Molecular Hydrogen and Extracorporeal Gas Exchange: A Match Made in Heaven? An In Vitro Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Donors
- should be healthy adults
- should be capable of donating > 300 mL of blood without complications
- should have an average hematocrit Hct ≥ 42%
- should not have experienced acute pyretic or other inflammatory incidents recently
- should not have any blood-associated conditions
- should not have a prescription for anticoagulation medication, etc.
2.2. In Vitro Inflammation
2.3. Experimental Setup
2.4. Test Protocol
2.5. Biomarkers of Inflammation and Oxidative Stress
2.6. Statistics
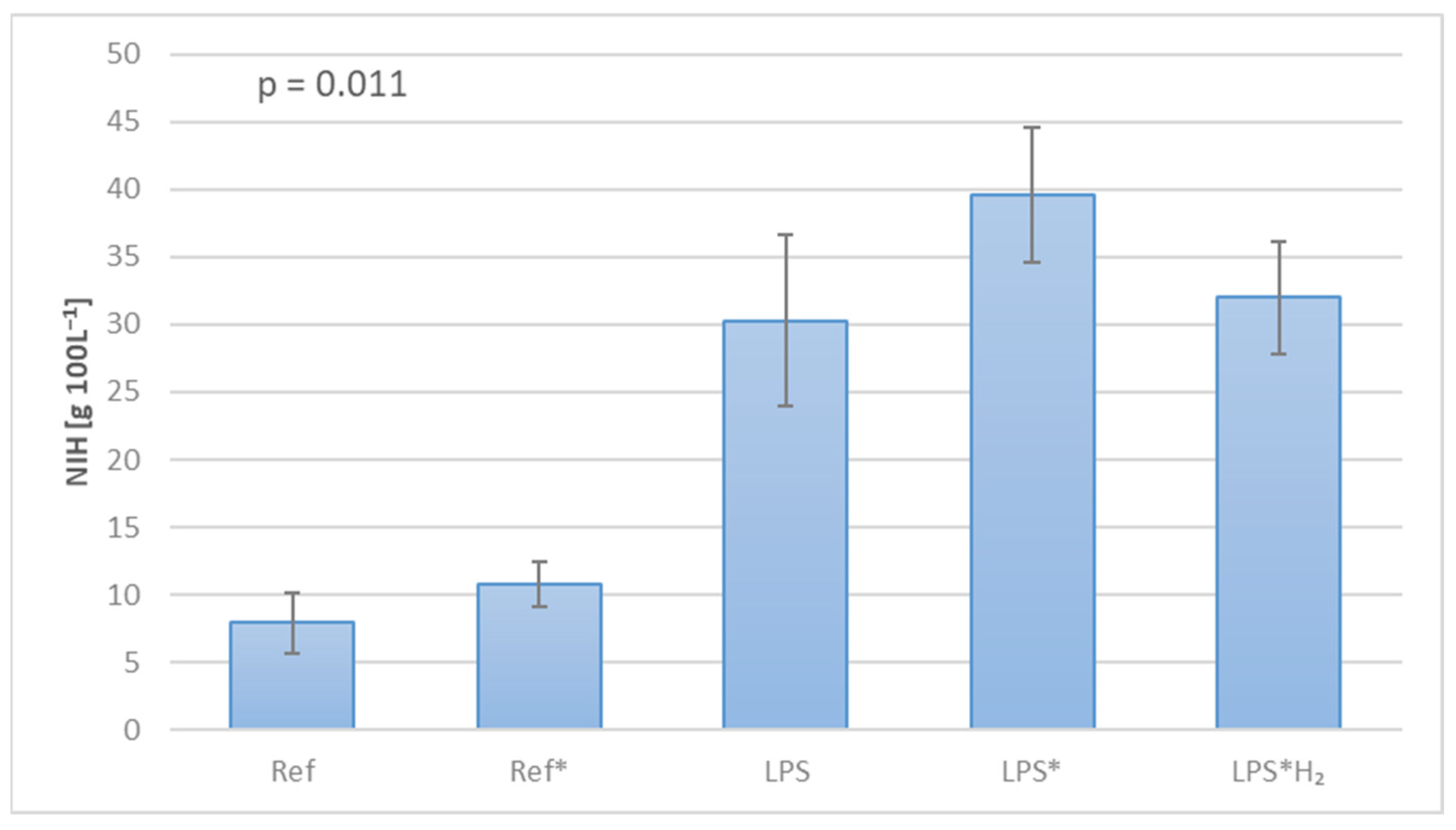
3. Results
4. Discussion
- MCP-1 levels remain practically unchanged throughout the investigation in all the circuits. This may indicate a low cell count that hampers any noticeable observation in MCP-1 expression when blood stems from healthy donors. The mild concentration increase in the LPS systems confirms the low number of cells being triggered.
- The majority of circuits exhibit a tendency towards the concentration in the Control with regard to MPO measurements. The concentration difference between the C and Ref/LPS systems leaves a small margin for H2 to act, similar to MCP-1. The slight variance between the different setups points towards time-dependent cell activation rather than any shear rate-induced stress. Nevertheless, these findings might provide a clue concerning in vivo investigations, where cell variability occurs over time, and in vivo activation mechanisms come into effect, potentially allowing for better evaluation of hydrogen’s efficacy.
- TRX1 expression does not seem to be affected by H2 as it is with MPO. This agrees with the fact that TRX1 and MPO are associated with anti-inflammatory and pro-inflammatory activity, respectively. Hence, lower MPO concentrations correspond to higher ones for TRX1.
- Varying levels of MDA expression can be witnessed among the systems, suggesting a combination of time-associated and mechanical stress. The inflammatory response to LPS is once again countered by the treatment with H2, although not to baseline levels. This agrees with Huang’s findings, where lower MDA concentrations were observed in the hydrogen-treated lungs (as opposed to the nitrogen-treated ones) [17,18].
- IL-6: apparent string inhibition by H2. In contrast to MDA, the unambiguously strong IL-6 suppression makes a compelling argument concerning hydrogen’s anti-inflammatory action and the specific pathways being inhibited, as already reported [43]. Furthermore, as with MPO, IL-6 also has pro-inflammatory characteristics, and since it is so heavily suppressed, the anti-inflammatory activity might naturally be reinforced (i.e., TRX1).
- the impact of blood volume on biomarker expression
- a wider spectrum of biomarkers
- the influence of hydrogen’s concentration on its curative efficacy
- hydrogen’s antioxidant and anti-inflammatory action at different administration patterns (e.g., continuous, intermittent, or delayed)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Firmin, R.K. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J. 1999, 45, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Kang, H.; Cheng, Y.; Su, X.; Wang, B. Inflammatory Progression in Patients Undergoing Extracorporeal Membrane Oxygenation. Curr. Mol. Med. 2024, 24, 844–855. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Jadzic, D.; Popovic Krneta, M.; Tauber, H.; Treml, B. Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2022, 11, 5147. [Google Scholar] [CrossRef] [PubMed]
- Durak, K.; Zayat, R.; Grottke, O.; Dreher, M.; Autschbach, R.; Marx, G.; Marx, N.; Spillner, J.; Kalverkamp, S.; Kersten, A. Extracorporeal membrane oxygenation in patients with COVID-19: 1-year experience. J. Thorac. Dis. 2021, 13, 5911–5924. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, T.; Hatam, N.; Durak, K.; Grottke, O.; Dreher, M.; Nubbemeyer, K.; Rossaint, R.; Marx, G.; Marx, N.; Spillner, J.; et al. Outcomes of Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome in COVID-19 Patients: A Propensity-Matched Analysis. J. Clin. Med. 2021, 10, 2547. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- McILwain, R.B.; Timpa, J.G.; Kurundkar, A.R.; Holt, D.W.; Kelly, D.R.; Hartman, Y.E.; Neel, M.L.; Karnatak, R.K.; Schelonka, R.L.; Anantharamaiah, G.M.; et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 2010, 90, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Yu, Y.; Zhang, Z.; Liu, W.; Pei, Y.; Xiong, L.; Hou, L.; Wang, G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock 2010, 34, 495–501. [Google Scholar] [CrossRef]
- Qi, B.; Yu, Y.; Wang, Y.; Wang, Y.; Yu, Y.; Xie, K. Perspective of Molecular Hydrogen in the Treatment of Sepsis. Curr. Pharm. Des. 2021, 27, 667–678. [Google Scholar] [CrossRef]
- Datzmann, T.; Träger, K. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 2018, 10 (Suppl. S5), S653–S660. [Google Scholar] [CrossRef]
- Sano, M.; Suzuki, M.; Homma, K.; Hayashida, K.; Tamura, T.; Matsuoka, T.; Katsumata, Y.; Onuki, S.; Sasaki, J. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med. Surg. 2017, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kabayama, S.; Nakano, H.; Zhu, W.J.; Terawaki, H.; Nakayama, K.; Katoh, K.; Satoh, T.; Ito, S. Biological effects of electrolyzed water in hemodialysis. Nephron Clin. Pract. 2009, 112, c9–c15. [Google Scholar] [CrossRef]
- Nakayama, M.; Kabayama, S.; Miyazaki, M. Application of Electrolyzed Hydrogen Water for Management of Chronic Kidney Disease and Dialysis Treatment-Perspective View. Antioxidants 2024, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Fu, Z. Molecular hydrogen is a potential protective agent in the management of acute lung injury. Mol. Med. 2022, 28, 27. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Lee, S.; Tochigi, N.; Shigemura, N.; Buchholz, B.M.; Kloke, J.D.; Billiar, T.R.; Toyoda, Y.; Nakao, A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit. Care 2010, 14, R234. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Kawamura, T.; Peng, X.; Tochigi, N.; Shigemura, N.; Billiar, T.R.; Nakao, A.; Toyoda, Y. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem. Biophys. Res. Commun. 2011, 408, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Becker, L.B.; Choudhary, R.C.; Takegawa, R.; Shoaib, M.; Shinozaki, K.; Endo, Y.; Homma, K.; Rolston, D.M.; Eguchi, S.; et al. Hydrogen gas with extracorporeal cardiopulmonary resuscitation improves survival after prolonged cardiac arrest in rats. J. Transl. Med. 2021, 19, 462. [Google Scholar] [CrossRef]
- Alwazeer, D.; Liu, F.F.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxid. Med. Cell Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef]
- Russell, G.; Rehman, M.; LeBaron, T.W.; Veal, D.; Adukwu, E.; Hancock, J. An overview of SARS-CoV-2 (COVID-19) infection and the importance of molecular hydrogen as an adjunctive therapy. React. Oxyg. Species 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Xu, K. Hydrogen-Oxygen Inhalation for Treatment of COVID-19: With Commentary from Zhong Nanshan; World Scientific: Singapore, 2020. [Google Scholar] [CrossRef]
- Stammers, A.H. Extracorporeal circulation in theory and practice. J. Extra Corpor. Technol. 2023, 55, 98–99. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology Functions and Disorders of the Immune System, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- ISO 7199:2016; Cardiovascular Implants and Artificial Organs Blood-Gas Exchangers (Oxygenators). International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/67607.html (accessed on 1 March 2024).
- FDA 510(k); Guidance for Cardiopulmonary Bypass Oxygenators 510(k). U.S. Department of Health and Human Services Food and Drug Administration: Rockville, MD, USA, 2000. Available online: https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/cardiopulmonary-bypass-oxygenators-510k-submissions-final-guidance-industry-and-fda-staff (accessed on 18 July 2018).
- Naito, K.; Mizuguchi, K.; Nosé, Y. The need for standardizing the index of hemolysis. Artif. Organs 1994, 18, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Suzuki, M.; Hayashida, K.; Kobayashi, Y.; Yoshizawa, J.; Shibusawa, T.; Sano, M.; Hori, S.; Sasaki, J. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J. Clin. Biochem. Nutr. 2020, 67, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Itami, N.; Suzuki, H.; Hamada, H.; Yamamoto, R.; Tsunoda, K.; Osaka, N.; Nakano, H.; Maruyama, Y.; Kabayama, S.; et al. Novel haemodialysis (HD) treatment employing molecular hydrogen (H2)-enriched dialysis solution improves prognosis of chronic dialysis patients: A prospective observational study. Sci. Rep. 2018, 8, 254. [Google Scholar] [CrossRef]
- Loria, V.; Dato, I.; Graziani, F.; Biasucci, L.M. Myeloperoxidase: A new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediat. Inflamm. 2008, 2008, 135625. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Satta, H.; Iwamoto, T.; Kawai, Y.; Koguchi, N.; Shibata, K.; Kobayashi, N.; Yoshida, M.; Nakayama, M. Amelioration of hemodialysis-induced oxidative stress and fatigue with a hemodialysis system employing electrolyzed water containing molecular hydrogen. Ren. Replace. Ther. 2021, 7, 37. [Google Scholar] [CrossRef]
- Matsuo, Y.; Yodoi, J. Extracellular thioredoxin: A therapeutic tool to combat inflammation. Cytokine Growth Factor. Rev. 2013, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: A clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Mouzakis, F.L.; Khadka, L.B.; Pereira da Silva, M.; Mottaghy, K. Application of Hydrogen in Hemodialysis: A Brief Review with Emphasis on the Quantification of Dissolved H2. In Molecular Hydrogen in Health and Disease. Advances in Biochemistry in Health and Disease; Slezak, J., Kura, B., Eds.; Springer: Cham, Switzerland, 2024; Volume 27, pp. 195–205. [Google Scholar] [CrossRef]
- Zayat, R.; Moza, A.; Grottke, O.; Grzanna, T.; Fechter, T.; Motomura, T.; Schmidt-Mewes, C.; Breuer, T.; Autschbach, R.; Rossaint, R.; et al. In vitro comparison of the hemocompatibility of two centrifugal left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2019, 157, 591–599. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, K.; Zhang, Y.; Duan, Y.; Tian, Y.; Yin, H.; Fu, X.; Ma, Z.; Zhou, J.; Yu, M.; et al. Potent anti-inflammatory responses: Role of hydrogen in IL-1α dominated early phase systemic inflammation. Front. Pharmacol. 2023, 14, 1138762. [Google Scholar] [CrossRef]
- Cornwell, A.; Badiei, A. From Gasotransmitter to Immunomodulator: The Emerging Role of Hydrogen Sulfide in Macrophage Biology. Antioxidants 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chi, Q.; Wang, D.; Chi, X.; Teng, X.; Li, S. Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotoxicol. Environ. Saf. 2018, 164, 201–209. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomed. Pharmacother. 2020, 130, 110589. [Google Scholar] [CrossRef] [PubMed]
- Khiji, M.N.; Arghidash, F.; Tanha, G.K.; Zadeh, R.H.; Ghorbani, E.; Khazaei, M.; Hassanian, S.M.; Gataa, I.S.; Lam, A.K.; Giovannetti, E.; et al. The Therapeutic Application of Hydrogen in Cancer: The Potential and Challenges. Curr. Pharm. Des. 2024, 30, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]




| System | Label | Total Volume Vprim [mL] | Blood Flow Rate QB [mL min−1] | Gas Mixture Content [%] | ||
|---|---|---|---|---|---|---|
| Air | CO2 | H2 | ||||
| Control | C | 1.5 | - | - | - | - |
| Reference 1 | Ref | 45 | 40 | - | - | - |
| Reference 2 | Ref* | 70 | 40 | 96 | 4 | - |
| LPS 1 | LPS | 45 | 40 | - | - | - |
| LPS 2 | LPS* | 70 | 40 | 96 | 4 | - |
| LPS 3 | LPS*H2 | 70 | 40 | 90 | 4 | 6 |
| Biomarker | Abbreviation | Classification |
|---|---|---|
| Monocyte chemoattractant protein-1 | MCP-1/CCL2 | oxidative stress/inflammation |
| Myeloperoxidase | MPO | oxidative stress/inflammation |
| Thioredoxin-1 | TRX1 | antioxidant/anti-inflammatory |
| Malondialdehyde | MDA | oxidative stress |
| Interleukin 6 | IL-6 | pro-inflammatory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouzakis, F.L.; Hima, F.; Kashefi, A.; Greven, J.; Rink, L.; van der Vorst, E.P.C.; Jankowski, J.; Mottaghy, K.; Spillner, J. Molecular Hydrogen and Extracorporeal Gas Exchange: A Match Made in Heaven? An In Vitro Pilot Study. Biomedicines 2024, 12, 1883. https://doi.org/10.3390/biomedicines12081883
Mouzakis FL, Hima F, Kashefi A, Greven J, Rink L, van der Vorst EPC, Jankowski J, Mottaghy K, Spillner J. Molecular Hydrogen and Extracorporeal Gas Exchange: A Match Made in Heaven? An In Vitro Pilot Study. Biomedicines. 2024; 12(8):1883. https://doi.org/10.3390/biomedicines12081883
Chicago/Turabian StyleMouzakis, Foivos Leonidas, Flutura Hima, Ali Kashefi, Johannes Greven, Lothar Rink, Emiel P. C. van der Vorst, Joachim Jankowski, Khosrow Mottaghy, and Jan Spillner. 2024. "Molecular Hydrogen and Extracorporeal Gas Exchange: A Match Made in Heaven? An In Vitro Pilot Study" Biomedicines 12, no. 8: 1883. https://doi.org/10.3390/biomedicines12081883
APA StyleMouzakis, F. L., Hima, F., Kashefi, A., Greven, J., Rink, L., van der Vorst, E. P. C., Jankowski, J., Mottaghy, K., & Spillner, J. (2024). Molecular Hydrogen and Extracorporeal Gas Exchange: A Match Made in Heaven? An In Vitro Pilot Study. Biomedicines, 12(8), 1883. https://doi.org/10.3390/biomedicines12081883










