. Oxygen 2023, 3, 300–321. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Ploska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Astier, A.L. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology 2008, 124, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kallstrom, H.; Islam, M.S.; Berggren, P.O.; Jonsson, A.B. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 1998, 273, 21777–21782. [Google Scholar] [CrossRef]
- Killick, J.; Hay, J.; Morandi, E.; Vermeren, S.; Kari, S.; Angles, T.; Williams, A.; Damoiseaux, J.; Astier, A.L. Vitamin D/CD46 Crosstalk in Human T Cells in Multiple Sclerosis. Front. Immunol. 2020, 11, 598727. [Google Scholar] [CrossRef]


| Cause of CKD | Patients | Percentage (%) | Male | Percentage (%) | Female | Percentage (%) |
|---|---|---|---|---|---|---|
| Hypertensive nephropathy | 10 | 55.55% | 4 | 40% | 6 | 60% |
| Diabetes | 4 | 22.22% | 3 | 75% | 1 | 25% |
| Polycystic kidney disease (PKD) | 2 | 11.11% | 2 | 100% | 0 | 0% |
| Glomerulopathies | 2 | 11.11% | 1 | 50% | 1 | 50% |
| CKD Stage | ||||||
| 3b | 7 | 38.88% | 3 | 42.85% | 4 | 57.15% |
| 4 | 5 | 27.77% | 2 | 40% | 3 | 60% |
| 5 | 6 | 33.33% | 5 | 83.3% | 1 | 16.66% |
| Before EPO Treatment | After EPO Treatment | p Value | |
|---|---|---|---|
| Laboratory Baseline | |||
| Ht (%) | 30.32 ± 2.641 | 39.33 ± 2.59 | <0.0001 |
| Hb (g/dL) | 9.82 ± 1.05 | 12.55 ± 0.9122 | <0.0001 |
| Ferritin (ng/mL) | 125.5 (30.15–189.5) | 76.5 (44.25–136) | 0.391 |
| Urea (mg/dL) | 96.08 ± 41.61 | 101.6 ± 42.5 | 0.6627 |
| Creatinine (mg/dL) | 3.146 ± 2.547 | 3.302 ± 2.628 | 0.1061 |
| Albumin (g/dL) | 4.169 ± 0.361 | 4.133 ± 0.297 | 0.8402 |
| eGFR-MDRD (mL/min/1.73 m2) | 26.29 ± 13.12 | 25.83 ± 13.74 | 0.3316 |
| PTH (pg/mL) | 104 (67.53–188.8) | 140.5 (67.50–236.5) | 0.4212 |
| Ca (mg/dL) | 9.2 ± 0.4147 | 9.272 ± 0.4336 | 0.4355 |
| P (mg/dL) | 4.033 ± 0.8702 | 4.356 ± 1.030 | 0.0171 |
| 25(OH)Vitamin.D (ng/mL) | 16.87 ± 6.323 | 22.03 ± 10.24 | 0.2324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadou, V.; Ampelakiotou, K.; Grigoriou, E.; Psarra, K.; Tsirogianni, A.; Valsami, S.; Pittaras, T.; Grapsa, E.; Detsika, M.G. Erythropoietin Effect on Complement Activation in Chronic Kidney Disease. Biomedicines 2024, 12, 1746. https://doi.org/10.3390/biomedicines12081746
Athanasiadou V, Ampelakiotou K, Grigoriou E, Psarra K, Tsirogianni A, Valsami S, Pittaras T, Grapsa E, Detsika MG. Erythropoietin Effect on Complement Activation in Chronic Kidney Disease. Biomedicines. 2024; 12(8):1746. https://doi.org/10.3390/biomedicines12081746
Chicago/Turabian StyleAthanasiadou, Virginia, Kleio Ampelakiotou, Eirini Grigoriou, Katherina Psarra, Alexandra Tsirogianni, Serena Valsami, Theodoros Pittaras, Eirini Grapsa, and Maria G. Detsika. 2024. "Erythropoietin Effect on Complement Activation in Chronic Kidney Disease" Biomedicines 12, no. 8: 1746. https://doi.org/10.3390/biomedicines12081746





