Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Platelets and Platelet-Derived Extracellular Vesicles (pEVs)
2.1. Platelets
2.2. Biogenesis
2.3. Biochemistry of pEVs
2.4. Isolation of pEVs
3. Biological Functions of pEVs
3.1. pEVs in Cancer
3.2. pEVs in Viral Infection
3.3. pEVs in Wound Healing
3.4. pEVs in Osteoarthritis
3.5. pEVs in Sepsis
3.6. pEVs and Cardiovascular Diseases
3.7. pEVs in Rheumatoid Arthritis
3.8. pEV in Atherothrombosis
4. Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petroni, D.; Fabbri, C.; Babboni, S.; Menichetti, L.; Basta, G.; Del Turco, S. Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking. Pharmaceutics 2023, 15, 1639. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, E.M.; Kruglik, S.G.; Swamy, S.; Latysheva, N.; Østerud, B.; Guigner, J.M.; Sureau, F.; Bonneau, S.; Kuzmin, A.N.; Prasad, P.N.; et al. Extracellular vesicles from activated platelets possess a phospholipid-rich biomolecular profile and enhance prothrombinase activity. J. Thromb. Haemost. JTH 2024, 22, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Saberian, M.; Abak, N. Hydrogel-mediated delivery of platelet-derived exosomes: Innovations in tissue engineering. Heliyon 2024, 10, e24584. [Google Scholar] [CrossRef] [PubMed]
- An, O.; Deppermann, C. Platelet lifespan and mechanisms for clearance. Curr. Opin. Hematol. 2024, 31, 6–15. [Google Scholar] [CrossRef] [PubMed]
- El-Mortada, F.; Landelouci, K.; Bertrand-Perron, S.; Aubé, F.A.; Poirier, A.; Bidias, A.; Jourdi, G.; Welman, M.; Gantier, M.P.; Hamilton, J.R.; et al. Megakaryocytes possess a STING pathway that is transferred to platelets to potentiate activation. Life Sci. Alliance 2023, 7, e202302211. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Litvinov, R.I.; Weisel, J.W. Blood clot contraction: Mechanisms, pathophysiology, and disease. Res. Pract. Thromb. Haemost. 2022, 7, 100023. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Tsioufis, K.; Tousoulis, D. Factors Associated with Platelet Activation-Recent Pharmaceutical Approaches. Int. J. Mol. Sci. 2022, 23, 3301. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9, 716184. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.; Braun, A.; Jansen, P.; Hartmann, M. Platelet-Therapeutics to Improve Tissue Regeneration and Wound Healing-Physiological Background and Methods of Preparation. Biomedicines 2021, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. (Wars. Pol.) 2016, 11, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhou, R.; Meng, P.; Wu, L.; Yang, L.; Liu, W.; Wu, J.; Cheng, Y.; Shi, L.; Zhang, Y. Establishment of a Bernard-Soulier syndrome model in zebrafish. Haematologica 2022, 107, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Solh, T.; Botsford, A.; Solh, M. Glanzmann’s thrombasthenia: Pathogenesis, diagnosis, and current and emerging treatment options. J. Blood Med. 2015, 6, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Veuthey, L.; Aliotta, A.; Bertaggia Calderara, D.; Pereira Portela, C.; Alberio, L. Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation-A Conceptual Review Summarizing Current Knowledge. Int. J. Mol. Sci. 2022, 23, 2536. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Guo, S.C.; Zhang, C.Q. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Brassart, B.; Da Silva, J.; Donet, M.; Seurat, E.; Hague, F.; Terryn, C.; Velard, F.; Michel, J.; Ouadid-Ahidouch, H.; Monboisse, J.C.; et al. Tumour cell blebbing and extracellular vesicle shedding: Key role of matrikines and ribosomal protein SA. Br. J. Cancer 2019, 120, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Weiss, R.; Karuthedom George, S.; Fischer, M.B.; Weber, V. Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.I.; Ma, J.; Carter, C.L.; Loudig, O. Circulating Exosome Cargoes Contain Functionally Diverse Cancer Biomarkers: From Biogenesis and Function to Purification and Potential Translational Utility. Cancers 2022, 14, 3350. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.P.; Unsworth, A.J.; Gibbins, J.M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. JTH 2016, 14, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhao, T.; Song, N.; Pan, K.; Yang, Y.; Zhu, X.; Chen, P.; Zhang, J.; Xia, C. Platelets and platelet extracellular vesicles in drug delivery therapy: A review of the current status and future prospects. Front. Pharmacol. 2022, 13, 1026386. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. Platelet Extracellular Vesicles Are Taken up by Canine T Lymphocytes but Do Not Play a Role in Their Proliferation, Differentiation and Cytokine Production In Vitro. Int. J. Mol. Sci. 2022, 23, 5504. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Mojarrad, M.G.; Safa, M. Tissue factor (coagulation factor III): A potential double-edge molecule to be targeted and re-targeted toward cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Przyborowski, K.; Kurpinska, A.; Wojkowska, D.; Kaczara, P.; Suraj-Prazmowska, J.; Karolczak, K.; Malinowska, A.; Pelesz, A.; Kij, A.; Kalvins, I.; et al. Protein disulfide isomerase-A1 regulates intraplatelet reactive oxygen species-thromboxane A2 -dependent pathway in human platelets. J. Thromb. Haemost. JTH 2022, 20, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J. Current progress in growth factors and extracellular vesicles in tendon healing. Int. Wound J. 2023, 20, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Romano, R.; Bucci, C.; Marzetti, E. Extracellular Vesicles and Damage-Associated Molecular Patterns: A Pandora’s Box in Health and Disease. Front. Immunol. 2020, 11, 601740. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Carnagarin, R.; Burger, D.; Nolde, J.M.; Chan, J.; Robinson, S.; Bosio, E.; Matthews, V.B.; Schlaich, M.P. Circulating platelet-derived extracellular vesicles correlate with night-time blood pressure and vascular organ damage and may represent an integrative biomarker of vascular health. J. Clin. Hypertens. (Greenwich Conn.) 2022, 24, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Kuravi, S.J.; Harrison, P.; Rainger, G.E.; Nash, G.B. Ability of Platelet-Derived Extracellular Vesicles to Promote Neutrophil-Endothelial Cell Interactions. Inflammation 2019, 42, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Catitti, G.; Cufaro, M.C.; De Bellis, D.; Cicalini, I.; Vespa, S.; Tonelli, F.; Miscia, G.; Secondi, L.; Simeone, P.; De Laurenzi, V.; et al. Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation. Int. J. Mol. Sci. 2022, 23, 14913. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, X.; Zhou, L.; Fang, X. The value of platelet-rich plasma-derived extracellular vesicles in modern medicine. Ann. Med. 2023, 55, 2287705. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in Platelet Rich Plasma-Derived Extracellular Vesicles for Regenerative Medicine: A Systematic-Narrative Review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.; Xia, H.F.; Gong, P.; Yu, Z.L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines 2023, 11, 2798. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Xue, F.; Xu, J.; Wang, W. Platelet-rich plasma-derived extracellular vesicles inhibit NF-κB/NLRP3 pathway-mediated pyroptosis in intervertebral disc degeneration via the MALAT1/microRNA-217/SIRT1 axis. Cell. Signal. 2024, 117, 111106. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Ferreira, A.F.; Soares, M.; Santos, S.; Tomé, P.; Machado-Simões, J.; Pais, A.S.; Sousa, A.P.; Paiva, A.; Almeida-Santos, T. Optimization of Platelet-Rich Plasma Preparation for Regenerative Medicine: Comparison of Different Anticoagulants and Resuspension Media. Bioengineering 2024, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Zarà, M.; Vismara, M.; Dona, G.; Trivigno, S.M.G.; Amadio, P.; Sandrini, L.; Guidetti, G.F.; Barbieri, S.S. The Impact of Platelet Isolation Protocol on the Release of Extracellular Vesicles. Front. Biosci. (Landmark Ed.) 2022, 27, 161. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Malekian, F.; Baghban, N.; Kodam, S.P.; Ullah, M. Methodologies to Isolate and Purify Clinical Grade Extracellular Vesicles for Medical Applications. Cells 2022, 11, 186. [Google Scholar] [CrossRef]
- Wu, Q.; Fu, S.; Xiao, H.; Du, J.; Cheng, F.; Wan, S.; Zhu, H.; Li, D.; Peng, F.; Ding, X.; et al. Advances in Extracellular Vesicle Nanotechnology for Precision Theranostics. Adv. Sci. 2023, 10, e2204814. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 10–3402. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.C.; Hage, D.S. Immunoaffinity chromatography: An introduction to applications and recent developments. Bioanalysis 2010, 2, 769–790. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J. Extracell. Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Park, K.; Shin, S. Rapid and Efficient Isolation of Exosomes by Clustering and Scattering. J. Clin. Med. 2020, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Yin, H. Polymer-Based Purification of Extracellular Vesicles. Methods Mol. Biol. (Clifton N. J.) 2017, 1660, 91–103. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Winnicka, A. Platelets Extracellular Vesicles as Regulators of Cancer Progression-An Updated Perspective. Int. J. Mol. Sci. 2020, 21, 5195. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Cavallini, L.; Spinelli, C.; Yang, J.; Reis-Sobreiro, M.; de Candia, P.; Minciacchi, V.R.; Di Vizio, D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int. J. Mol. Sci. 2016, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S.; Kim, S. Shedding Light on the Cell Biology of Platelet-Derived Extracellular Vesicles and Their Biomedical Applications. Life 2023, 13, 1403. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Yin, S.; Yang, S.; Jiang, X.; Wang, J.; Zhang, M.; Zhang, L. Roles of platelets in tumor invasion and metastasis: A review. Heliyon 2022, 8, e12072. [Google Scholar] [CrossRef]
- Aspriţoiu, V.M.; Stoica, I.; Bleotu, C.; Diaconu, C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021, 9, 689962. [Google Scholar] [CrossRef] [PubMed]
- Happonen, K.E.; Tran, S.; Mörgelin, M.; Prince, R.; Calzavarini, S.; Angelillo-Scherrer, A.; Dahlbäck, B. The Gas6-Axl Protein Interaction Mediates Endothelial Uptake of Platelet Microparticles. J. Biol. Chem. 2016, 291, 10586–10601. [Google Scholar] [CrossRef] [PubMed]
- Goyette, M.A.; Côté, J.F. AXL Receptor Tyrosine Kinase as a Promising Therapeutic Target Directing Multiple Aspects of Cancer Progression and Metastasis. Cancers 2022, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, X.L.; Zhang, D.; Liu, F.; Cheng, Y.J.; Ma, Y.; Zhou, Y.J.; Zhao, Y.X. Platelet-Derived Exosomes Affect the Proliferation and Migration of Human Umbilical Vein Endothelial Cells Via miR-126. Curr. Vasc. Pharmacol. 2019, 17, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chou, M.L.; Lundy, D.J.; Chuang, E.Y.; Tseng, C.L.; Goubran, H. Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery. J. Biomed. Sci. 2023, 30, 79. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Wu, Y.W.; Blyth, C.; Lichtfuss, G.; Goubran, H.; Burnouf, T. Prospective Therapeutic Applications of Platelet Extracellular Vesicles. Trends Biotechnol. 2021, 39, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yan, X.; Pan, Y.; Wang, Y.; Wang, N.; Li, L.; Liu, Y.; Chen, X.; Zhang, C.Y.; Gu, H.; et al. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol. Cancer 2015, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tao, Z.; Chen, Y.; Lin, S.; Zhu, M.; Ji, W.; Liu, X.; Li, T.; Hu, X. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res. Treat. 2022, 193, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Anene, C.; Graham, A.M.; Boyne, J.; Roberts, W. Platelet microparticle delivered microRNA-Let-7a promotes the angiogenic switch. Biochim. Et. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Jászai, J.; Schmidt, M.H.H. Trends and Challenges in Tumor Anti-Angiogenic Therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Jiang, L.; Lin, Y.; Wu, X.; Wang, K.; He, Q.; Wang, X.; Li, W. Platelet microparticle-mediated transfer of miR-939 to epithelial ovarian cancer cells promotes epithelial to mesenchymal transition. Oncotarget 2017, 8, 97464–97475. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Qi, L.; Zhou, F.; Wang, Y.; Liu, J. MiR-939 promotes the proliferation of human ovarian cancer cells by repressing APC2 expression. Biomed. Pharmacother. 2015, 71, 64–69. [Google Scholar] [CrossRef]
- Dashevsky, O.; Varon, D.; Brill, A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int. J. Cancer 2009, 124, 1773–1777. [Google Scholar] [CrossRef]
- Vismara, M.; Negri, S.; Scolari, F.; Brunetti, V.; Trivigno, S.M.G.; Faris, P.; Galgano, L.; Soda, T.; Berra-Romani, R.; Canobbio, I.; et al. Platelet-Derived Extracellular Vesicles Stimulate Migration through Partial Remodelling of the Ca2+ Handling Machinery in MDA-MB-231 Breast Cancer Cells. Cells 2022, 11, 3120. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Feng, J.; Dong, N.; Zhou, P.; Huang, Y.; Zhang, H. Platelet-derived extracellular vesicles play an important role in platelet transfusion therapy. Platelets 2023, 34, 2242708. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Ding, J.; Dai, M.; Liu, L.; Fang, Q.; Wang, D.W.; Wu, L.; Wang, Y. Insights Into Platelet-Derived MicroRNAs in Cardiovascular and Oncologic Diseases: Potential Predictor and Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 879351. [Google Scholar] [CrossRef] [PubMed]
- Vasina, E.M.; Cauwenberghs, S.; Feijge, M.A.H.; Heemskerk, J.W.M.; Weber, C.; Koenen, R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011, 2, e233. [Google Scholar] [CrossRef]
- Vismara, M.; Manfredi, M.; Zarà, M.; Trivigno, S.M.G.; Galgano, L.; Barbieri, S.S.; Canobbio, I.; Torti, M.; Guidetti, G.F. Proteomic and functional profiling of platelet-derived extracellular vesicles released under physiological or tumor-associated conditions. Cell Death Discov. 2022, 8, 467. [Google Scholar] [CrossRef]
- Contursi, A.; Fullone, R.; Szklanna-Koszalinska, P.; Marcone, S.; Lanuti, P.; Taus, F.; Meneguzzi, A.; Turri, G.; Dovizio, M.; Bruno, A.; et al. Tumor-Educated Platelet Extracellular Vesicles: Proteomic Profiling and Crosstalk with Colorectal Cancer Cells. Cancers 2023, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, C. Platelet-derived extracellular vesicles for drug delivery. Biomater. Sci. 2023, 11, 5758–5768. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, V.; Vangapandu, C.; Savini, I.; Ventimiglia, G.; Adorno, G.; Catani, M.V. Polyunsaturated fatty acids modulate the delivery of platelet microvesicle-derived microRNAs into human breast cancer cell lines. J. Nutr. Biochem. 2019, 74, 108242. [Google Scholar] [CrossRef] [PubMed]
- Pottoo, F.H.; Iqubal, A.; Iqubal, M.K.; Salahuddin, M.; Rahman, J.U.; AlHajri, N.; Shehadeh, M. miRNAs in the Regulation of Cancer Immune Response: Effect of miRNAs on Cancer Immunotherapy. Cancers 2021, 13, 6145. [Google Scholar] [CrossRef] [PubMed]
- Sibilano, M.; Tullio, V.; Adorno, G.; Savini, I.; Gasperi, V.; Catani, M.V. Platelet-Derived miR-126-3p Directly Targets AKT2 and Exerts Anti-Tumor Effects in Breast Cancer Cells: Further Insights in Platelet-Cancer Interplay. Int. J. Mol. Sci. 2022, 23, 5484. [Google Scholar] [CrossRef]
- Ahmad, F.; Kannan, M.; Ansari, A.W. Role of SARS-CoV-2 -induced cytokines and growth factors in coagulopathy and thromboembolism. Cytokine Growth Factor Rev. 2022, 63, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Portier, I.; Campbell, R.A. Role of Platelets in Detection and Regulation of Infection. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 70–78. [Google Scholar] [CrossRef]
- Sandor-Keri, J.; Benedek, I.; Polexa, S.; Benedek, I. The Link between SARS-CoV-2 Infection, Inflammation and Hypercoagulability-Impact of Hemorheologic Alterations on Cardiovascular Mortality. J. Clin. Med. 2021, 10, 3015. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Flamand, L.; Boilard, E. Platelet extracellular vesicles in COVID-19: Potential markers and makers. J. Leukoc. Biol. 2022, 111, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Caillon, A.; Trimaille, A.; Favre, J.; Jesel, L.; Morel, O.; Kauffenstein, G. Role of neutrophils, platelets, and extracellular vesicles and their interactions in COVID-19-associated thrombopathy. J. Thromb. Haemost. JTH 2022, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, C.; Bonifay, A.; Burtey, S.; Sabatier, F.; Cauchois, R.; Abdili, E.; Arnaud, L.; Lano, G.; Pietri, L.; Robert, T.; et al. Dissemination of extreme levels of extracellular vesicles: Tissue factor activity in patients with severe COVID-19. Blood Adv. 2021, 5, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Perez-Toledo, M.; Beristain-Covarrubias, N. A new player in the game: Platelet-derived extracellular vesicles in dengue hemorrhagic fever. Platelets 2020, 31, 412–414. [Google Scholar] [CrossRef]
- Vedpathak, S.; Sharma, A.; Palkar, S.; Bhatt, V.R.; Patil, V.C.; Kakrani, A.L.; Mishra, A.; Bhosle, D.; Arankalle, V.A.; Shrivastava, S. Platelet derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients. Front. Immunol. 2024, 14, 1285162. [Google Scholar] [CrossRef] [PubMed]
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Lauková, L.; Weber, V. Activated Platelets and Platelet-Derived Extracellular Vesicles Mediate COVID-19-Associated Immunothrombosis. Front. Cell Dev. Biol. 2022, 10, 914891. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.C.; Burnouf, T.; Chiang, C.W.; Jheng, P.R.; Szunerits, S.; Yang, J.C.; Chuang, E.Y. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J. Nanobiotechnology 2023, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; De Bari, C. Osteoarthritis year in review 2023: Biology. Osteoarthr. Cartil. 2024, 32, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mi, Z.; Dong, Z.; Chen, X.; Ji, G.; Kang, H.; Li, K.; Zhao, B.; Wang, F. Platelet-Derived Exosomes Alleviate Knee Osteoarthritis by Attenuating Cartilage Degeneration and Subchondral Bone Loss. Am. J. Sports Med. 2023, 51, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Jt. Res. 2020, 9, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Guliciuc, M.; Maier, A.C.; Maier, I.M.; Kraft, A.; Cucuruzac, R.R.; Marinescu, M.; Şerban, C.; Rebegea, L.; Constantin, G.B.; Firescu, D. The Urosepsis-A Literature Review. Medicina 2021, 57, 872. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Caballero, C.L.; Del Rio-Pertuz, G.; Gomez, H. Sepsis-Associated Acute Kidney Injury. Crit. Care Clin. 2021, 37, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, D.; Lu, X.; Jiang, T.; Zhang, L.; Chen, M.; Chen, S. Platelet-derived extracellular vesicles are associated with kidney injury in patients with urosepsis. Mol. Cell. Probes 2024, 73, 101949. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, G.; Gao, Y.; Chen, Q.; Sun, S.; Mao, W.; Zhang, N.; Zhu, Z.; Wang, D.; Zhang, G.; et al. Platelet-derived extracellular vesicles aggravate septic acute kidney injury via delivering ARF6. Int. J. Biol. Sci. 2023, 19, 5055–5073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Qu, M.; Li, W.; Wu, D.; Cata, J.P.; Miao, C. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin. Transl. Med. 2023, 13, e1170. [Google Scholar] [CrossRef]
- Eustes, A.S.; Dayal, S. The Role of Platelet-Derived Extracellular Vesicles in Immune-Mediated Thrombosis. Int. J. Mol. Sci. 2022, 23, 7837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaspar, R.S.; Ferreira, P.M.; Mitchell, J.L.; Pula, G.; Gibbins, J.M. Platelet-derived extracellular vesicles express NADPH oxidase-1 (Nox-1), generate superoxide and modulate platelet function. Free Radic. Biol. Med. 2021, 165, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Al-Kassou, B.; Jamme, P.; Rolfes, V.; Steffen, E.; Bulic, M.; Hosen, M.R.; Goody, P.R.; Tiyerili, V.; Zimmer, S.; et al. Large extracellular vesicles in the left atrial appendage in patients with atrial fibrillation-the missing link? Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2022, 111, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Chimen, M.; Evryviadou, A.; Box, C.L.; Harrison, M.J.; Hazeldine, J.; Dib, L.H.; Kuravi, S.J.; Payne, H.; Price, J.M.J.; Kavanagh, D.; et al. Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica 2020, 105, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Vats, R.; Brzoska, T.; Bennewitz, M.F.; Jimenez, M.A.; Pradhan-Sundd, T.; Tutuncuoglu, E.; Jonassaint, J.; Gutierrez, E.; Watkins, S.C.; Shiva, S.; et al. Platelet Extracellular Vesicles Drive Inflammasome-IL-1β-Dependent Lung Injury in Sickle Cell Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Tessandier, N.; Melki, I.; Cloutier, N.; Allaeys, I.; Miszta, A.; Tan, S.; Milasan, A.; Michel, S.; Benmoussa, A.; Lévesque, T.; et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padró, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell. Mol. Life Sci. CMLS 2022, 79, 190. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.R.; Alexander, W.; Hassoune, A.; Chen, Q.; Brzoska, T.; Alvikas, J.; Liu, Y.; Haldeman, S.; Plautz, W.; Loughran, P.; et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J. Thromb. Haemost. JTH 2019, 17, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fan, Q.; Xu, J.; Bai, J.; Han, X.; Dong, Z.; Zhou, X.; Liu, Z.; Gu, Z.; Wang, C. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter 2020, 3, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Böing, A.N.; Filipiak, K.J.; Nieuwland, R. Platelet extracellular vesicles as biomarkers for arterial thrombosis. Platelets 2017, 28, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Beetler, D.J.; Bruno, K.A.; Watkins, M.M.; Xu, V.; Chekuri, I.; Giresi, P.; Di Florio, D.N.; Whelan, E.R.; Edenfield, B.H.; Walker, S.A.; et al. Reconstituted Extracellular Vesicles from Human Platelets Decrease Viral Myocarditis in Mice. Small 2023, 19, e2303317. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Nolde, J.M.; Carnagarin, R.; Burger, D.; Chan, J.; Robinson, S.; Bosio, E.; Matthews, V.B.; Schlaich, M.P. Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation. Int. J. Mol. Sci. 2022, 23, 10524. [Google Scholar] [CrossRef]
- Ostermeier, B.; Soriano-Sarabia, N.; Maggirwar, S.B. Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy. Int. J. Mol. Sci. 2022, 23, 2321. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, J.; Wang, W.; Chen, B.; Chen, J.; Shen, Z.; Hou, J.; Mei, Y.; Liu, S.; Zhang, L.; et al. Platelet extracellular vesicles enhance the proangiogenic potential of adipose-derived stem cells in vivo and in vitro. Stem Cell Res. Ther. 2021, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, M.; Liu, Q.; Yu, A.; Cheng, K.; Ma, J.; Murphy, S.; McNutt, P.M.; Zhang, Y. Insights into optimizing exosome therapies for acute skin wound healing and other tissue repair. Front. Med. 2024, 18, 258–284. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Mirshahvaladi, S.; Kashani, S.A.; Paulo, J.A.; Amirkhani, A.; Mehryab, F.; Seydi, H.; Moradpour, N.; Jodeiryjabarzade, S.; Mirzaei, M.; et al. Proteomic profiling of mesenchymal stem cell-derived extracellular vesicles: Impact of isolation methods on protein cargo. J. Extracell. Biol. 2024, 3, e159. [Google Scholar] [CrossRef] [PubMed]


| Feature | Platelet-Rich Plasma Extracellular Vesicles (PRP-EVs) | Platelet-Derived Extracellular Vesicles (pEVs) |
|---|---|---|
| Origin | Derived from platelet-rich plasma obtained by centrifugation of whole blood [34] | Produced by activated platelets during physiological or pathological processes [23] |
| Formation | Formed intracellularly within bilayer lipid bodies [34] | Released from platelets in response to various triggers such as Ca2+ ionophore, ADP, thrombin, collagen, and epinephrine [24] |
| Common Surface Markers | CD9, CD63, CD81, HSP101 [34] | CD9, CD63, TSG101, PDCD6IP (ALIX) [32,33] |
| Key Clotting Factors | - | Tissue factor (TF), factor Va (FVa), factor VIII (FVIII) [27] |
| Adhesion Molecules | CD31, CD41, CD42a, CD61, CD62p, CD63 and PS, GPIbα [34] | CD41/61 (GPIIb/IIIa), CD31, CD62P (P-selectin) [28] |
| Enzymes | Thrombin, matrix metalloproteinases, alkaline phosphatase [35] | Heparanase, protein disulfide isomerase (PDI), NADPH oxidase 1, glutathione peroxidase, alkaline phosphatase, phospholipase A2 [28] |
| Bioactive Lipids | Ceramides, phosphatidylserine [36] | Lysophospholipids (LPLs), glycerolipids (GLs), phospholipids (PLs), sphingolipids (SLs), L-carnitine, arachidonic acid (AA), thromboxane A2 (TXA2) [28] |
| Growth Factors | PDGF, TGF-β, VEGF, IL-1β, TNF-α [36] | TGF-β1, PDGF, VEGF [29] |
| Chemokines | PF4, PPBP, CCL5 (RANTES) [36] | CXCR1, PF4, PPBP [30] |
| Immune Response Molecules | IL-1β, IL-6, IL-8, IL-10, CXCL1, CXCL2, CXCL8 [36] | CCL5, CCL23, IL-1β, complement components (C5b-9) [30] |
| Components Related to Apoptosis | Caspase, Bcl-2 family, Apaf-1 [31] | Phosphatidylserine, caspase-3, caspase-9, DAMPs [31] |
| Signaling Pathways | Wnt/β-catenin, AKT/ERK, PI3K/Akt, Keap1-Nrf2 [36] | TGF-beta, NF-κB, MAPK/ERK, PI3K/Akt, Wnt/β-catenin, Notch [36] |
| Functions | Supports tissue regeneration and healing Reduces inflammation, apoptosis, oxidative stress, and senescence [36] | Clotting, immune response, programmed cell death, growth factor signaling, adhesion [25] |
| Applications | Tissue regeneration, healing [37,38] | Diagnostics, therapeutic applications in cardiovascular disorders, cancer, regenerative medicine [37,38] |
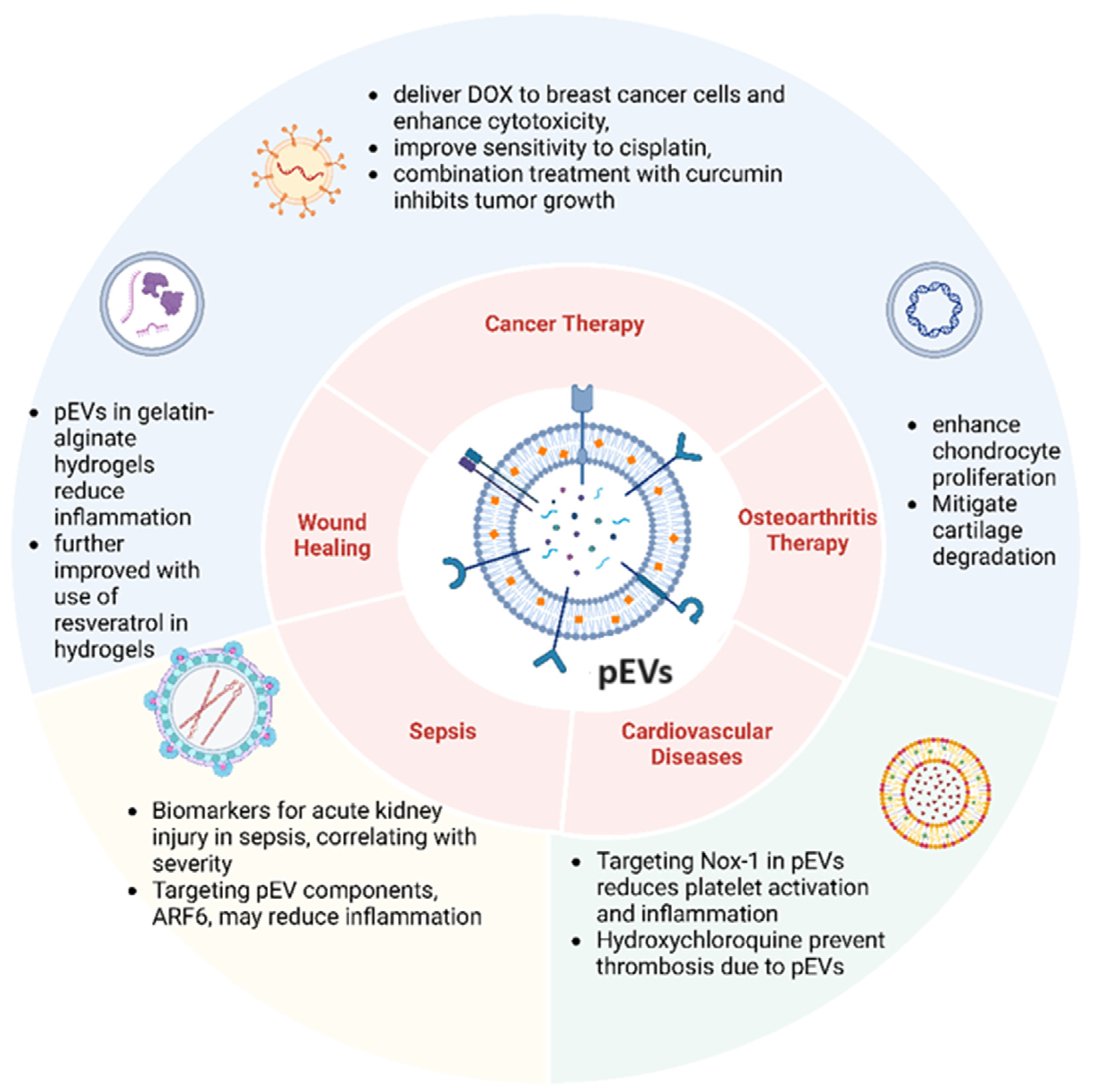
| Disease/Condition | Role of pEVs | Potential Therapeutic Application | References |
|---|---|---|---|
| Cancer | pEVs contribute to tumor growth, metastasis, angiogenesis, and epithelial-to-mesenchymal transition, and they transfer bioactive molecules to cancer cells and the tumor microenvironment. | Targeting pEV pathways or specific miRNAs for treatment; drug-loaded pEVs for targeted therapy. | [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] |
| Viral Infection (COVID-19) | Elevated pEV levels observed in patients, linked to blood clotting issues and inflammation. | Potential as diagnostic biomarkers; targeting pEVs to mitigate inflammatory and thrombotic responses. | [81,82,83,84,85,86] |
| Viral Infection (Dengue) | pEVs promote neutrophil activity and inflammation, disrupt blood vessel integrity, and increase vascular leakage. | Investigating pEVs as diagnostic tools and therapeutic targets to manage inflammation and vascular damage. | [87,88,89] |
| Wound Healing | pEV-loaded hydrogels promote wound healing by scavenging reactive oxygen species and modulating immune responses. | Developing pEV-loaded hydrogels for chronic wound treatment, particularly in diabetic patients. | [90,91,92] |
| Osteoarthritis (OA) | pEVs enhance chondrocyte function and cartilage regeneration, alleviate symptoms by promoting chondrocyte proliferation and migration. | Developing pEV-based therapies for cartilage regeneration and OA treatment. | [93,94,95] |
| Sepsis | pEVs contribute to inflammation, apoptosis, oxidative stress, and endothelial dysfunction in sepsis, leading to kidney injury and worsened outcomes. | Targeting pEVs to mitigate sepsis-related kidney injury and inflammatory responses. | [96,97,98,99,100,101] |
| Cardiovascular Diseases | pEVs drive platelet activation, superoxide generation, monocyte recruitment, and thrombo-inflammatory conditions. | Inhibiting pEV pathways to reduce platelet activation and control inflammatory responses. | [102,103,104,105] |
| Rheumatoid Arthritis (RA) | pEVs spread inflammatory signals by transporting miRNAs and cytokines, contributing to disease progression and cardiovascular risks. | Investigating pEVs as therapeutic targets to manage inflammation and reduce cardiovascular risks in RA patients. | [106] |
| Atherothrombosis | pEVs carry stress-related proteins and modulate redox states, facilitating thrombus formation and platelet activation. | Targeting proteins carried by pEVs to prevent atherothrombosis progression; using hydroxychloroquine to reduce pEV-induced thrombosis risks. | [107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines 2024, 12, 1850. https://doi.org/10.3390/biomedicines12081850
Muttiah B, Ng SL, Lokanathan Y, Ng MH, Law JX. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines. 2024; 12(8):1850. https://doi.org/10.3390/biomedicines12081850
Chicago/Turabian StyleMuttiah, Barathan, Sook Luan Ng, Yogeswaran Lokanathan, Min Hwei Ng, and Jia Xian Law. 2024. "Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles" Biomedicines 12, no. 8: 1850. https://doi.org/10.3390/biomedicines12081850








