Influence of Metamizole on Antitumour Activity of Risedronate Sodium in In Vitro Studies on Canine (D-17) and Human (U-2 OS) Osteosarcoma Cell Lines
Abstract
1. Introduction
- primary or secondary;
- osteoblastic, chondroblastic, fibroblastic, or mixed;
- extraosseous or intraosseous (central, periosteal, paraosteal);
- low or high grade;
- classic, telangiectatic, or small cell.
- amputation of the affected limb, resection of the affected tissues, or less traumatic limb-sparing methods;
- adjuvant chemotherapy using platinum derivatives (cisplatin and carboplatin), doxorubicin or, less commonly, etoposide;
- in humans, neoadjuvant chemotherapy is sometimes used, which involves administering chemotherapy not after but before surgery.
2. Materials and Methods
2.1. Cell Cultures and Selected Drug Preparations
2.2. Selected Drugs
2.3. MTT Assay—Cell Viability Assessment
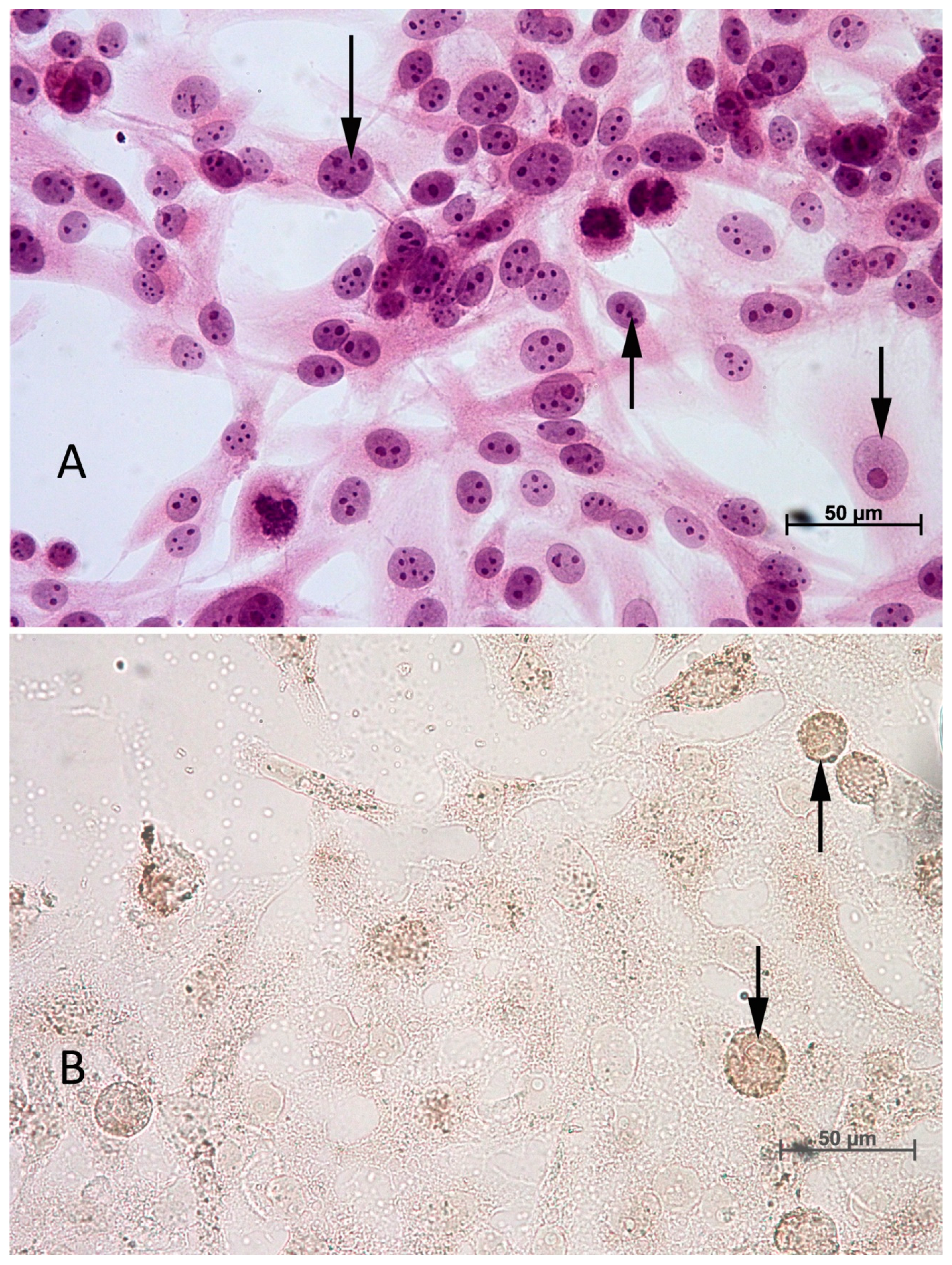
2.4. TUNEL Method—Assessing the Percentage of Apoptotic Cells
2.5. Flow Cytometry—Cell Cycle Assessment
2.6. Statistical Analysis
3. Results
3.1. EC50 Values
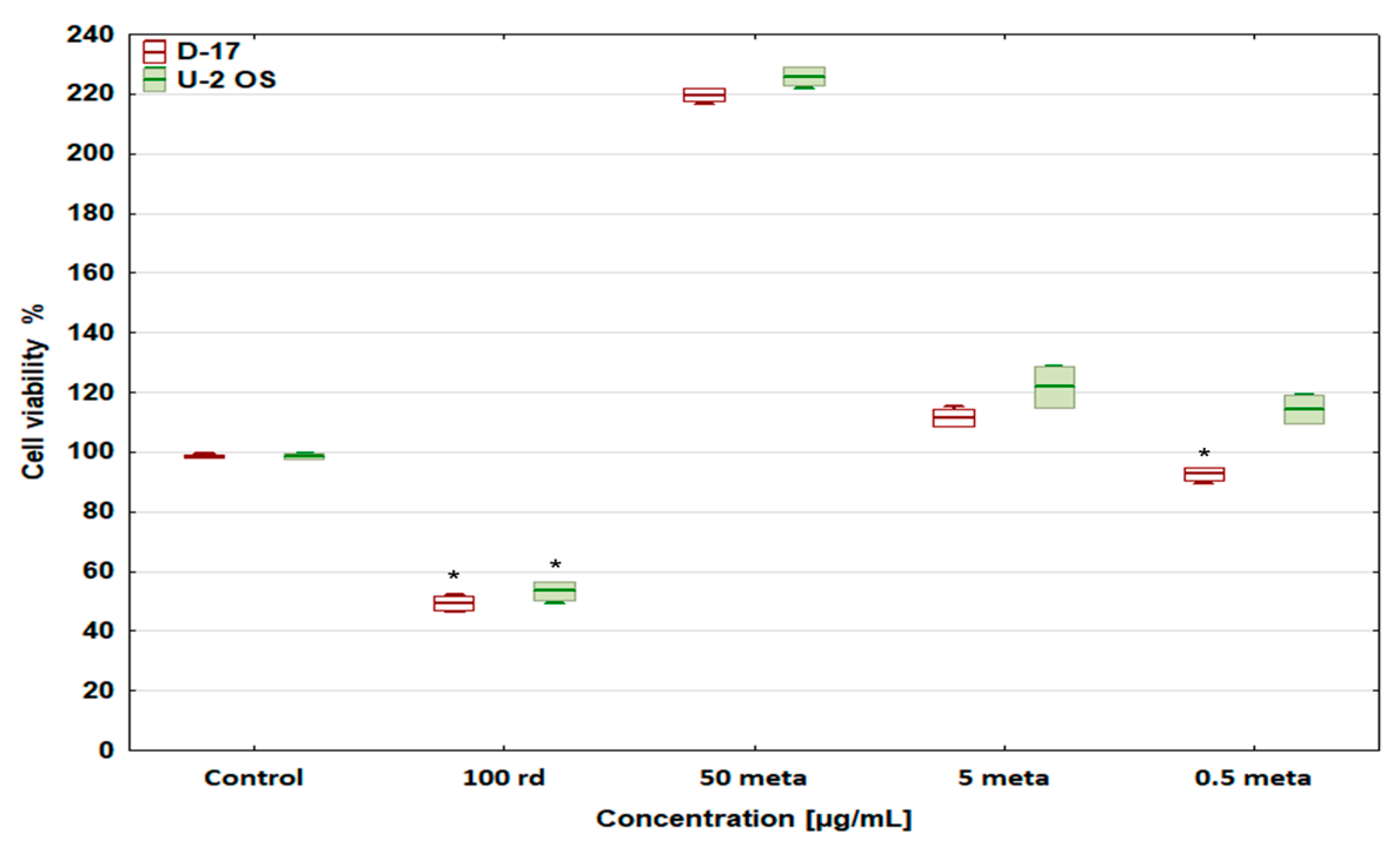
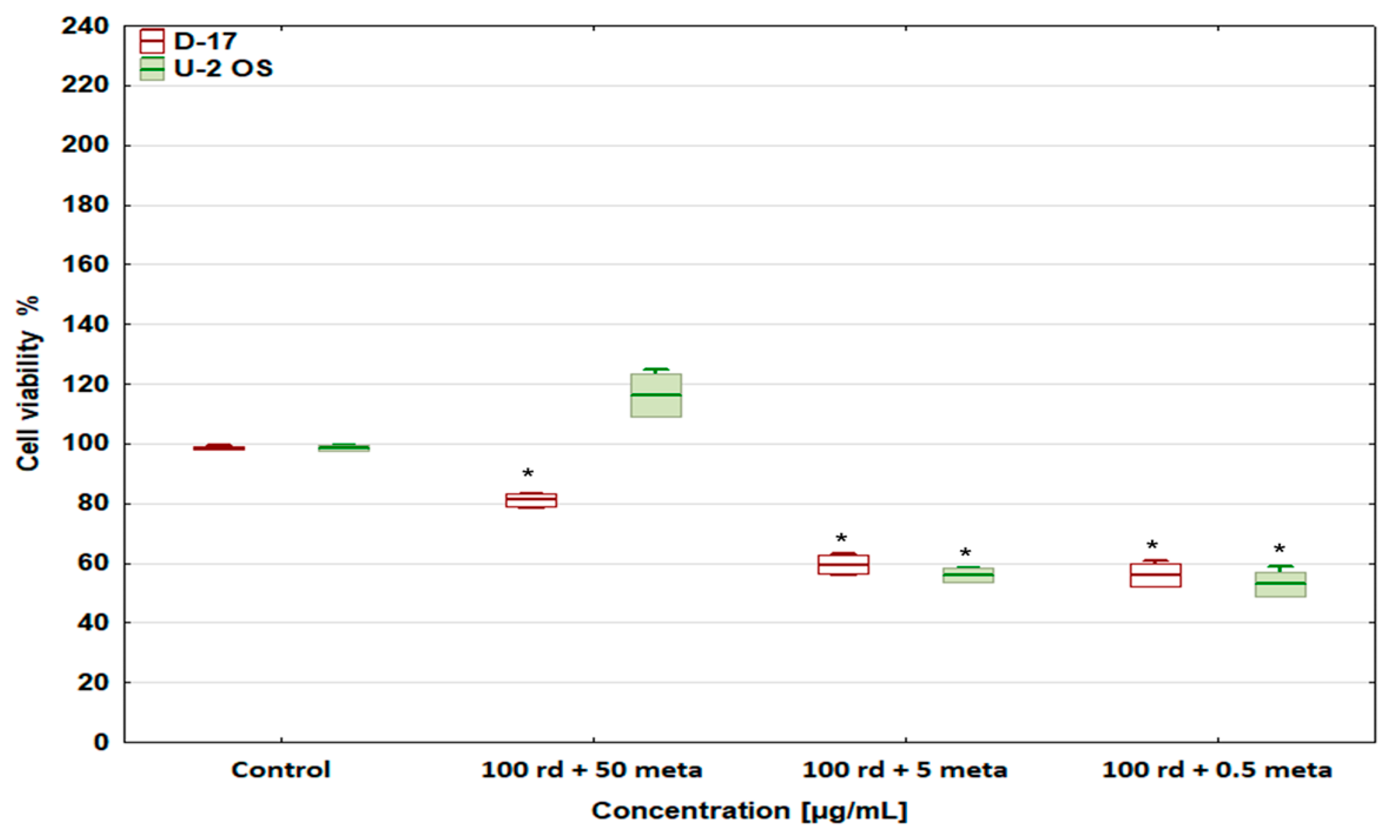
3.2. Cell Viability
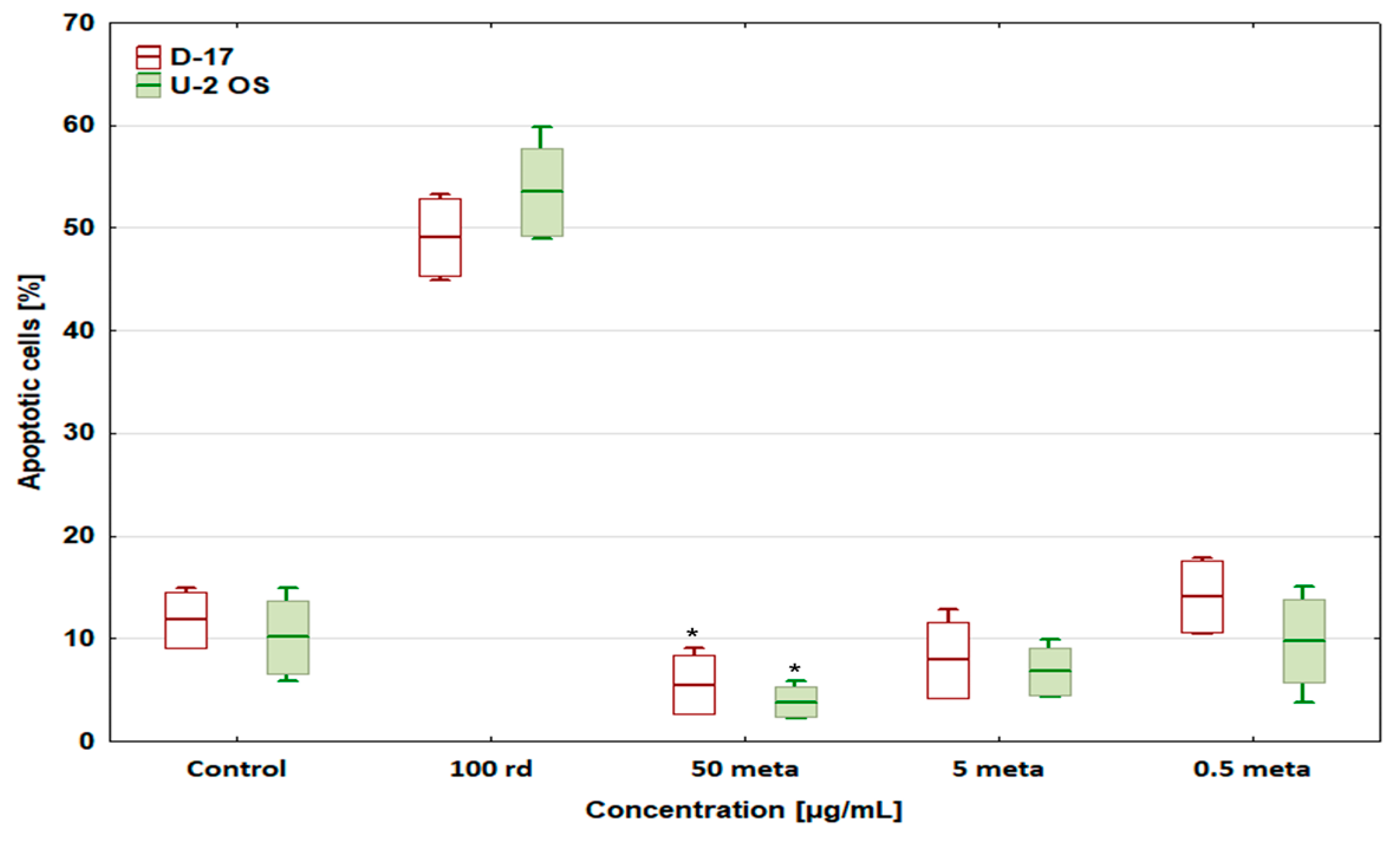
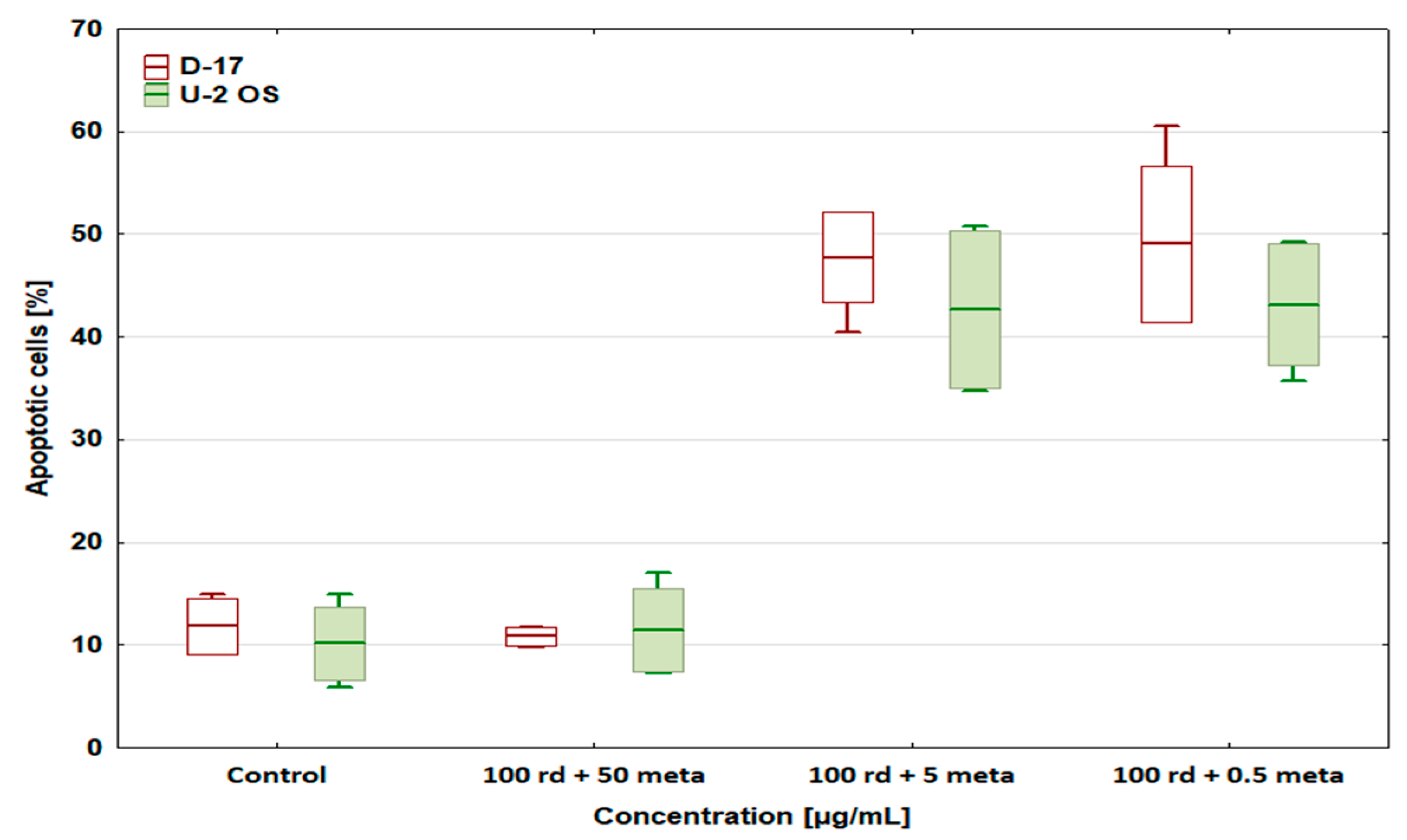
3.3. Percentage of Apoptotic Cells
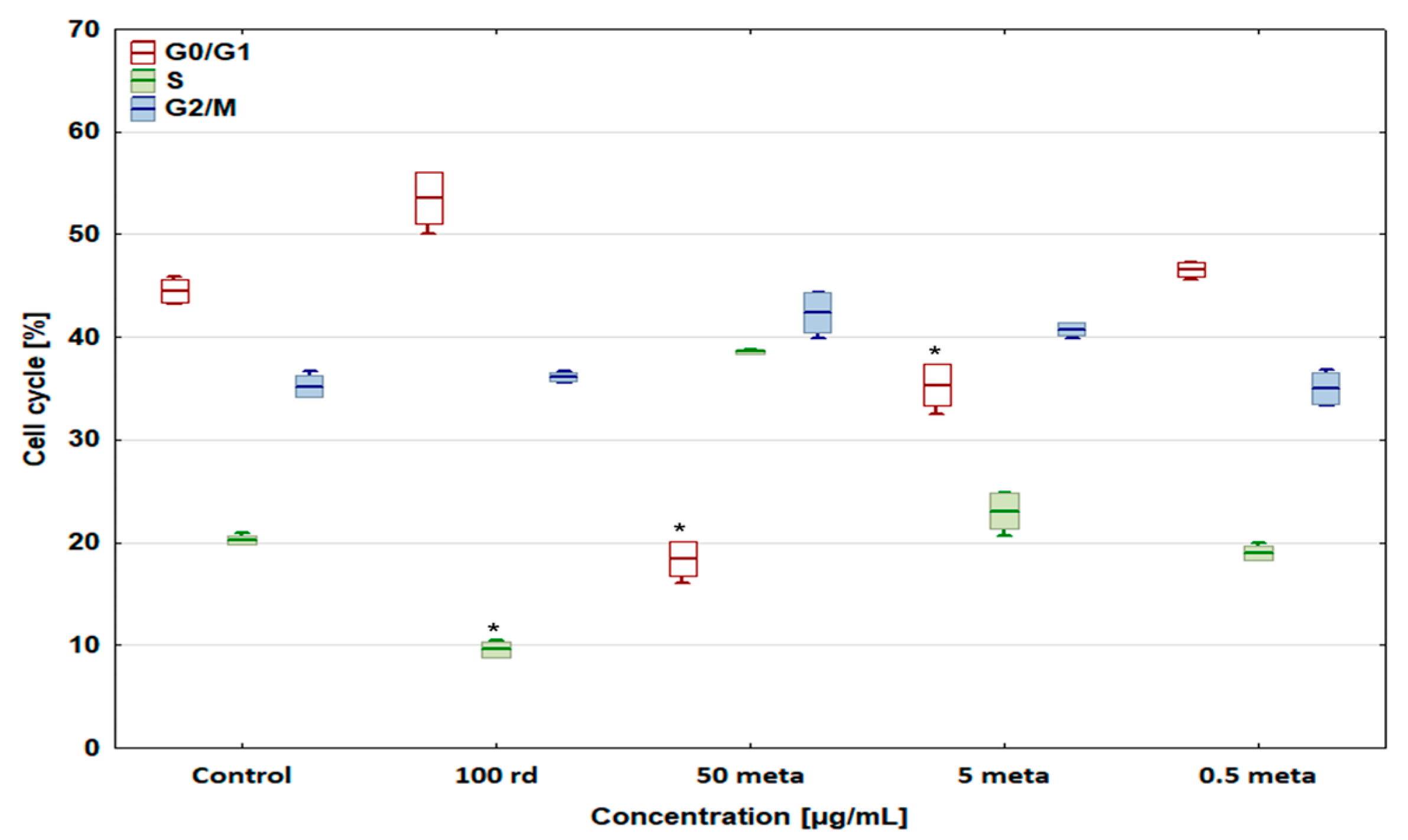
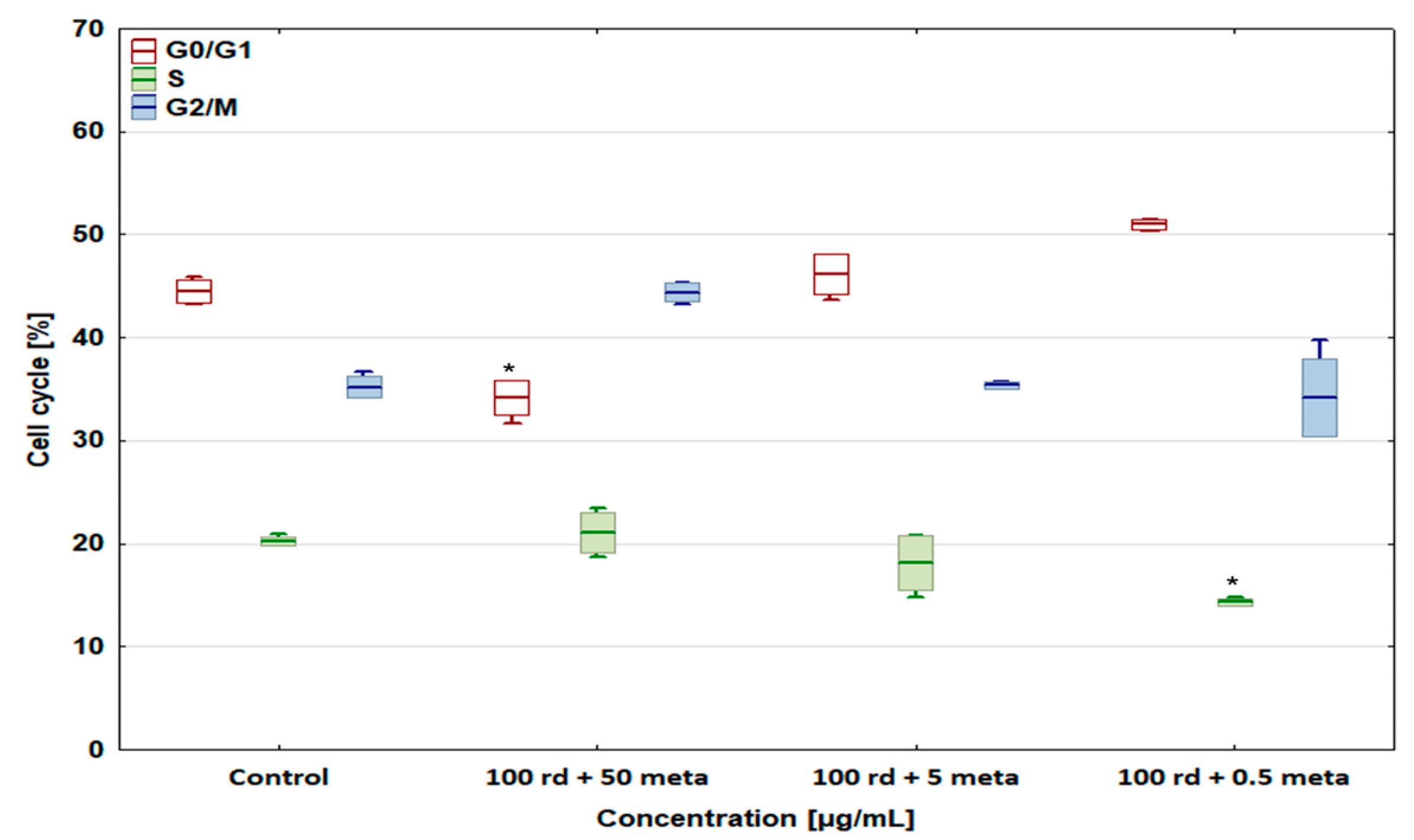
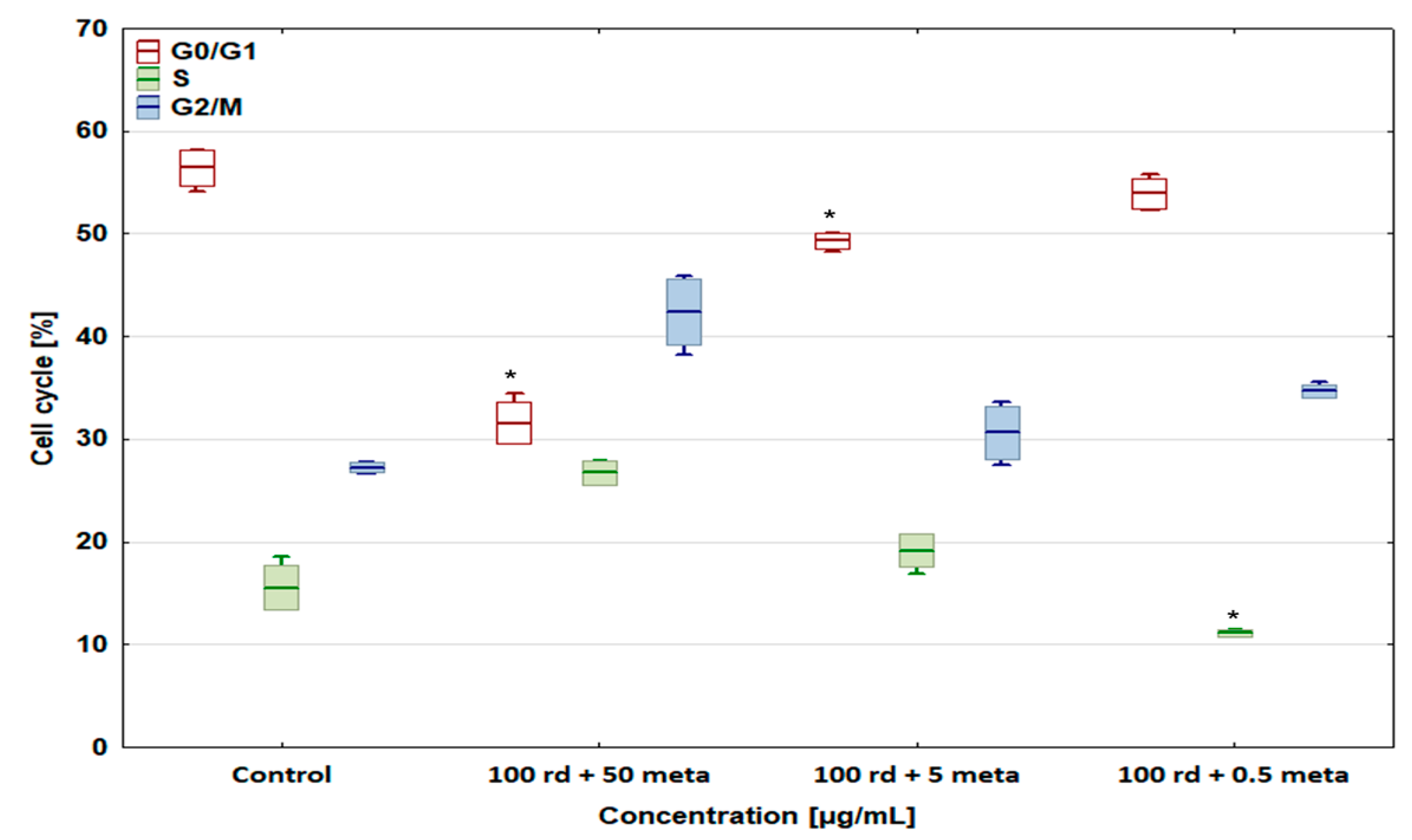
3.4. Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO classification of soft tissue tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Slayter, M.V.; Boosinger, T.R.; Pool, R.R.; Dammrich, K.; Misdrop, W.; Larsen, S.; World Health Organization. International Histologic Classification of Tumors of Domestic Animals, Histological Classification of Bone and Joint Tumors of Domestic Animals, 1st ed.; Armed Forces Institute of Pathology American Registry of Pathology: Washington, DC, USA, 1994. [Google Scholar]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.K.; Wood, G.A.; Viloria-Petit, A.M. Recent advances in the discovery of biomarkers for canine osteosarcoma. Front. Vet. Sci. 2021, 8, 734965. [Google Scholar] [CrossRef]
- Szewczyk, M.; Lechowski, R.; Zabielska, K. What do we know about canine osteosarcoma treatment?—Review. Vet. Res. Commun. 2015, 39, 61–67. [Google Scholar] [CrossRef]
- Tuohy, J.L.; Shaevitz, M.H.; Garrett, L.D.; Ruple, A.; Selmic, L.E. Demographic characteristics, site and phylogenetic distribution of dogs with appendicular osteosarcoma: 744 dogs (2000–2015). PLoS ONE 2019, 14, e0223243. [Google Scholar] [CrossRef]
- Simpson, S.; Rizvanov, A.A.; Jeyapalan, J.N.; de Brot, S.; Rutland, C.S. Canine osteosarcoma in comparative oncology: Molecular mechanisms through to treatment discovery. Front. Vet. Sci. 2022, 9, 965391. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar]
- Picci, P. Osteosarcoma (Osteogenic sarcoma). Orphanet J. Rare Dis. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk factors for development of canine and human osteosarcoma: A comparative review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Ru, G.; Terracini, B.; Glickman, L.T. Host related risk factors for canine osteosarcoma. Vet. J. 1998, 156, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Legare, M.E.; Bush, J.; Ashley, A.K.; Kato, T.; Hanneman, W.H. Cellular and phenotypic characterization of canine osteosarcoma cell lines. J. Cancer 2011, 2, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar]
- Gârjoabă, I.; Tudor, N.; Soare, T.; Tănase, A.; Alistar, A.; Vlăgioiu, C. A study on the prevalence of skeletal osteosarcoma in dogs and cats. Lucr. Sti. Med. Vet. 2009, 42, 102–106. [Google Scholar]
- Withrow, S.J.; Vail, M.D. Withrow and Macewen’s Small Animal Clinical Oncology, Saunders, 4th ed.; Saunders: Denver, CO, USA, 2007. [Google Scholar]
- Selvarajah, G.T.; Kirpensteijn, J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet. J. 2010, 185, 28–35. [Google Scholar] [CrossRef]
- Burk, R.; Feeney, A. Small Animal Radiology and Ultrasonography a Diagnostic Atlas and Text, 3rd ed.; Saunders: Denver, CO, USA, 2003. [Google Scholar]
- Holmberg, B.J.; Farese, J.P.; Taylor, D.; Uhl, E.W. Osteosarcoma of the humeral head associated with osteocondritis dissecans in a dog. J. Am. Anim. Hosp. Assoc. 2004, 40, 246–249. [Google Scholar] [CrossRef]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Choong, P.F.M.; Dunstan, D.E. Osteosarcoma treatment: State of the art. Cancer Metastasis Rev. 2009, 28, 247–263. [Google Scholar] [CrossRef]
- Li, J.; Luan, F.; Song, J.; Dong, J.; Shang, M. Clinical efficacy of controlled-release morphine tablets combined with celecoxib in pain management and the effects on WNK1 expression. Clinics 2021, 76, e1907. [Google Scholar] [CrossRef]
- Schneider, G.; Voltz, R.; Gaertner, J. Cancer pain management and bone metastases: An Update for the Clinician. Breast Care 2012, 7, 113–120. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Pain Relief, 1st ed.; World Health Press: Geneva, Switzerland, 1986. [Google Scholar]
- Silva, L.C.; Castor, M.G.; Navarro, L.C.; Romero, T.R.; Duarte, I.D. Kappa-opioid receptor participates of NSAIDs peripheral antinociception. Neurosci. Lett. 2016, 622, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Cascorbi, I. The Uncertainties of Metamizole Use. Clin. Pharmacol. Ther. 2021, 109, 1373–1375. [Google Scholar] [CrossRef]
- Sznejder, H.; Amand, C.; Stewart, A.; Salazar, R.; Scala, W.A.R. Real world evidence of the use of metamizole (dipyrone) by the Brazilian population. A retrospective cohort with over 380,000 patients. Einstein 2022, 20, eAO6353. [Google Scholar] [CrossRef]
- Russell, R.G.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Suva, L.J.; Cooper, A.; Watts, A.E.; Ebetino, F.H.; Price, J.; Gaddy, D. Bisphosphonates in veterinary medicine: The new horizon for use. Bone 2021, 142, 115711. [Google Scholar] [CrossRef]
- Farese, J.P.; Ashton, J.; Milner, R.; Ambrose, L.L.; Van Gilder, J. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell. Dev. Biol. Anim. 2004, 40, 113–117. [Google Scholar] [CrossRef]
- Poirier, V.J.; Huelsmeyer, M.K.; Kurzman, I.D.; Thamm, D.H.; Vail, D.M. The bisphosphonates alendronate and zoledronate are inhibitors of canine and human osteosarcoma cell growth in vitro. Vet. Comp. Oncol. 2003, 1, 207–215. [Google Scholar] [CrossRef]
- Poradowski, D.; Chrószcz, A.; Spychaj, R.; Onar, V. Potential cytoprotective and anti-apoptotic effect of metamizole alone and in combination with cytostatic drugs observed in vitro in canine (D-17) and human (U-2 OS) osteosarcoma cell lines. Biomedicines 2024, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 10993-5: 2012; Biological Evaluation of Medical Devices. Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2012.
- Poradowski, D.; Chrószcz, A.; Obmińska-Mrukowicz, B. Synergistic antitumor interaction of risedronate sodium and standard anticancer agents in canine (D-17) and human osteosarcoma (U-2 OS) cell lines. Animals 2022, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Akgun, F.S.; Sirin, D.Y.; Yilmaz, I.; Karaarslan, N.; Ozbek, H.; Simsek, A.T.; Kaya, Y.E.; Kaplan, N.; Akyuva, Y.; Caliskan, T.; et al. Investigation of the effect of dipyrone on cells isolated from intervertebral disc tissue. Exp. Ther. Med. 2019, 18, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Pompeia, C.; Boaventura, M.F.; Curi, R. Antiapoptotic effect of dipyrone on HL-60, Jurkat and Raji cell lines submitted to UV irradiation, arachidonic acid and cycloheximide treatments. Int. Immunopharmacol. 2001, 1, 2173–2182. [Google Scholar] [CrossRef] [PubMed]

| Drug Name | ||
|---|---|---|
| Risedronate Sodium (rd) | Metamizole (meta) | |
| Concentration (µg/mL) | 300 | 50 |
| 150 | 20 | |
| 100 | 10 | |
| 30 | 5 | |
| 15 | 1 | |
| 10 | 0.5 | |
| 3 | 0.1 | |
| 1.5 | ||
| 1 | ||
| EC50 (µg/mL) | ||
|---|---|---|
| Drug Name | D-17 | U-2 OS |
| Risedronate sodium | 144.83 ± 6.22 µg/mL | 98.1 ± 5.4 µg/mL |
| Metamizole | >100 µg/mL | >100 µg/mL |
| Drug Combinations (µg/mL) |
|---|
| 100 rd + 50 meta |
| 100 rd + 5 meta |
| 100 rd + 0.5 meta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poradowski, D.; Chrószcz, A.; Spychaj, R.; Wolińska, J.; Onar, V. Influence of Metamizole on Antitumour Activity of Risedronate Sodium in In Vitro Studies on Canine (D-17) and Human (U-2 OS) Osteosarcoma Cell Lines. Biomedicines 2024, 12, 1869. https://doi.org/10.3390/biomedicines12081869
Poradowski D, Chrószcz A, Spychaj R, Wolińska J, Onar V. Influence of Metamizole on Antitumour Activity of Risedronate Sodium in In Vitro Studies on Canine (D-17) and Human (U-2 OS) Osteosarcoma Cell Lines. Biomedicines. 2024; 12(8):1869. https://doi.org/10.3390/biomedicines12081869
Chicago/Turabian StylePoradowski, Dominik, Aleksander Chrószcz, Radosław Spychaj, Joanna Wolińska, and Vedat Onar. 2024. "Influence of Metamizole on Antitumour Activity of Risedronate Sodium in In Vitro Studies on Canine (D-17) and Human (U-2 OS) Osteosarcoma Cell Lines" Biomedicines 12, no. 8: 1869. https://doi.org/10.3390/biomedicines12081869
APA StylePoradowski, D., Chrószcz, A., Spychaj, R., Wolińska, J., & Onar, V. (2024). Influence of Metamizole on Antitumour Activity of Risedronate Sodium in In Vitro Studies on Canine (D-17) and Human (U-2 OS) Osteosarcoma Cell Lines. Biomedicines, 12(8), 1869. https://doi.org/10.3390/biomedicines12081869







