Platelet and Lymphocyte-Related Parameters as Potential Markers of Osteoarthritis Severity: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Radiologic Assessment
2.3. Laboratory Parameters
2.4. Statistical Analysis
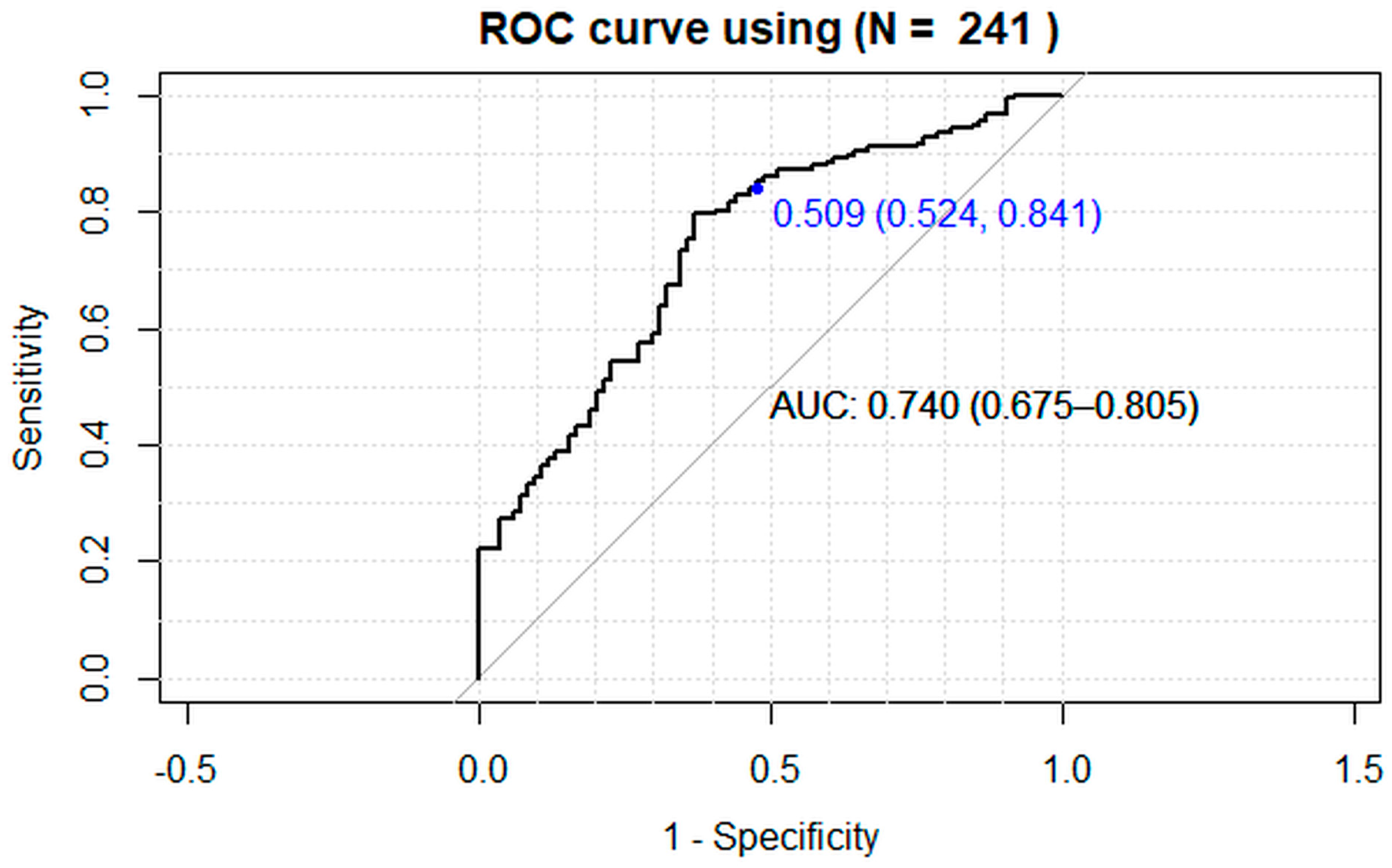
3. Results
4. Discussion
5. Conclusions
- This study provides valuable information on biomarkers related to osteoarthritis (OA) severity.
- The identification of PLR and age as key predictors of OA severity has important clinical implications.
- Monitoring these parameters in OA patients could help in the early identification of individuals at higher risk of disease worsening.
- This could enable timely interventions to mitigate disease progression, improve patient outcomes, and reduce the burden of OA on healthcare systems.
- Population stratification could optimize access to the most invasive and expensive tests.
- Early identification based on these biomarkers could lead to more personalized treatment strategies.
- Future studies should aim to validate these findings in larger, more diverse cohorts and explore additional factors that may identify OA severity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, R.J.; Alvarez, C.; Schwartz, T.A.; Losina, E.; Renner, J.B.; Jordan, J.M.; Callahan, L.F. The impact of painful knee osteoarthritis on mortality: A community-based cohort study with over 24 years of follow-up. Osteoarthr. Cartil. 2019, 27, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.D.T.; Saunders, F.R.; Martin, K.R. Sex Difference in OA: Should We Blame Estrogen? Eur. J. Rheumatol. 2023. [Google Scholar] [CrossRef]
- Li, X.X.; Cao, F.; Zhao, C.N.; Ge, M.; Wei, H.F.; Tang, J.; Xu, W.L.; Wang, S.; Gao, M.; Wang, P.; et al. Global burden of osteoarthritis: Prevalence and temporal trends from 1990 to 2019. Int. J. Rheum. Dis. 2024, 27, e15285. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.B.; Kim, J.H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Boffa, A.; Merli, G.; Andriolo, L.; Lattermann, C.; Salzmann, G.M.; Filardo, G. Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria. Cartilage 2021, 13, 82S–103S. [Google Scholar] [CrossRef]
- Kavanaugh, A. The role of the laboratory in the evaluation of rheumatic diseases. Clin. Cornerstone 1999, 2, 11–25. [Google Scholar] [CrossRef]
- Sandhaus, L.M.; Meyer, P. How useful are CBC and reticulocyte reports to clinicians? Am. J. Clin. Pathol. 2002, 118, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Butterworth, R.J. Platelet size: Measurement, physiology and vascular disease. Blood Coagul. Fibrinolysis 1996, 7, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Endler, G.; Klimesch, A.; Sunder-Plassmann, H.; Schillinger, M.; Exner, M.; Mannhalter, C.; Jordanova, N.; Christ, G.; Thalhammer, R.; Huber, K.; et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br. J. Haematol. 2002, 117, 399–404. [Google Scholar] [CrossRef]
- Kapsoritakis, A.N.; Koukourakis, M.I.; Sfiridaki, A.; Potamianos, S.P.; Kosmadaki, M.G.; Koutroubakis, I.E.; Kouroumalis, E.A. Mean platelet volume: A useful marker of inflammatory bowel disease activity. Am. J. Gastroenterol. 2001, 96, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Wollheim, F.A. Markers of disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2000, 12, 200–204. [Google Scholar] [CrossRef]
- Colglazier, C.L.; Sutej, P.G. Laboratory testing in the rheumatic diseases: A practical review. South Med. J. 2005, 98, 185–191. [Google Scholar] [CrossRef]
- Nakamura, R.M. Progress in the use of biochemical and biological markers for evaluation of rheumatoid arthritis. J. Clin. Lab. Anal. 2000, 14, 305–313. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mikhailidis, D.M.; Kitas, G.D. Mean platelet volume: A link between thrombosis and inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef]
- Tasoglu, O.; Sahin, A.; Karatas, G.; Koyuncu, E.; Taşoğlu, İ.; Tecimel, O.; Özgirgin, N. Blood mean platelet volume and platelet lymphocyte ratio as new predictors of hip osteoarthritis severity. Medicine 2017, 96, e6073. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Koh, I.H.; Chung, K.; Lee, Y.J.; Kim, H.S. Association between platelet count and osteoarthritis in women older than 50 years. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20912861. [Google Scholar] [CrossRef]
- Korkmaz, M.D.; Menekşeoğlu, A.K.; Yakşi, E. Are inflammatory parameters an independent predictor of hip osteoarthritis severity? A prospective cross-sectional study. Rev. Assoc. Med. Bras. 2022, 68, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramirez, D.C.; van der Leeden, M.; van der Esch, M.; Gerritsen, M.; Roorda, L.D.; Verschueren, S.; van Dieën, J.; Dekker, J.; Lems, W.F. Association of serum C-reactive protein and erythrocyte sedimentation rate with muscle strength in patients with knee osteoarthritis. Rheumatology 2013, 52, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Boffa, A.; Andriolo, L.; Raggi, F.; Zaffagnini, S.; Filardo, G. Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review. J. Clin. Med. 2022, 11, 6663. [Google Scholar] [CrossRef]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2021, 13, 364S–375S. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E. PRP: Product Rich in Placebo? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3702–3703. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef]
- Kverneland, A.H.; Streitz, M.; Geissler, E.; Hutchinson, J.; Vogt, K.; Boës, D.; Niemann, N.; Pedersen, A.E.; Schlickeiser, S.; Sawitzki, B. Age and gender leucocytes variances and references values generated using the standardized ONE-study protocol. Cytom. A 2016, 89, 543–564. [Google Scholar] [CrossRef]
- Andreu-Ballester, J.C.; Garcia-Ballesteros, C.; Benet-Campos, C.; Amigó, V.; Almela-Quilis, A.; Mayans, J.; Ballester, F. Values for αβ and γδ T-lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: Assessment by age and gender. Cytom. B Clin. Cytom. 2012, 82, 238–244. [Google Scholar] [CrossRef]
- Biino, G.; Santimone, I.; Minelli, C.; Sorice, R.; Frongia, B.; Traglia, M.; Ulivi, S.; Di Castelnuovo, A.; Gögele, M.; Nutile, T.; et al. Age- and sex-related variations in platelet count in Italy: A proposal of reference ranges based on 40987 subjects’ data. PLoS ONE 2013, 8, e54289. [Google Scholar] [CrossRef]
- Sayit, A.T.; Gunbey, P.H.; Terzi, Y. Is the Mean Platelet Volume in Patients with Acute Cholecystitis an Inflammatory Marker? J. Clin. Diagn. Res. 2015, 9, TC05–TC07. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Barbieri, L.; Schaffer, A.; Bellomo, G.; Marino, P.; De Luca, G.; Novara Atherosclerosis Study (NAS) Group. Impact of renal function on mean platelet volume and its relationship with coronary artery disease: A single-centre cohort study. Thromb. Res. 2016, 141, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bessman, J.D.; Williams, L.J.; Gilmer, P.R., Jr. Mean platelet volume. The inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am. J. Clin. Pathol. 1981, 76, 289–293. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Justo, D.; Rogowski, O.; Saar, N.; Abu-Abeid, S.; Shenkerman, G.; Shapira, I.; Berliner, S.; Tomer, A. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008, 2008, 834153. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.; Tien, P.C.; Feldman, J.; Shin, J.H.; Fennie, K.; Anastos, K.; Cohen, M.H.; Bacon, M.C.; Minkoff, H. Obesity and immune cell counts in women. Metabolism 2007, 56, 998–1004. [Google Scholar] [CrossRef]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteoarthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 30 August 2024).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Uslu, A.U.; Küçük, A.; Sahin, A.; Ugan, Y.; Yılmaz, R.; Güngör, T.; Bağcacı, S.; Küçükşen, S. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int. J. Rheum. Dis. 2015, 18, 731–735. [Google Scholar] [CrossRef]
- Fu, H.; Qin, B.; Hu, Z.; Ma, N.; Yang, M.; Wei, T.; Tang, Q.; Huang, Y.; Huang, F.; Liang, Y.; et al. Neutrophil-and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin. Lab. 2015, 61, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2007–2009. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1261–1265. [Google Scholar]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Calcada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136–137, 138–147. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef]
- Salamanna, F.; Maglio, M.; Sartori, M.; Tschon, M.; Fini, M. Platelet Features and Derivatives in Osteoporosis: A Rational and Systematic Review on the Best Evidence. Int. J. Mol. Sci. 2020, 21, 1762. [Google Scholar] [CrossRef] [PubMed]

| Patients | KL < 3 | KL ≥ 3 | p-Value | ||
|---|---|---|---|---|---|
| N | 245 | 88 | 157 | ||
| Sex | F | 126 | 50 | 76 | 0.232 * |
| M | 119 | 38 | 81 | ||
| Age | yrs | 65 [64, 66] | 59 [58, 61] | 68 [66, 69] | <0.0005 ° |
| 40–60 | 96 | 53 | 43 | <0.0005 * | |
| >60 | 149 | 35 | 114 | ||
| BMI | kg/m2 | 25.8 [25.6, 26.1] | 25.6 [25.2, 26.0] | 26.0 [25.6, 26.3] | 0.2879 ° |
| <25 kg/m2 | 83 | 32 | 51 | 0.575 * | |
| 25–30 kg/m2 | 162 | 56 | 106 |
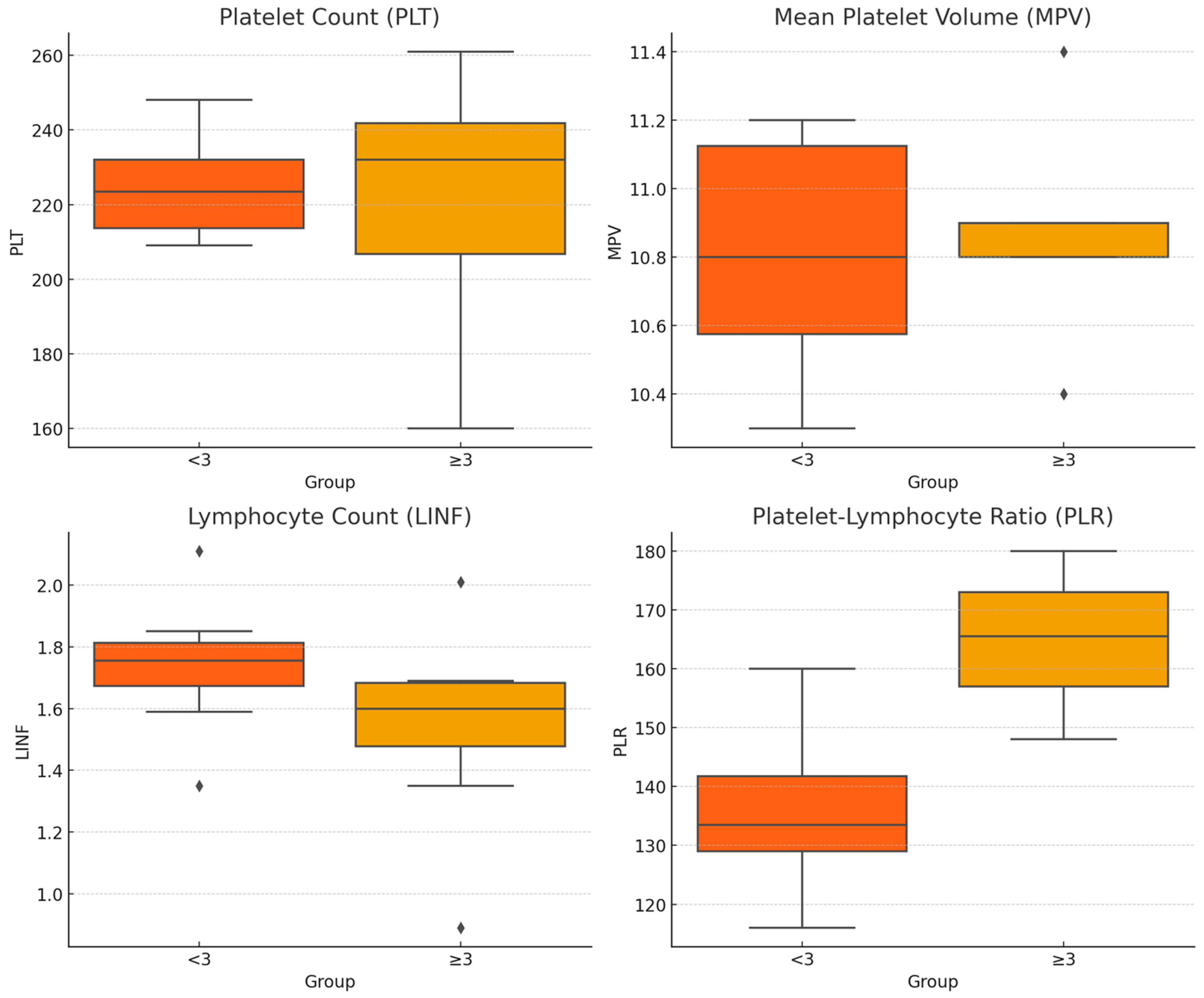
| OA_KL Grade | Sex | Age | BMI | N | PLT 109/L | MPV fL | LINF 109/L | PLR 109/L |
|---|---|---|---|---|---|---|---|---|
| <3 | F | 40–60 | <25 | 15 | 215 [205, 225] | 11.2 [11.0, 11.4] | 1.70 [1.60, 1.80] | 135 [125, 145] |
| 25–30 | 13 | 232 [213, 251] | 11.2 [10.9, 11.5] | 1.85 [1.77, 1.93] | 129 [116, 142] | |||
| >60 | <25 | 5 | 216 [201, 231] | 10.7 [10.4, 11.0] | 1.59 [1.50, 1.68] | 137 [126, 148] | ||
| 25–30 | 17 | 248 [232, 264] | 10.9 [10.7, 11.1] | 1.71 [1.56, 1.86] | 160 [145, 175] | |||
| M | 40–60 | <25 | 9 | 231 [223, 239] | 10.6 [10.3, 10.9] | 1.80 [1.67, 1.93] | 132 [123, 141] | |
| 25–30 | 16 | 232 [218, 246] | 11.1 [10.9, 11.3] | 2.11 [1.96, 2.26] | 116 [107, 125] | |||
| >60 | <25 | 3 | 209 [138, 280] | 10.3 [9.6, 11.0] | 1.35 [1.00, 1.70] | 156 [127, 185] | ||
| 25–30 | 10 | 210 [193, 227] | 10.5 [10.2, 10.8] | 1.80 [1.59, 2.01] | 129 [114, 144] | |||
| ≥3 | F | 40–60 | <25 | 11 | 261 [237, 285] | 10.9 [10.7, 11.1] | 2.01 [1.74, 2.28] | 148 [128, 168] |
| 25–30 | 13 | 250 [235, 265] | 10.9 [10.7, 11.1] | 1.69 [1.56, 1.82] | 154 [144, 164] | |||
| >60 | <25 | 18 | 236 [221, 251] | 10.8 [10.6, 11.0] | 1.59 [1.45, 1.73] | 173 [153, 193] | ||
| 25–30 | 34 | 239 [226, 252] | 10.8 [10.6, 11.0] | 1.68 [1.59, 1.77] | 158 [146, 170] | |||
| M | 40–60 | <25 | 2 | 160 [128, 192] | 11.4 [10.7, 12.1] | 0.89 [0.71, 1.07] | 180 [152, 208] | |
| 25–30 | 17 | 206 [195, 217] | 10.9 [10.7, 11.1] | 1.35 [1.22, 1.48] | 173 [158, 188] | |||
| >60 | <25 | 20 | 228 [216, 240] | 10.4 [9.9, 10.9] | 1.61 [1.41, 1.81] | 168 [153, 183] | ||
| 25–30 | 42 | 207 [198, 216] | 10.9 [10.8, 11.0] | 1.52 [1.40, 1.64] | 163 [151, 175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanna, F.; Pagani, S.; Filardo, G.; Contartese, D.; Boffa, A.; Angelelli, L.; Maglio, M.; Fini, M.; Zaffagnini, S.; Giavaresi, G. Platelet and Lymphocyte-Related Parameters as Potential Markers of Osteoarthritis Severity: A Cross-Sectional Study. Biomedicines 2024, 12, 2052. https://doi.org/10.3390/biomedicines12092052
Salamanna F, Pagani S, Filardo G, Contartese D, Boffa A, Angelelli L, Maglio M, Fini M, Zaffagnini S, Giavaresi G. Platelet and Lymphocyte-Related Parameters as Potential Markers of Osteoarthritis Severity: A Cross-Sectional Study. Biomedicines. 2024; 12(9):2052. https://doi.org/10.3390/biomedicines12092052
Chicago/Turabian StyleSalamanna, Francesca, Stefania Pagani, Giuseppe Filardo, Deyanira Contartese, Angelo Boffa, Lucia Angelelli, Melania Maglio, Milena Fini, Stefano Zaffagnini, and Gianluca Giavaresi. 2024. "Platelet and Lymphocyte-Related Parameters as Potential Markers of Osteoarthritis Severity: A Cross-Sectional Study" Biomedicines 12, no. 9: 2052. https://doi.org/10.3390/biomedicines12092052







