Mortality Risk and Urinary Proteome Changes in Acute COVID-19 Survivors in the Multinational CRIT-COV-U Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Urinary Peptidomics
2.3. Statistical Analysis
2.4. Classifier Development
3. Results
3.1. Assessment of Mortality in Acute COVID-19 Survivors
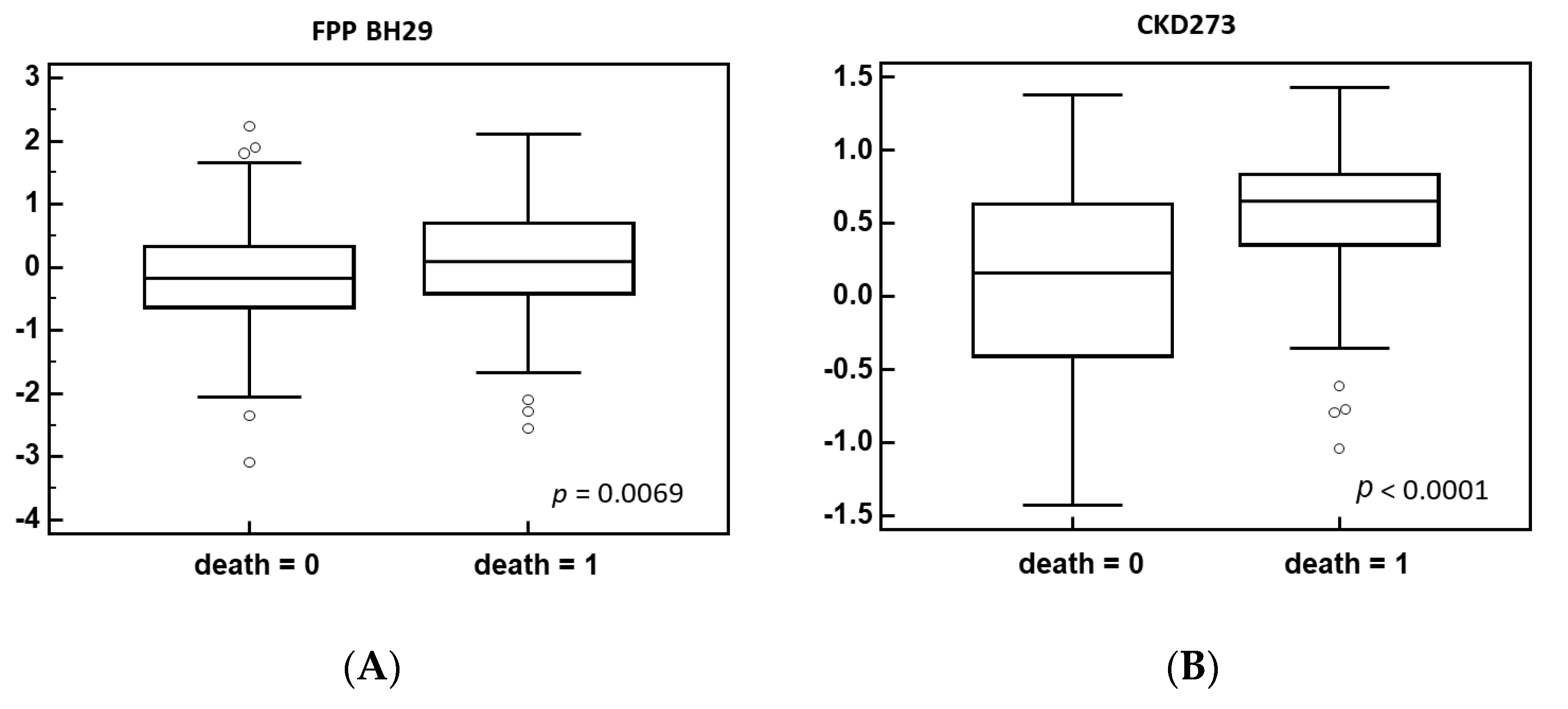
3.2. Identification of Biomarkers Associated with Post-Acute COVID-19 Mortality
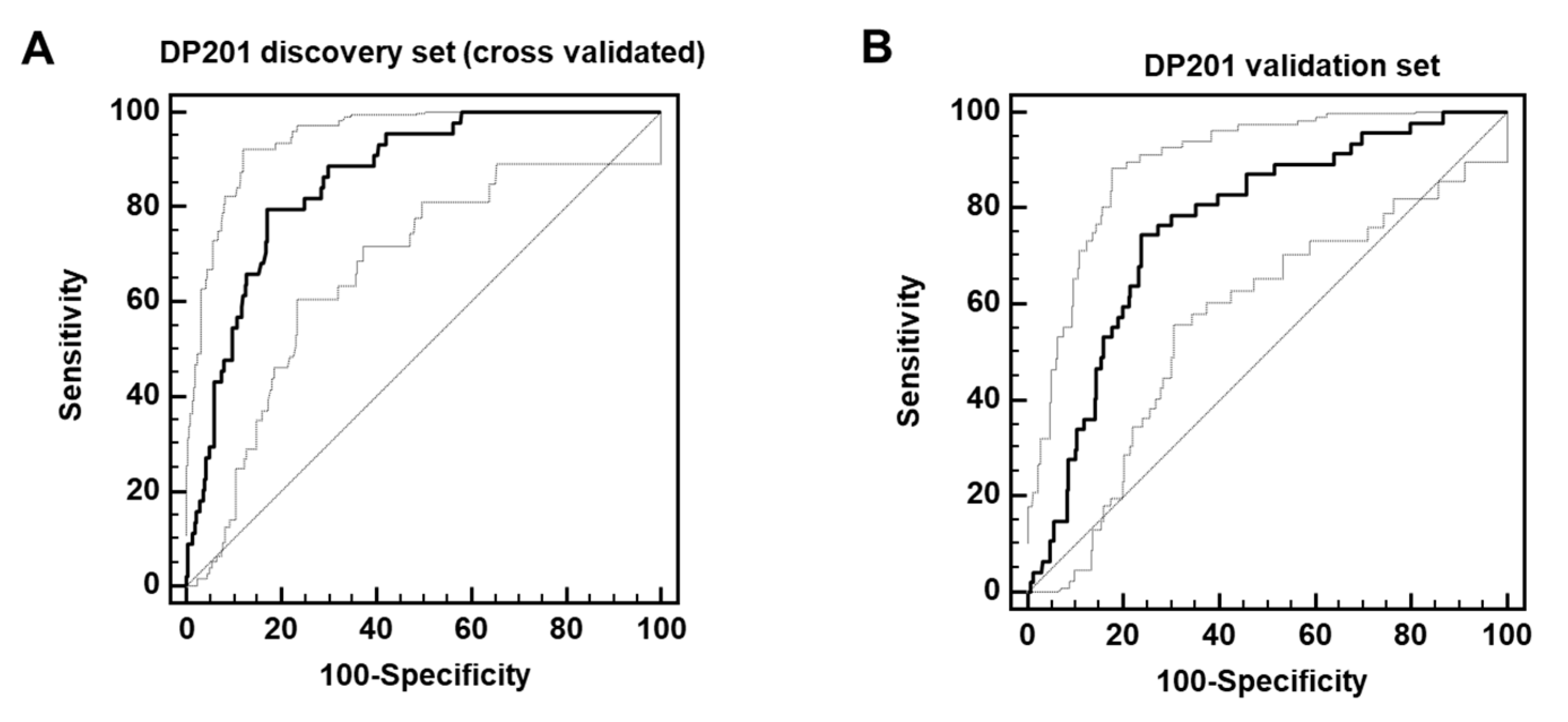
3.3. Establishment and Validating a Classifier Predicting Post-Acute COVID-19 Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wang, F.; Shen, Y.; Zhang, X.; Cen, Y.; Wang, B.; Zhao, S.; Zhou, Y.; Hu, B.; Wang, M.; et al. Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge From Hospitals in Wuhan, China. JAMA Netw. Open 2021, 4, e2127403. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Demographics Collaborators. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20,133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Apea, V.J.; Wan, Y.I.; Dhairyawan, R.; Puthucheary, Z.A.; Pearse, R.M.; Orkin, C.M.; Prowle, J.R. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: An observational cohort study. BMJ Open 2021, 11, e042140. [Google Scholar] [CrossRef]
- Vivaldi, G.; Pfeffer, P.E.; Talaei, M.; Basera, T.J.; Shaheen, S.O.; Martineau, A.R. Long-term symptom profiles after COVID-19 vs. other acute respiratory infections: An analysis of data from the COVIDENCE UK study. EClinicalMedicine 2023, 65, 102251. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.L.; Meyrignac, O.; Noel, N.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Ely, E.W.; Brown, L.M.; Fineberg, H.V. Long Covid Defined. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Chou, R.; Herman, E.; Ahmed, A.; Anderson, J.; Selph, S.; Dana, T.; Williams, L.; Ivlev, I. Long COVID Definitions and Models of Care: A Scoping Review. Ann. Intern. Med. 2024, 177, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long COVID in People Infected With Severe Acute Respiratory Syndrome Coronavirus 2 after 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac464. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Postacute Sequelae of SARS-CoV-2 Infection in the Pre-Delta, Delta, and Omicron Eras. N. Engl. J. Med. 2024, 391, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Munoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Ghafari, M.; Hall, M.; Golubchik, T.; Ayoubkhani, D.; House, T.; MacIntyre-Cockett, G.; Fryer, H.R.; Thomson, L.; Nurtay, A.; Kemp, S.A.; et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 2024, 626, 1094–1101. [Google Scholar] [CrossRef]
- Hampshire, A.; Azor, A.; Atchison, C.; Trender, W.; Hellyer, P.J.; Giunchiglia, V.; Husain, M.; Cooke, G.S.; Cooper, E.; Lound, A.; et al. Cognition and Memory after Covid-19 in a Large Community Sample. N. Engl. J. Med. 2024, 390, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalova, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Talla, A.; Vasaikar, S.V.; Szeto, G.L.; Lemos, M.P.; Czartoski, J.L.; MacMillan, H.; Moodie, Z.; Cohen, K.W.; Fleming, L.B.; Thomson, Z.; et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 2023, 14, 3417. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19, retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Staessen, J.A.; Wendt, R.; Yu, Y.L.; Kalbitz, S.; Thijs, L.; Siwy, J.; Raad, J.; Metzger, J.; Neuhaus, B.; Papkalla, A.; et al. Predictive performance and clinical application of COV50, a urinary proteomic biomarker in early COVID-19 infection: A prospective multicentre cohort study. Lancet Digit. Health 2022, 10, e727–e737. [Google Scholar] [CrossRef]
- Latosinska, A.; Siwy, J.; Mischak, H.; Frantzi, M. Peptidomics and proteomics based on CE-MS as a robust tool in clinical application: The past, the present, and the future. Electrophoresis 2019, 40, 2294–2308. [Google Scholar] [CrossRef]
- Mavrogeorgis, E.; Mischak, H.; Latosinska, A.; Siwy, J.; Jankowski, V.; Jankowski, J. Reproducibility Evaluation of Urinary Peptide Detection Using CE-MS. Molecules 2021, 26, 7260. [Google Scholar] [CrossRef]
- Jantos-Siwy, J.; Schiffer, E.; Brand, K.; Schumann, G.; Rossing, K.; Delles, C.; Mischak, H.; Metzger, J. Quantitative Urinary Proteome Analysis for Biomarker Evaluation in Chronic Kidney Disease. J. Proteome Res. 2009, 8, 268–281. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B (Methodol.) 1995, 57, 125–133. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Good, D.M.; Zürbig, P.; Argiles, A.; Bauer, H.W.; Behrens, G.; Coon, J.J.; Dakna, M.; Decramer, S.; Delles, C.; Dominiczak, A.F.; et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell Proteom. 2010, 9, 2424–2437. [Google Scholar] [CrossRef]
- Farmakis, D.; Koeck, T.; Mullen, W.; Parissis, J.; Gogas, B.D.; Nikolaou, M.; Lekakis, J.; Mischak, H.; Filippatos, G. Urine proteome analysis in heart failure with reduced ejection fraction complicated by chronic kidney disease: Feasibility, and clinical and pathogenetic correlates. Eur. J. Heart Fail. 2016, 18, 822–829. [Google Scholar] [CrossRef]
- Wendt, R.; Thijs, L.; Kalbitz, S.; Mischak, H.; Siwy, J.; Raad, J.; Metzger, J.; Neuhaus, B.; Leyen, H.V.; Dudoignon, E.; et al. A urinary peptidomic profile predicts outcome in SARS-CoV-2-infected patients. EClinicalMedicine 2021, 36, 100883. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G., III; Rooks, B.J.; Wu, V.; Orlando, F.A. COVID-19 Post-acute Sequelae Among Adults: 12 Month Mortality Risk. Front. Med. 2021, 8, 778434. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.C.; Reichardt, B.; Wagenlechner, C.; Krotka, P.; Traxler-Weidenauer, D.; Mildner, M.; Mascherbauer, J.; Aigner, C.; Auer, J.; Wendt, R. Baseline Drug Treatments and Long-Term Outcomes in COVID-19-Hospitalized Patients: Results of the 2020 AUTCOV Study. medRxiv 2024. [Google Scholar] [CrossRef]
- Oseran, A.S.; Song, Y.; Xu, J.; Dahabreh, I.J.; Wadhera, R.K.; de Lemos, J.A.; Das, S.R.; Sun, T.; Yeh, R.W.; Kazi, D.S. Long term risk of death and readmission after hospital admission with covid-19 among older adults: Retrospective cohort study. BMJ 2023, 382, e076222. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Seelye, S.; Berkowitz, T.S.; Pura, J.; Bohnert, A.S.B.; Bowling, C.B.; Boyko, E.J.; Hynes, D.M.; Ioannou, G.N.; Maciejewski, M.L.; et al. Late Mortality After COVID-19 Infection Among US Veterans vs Risk-Matched Comparators: A 2-Year Cohort Analysis. JAMA Intern. Med. 2023, 183, 1111–1119. [Google Scholar] [CrossRef]
- Cai, M.; Xie, Y.; Topol, E.J.; Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 2024, 30, 1564–1573. [Google Scholar] [CrossRef]
- Maruyama, Y.; Nakayama, M.; Abe, M.; Yokoo, T.; Minakuchi, J.; Nitta, K. Association between serum beta2-microglobulin and mortality in Japanese peritoneal dialysis patients: A cohort study. PLoS ONE 2022, 17, e0266882. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Rios, G.; Katz, R.; Levitan, E.B.; Cushman, M.; Parikh, C.R.; Kimmel, P.L.; Bonventre, J.V.; Waikar, S.S.; Schrauben, S.J.; Greenberg, J.H.; et al. Urinary Biomarkers of Kidney Tubule Health and Mortality in Persons with CKD and Diabetes Mellitus. Kidney360 2023, 4, e1257–e1264. [Google Scholar] [CrossRef] [PubMed]
- Mina, I.K.; Mavrogeorgis, E.; Siwy, J.; Stojanov, R.; Mischak, H.; Latosinska, A.; Jankowski, V. Multiple urinary peptides display distinct sex-specific distribution. Proteomics 2023, 24, e2300227. [Google Scholar] [CrossRef]
- Drum, C.L.; Tan, W.K.Y.; Chan, S.P.; Pakkiri, L.S.; Chong, J.P.C.; Liew, O.W.; Ng, T.P.; Ling, L.H.; Sim, D.; Leong, K.G.; et al. Thymosin Beta-4 Is Elevated in Women With Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e005586. [Google Scholar] [CrossRef]
- Rudnicki, M.; Siwy, J.; Wendt, R.; Lipphardt, M.; Koziolek, M.J.; Maixnerova, D.; Peters, B.; Kerschbaum, J.; Leierer, J.; Neprasova, M.; et al. Urine proteomics for prediction of disease progression in patients with IgA nephropathy. Nephrol. Dial. Transpl. 2020, 37, 42–52. [Google Scholar] [CrossRef]
- Didriksson, I.; Lengquist, M.; Spangfors, M.; Leffler, M.; Sievert, T.; Lilja, G.; Frigyesi, A.; Friberg, H.; Schiopu, A. Increasing plasma calprotectin (S100A8/A9) is associated with 12-month mortality and unfavourable functional outcome in critically ill COVID-19 patients. J. Intensive Care 2024, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Barratt-Due, A.; Pettersen, K.; Borresdatter-Dahl, T.; Holter, J.C.; Gronli, R.H.; Dyrhol-Riise, A.M.; Lerum, T.V.; Holten, A.R.; Tonby, K.; Troseid, M.; et al. Escalated complement activation during hospitalization is associated with higher risk of 60-day mortality in SARS-CoV-2-infected patients. J. Intern. Med. 2024, 296, 80–92. [Google Scholar] [CrossRef]
- Keller, F.; Beige, J.; Siwy, J.; Mebazaa, A.; An, D.; Mischak, H.; Schanstra, J.P.; Mokou, M.; Perco, P.; Staessen, J.A.; et al. Urinary peptides provide information about the risk of mortality across a spectrum of diseases and scenarios. J. Transl. Med. 2023, 21, 663. [Google Scholar] [CrossRef]
- Magalhães, P.; Pejchinovski, M.; Markoska, K.; Banasik, M.; Klinger, M.; Svec-Billa, D.; Rychlik, I.; Rroji, M.; Restivo, A.; Capasso, G.; et al. Association of kidney fibrosis with urinary peptides: A path towards non-invasive liquid biopsies? Sci. Rep. 2017, 7, 16915. [Google Scholar] [CrossRef]
- Catanese, L.; Siwy, J.; Mavrogeorgis, E.; Amann, K.; Mischak, H.; Beige, J.; Rupprecht, H. A Novel Urinary Proteomics Classifier for Non-Invasive Evaluation of Interstitial Fibrosis and Tubular Atrophy in Chronic Kidney Disease. Proteomes 2021, 9, 32. [Google Scholar] [CrossRef]
- Siwy, J.; Wendt, R.; Albalat, A.; He, T.; Mischak, H.; Mullen, W.; Latosinska, A.; Lubbert, C.; Kalbitz, S.; Mebazaa, A.; et al. CD99 and polymeric immunoglobulin receptor peptides deregulation in critical COVID-19, A potential link to molecular pathophysiology? Proteomics 2021, 21, e2100133. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Setoh, K.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Matsuda, F. Association between serum alpha1-antitrypsin levels and all-cause mortality in the general population: The Nagahama study. Sci. Rep. 2021, 11, 17241. [Google Scholar] [CrossRef] [PubMed]
- Jaimes Campos, M.A.; Andujar, I.; Keller, F.; Mayer, G.; Rossing, P.; Staessen, J.A.; Delles, C.; Beige, J.; Glorieux, G.; Clark, A.L.; et al. Prognosis and Personalized In Silico Prediction of Treatment Efficacy in Cardiovascular and Chronic Kidney Disease: A Proof-of-Concept Study. Pharmaceuticals 2023, 16, 1298. [Google Scholar] [CrossRef] [PubMed]


| No Death (n = 560) | Death (n = 91) | p-Value | |
|---|---|---|---|
| Age | 60 (45–73) | 78 (70–83) | <0.0001 |
| BMI [kg/m2] | 27.1 (24.5–30.3) | 26.5 (23.7–29.9) | 0.1929 |
| Number of comorbidities | 0.0 (0.0–1.0) | 1 (0.3–2.0) | <0.0001 |
| eGFR [mL/min/1.73 m2] | 92.47 (76.00–112.17) | 69 (52.00–90.00) | <0.0001 |
| Heart rate [beats per min] | 80.0 (72.0–80.0) | 80.0 (70.0–86.5) | 0.1827 |
| Diastolic blood pressure [mm Hg] | 78.0 (70.0–82.0) | 73.0 (64.3–80.0) | 0.0494 |
| Systolic blood pressure [mm Hg] | 128 (115.0–140.0) | 128 (110.0–140.0.) | 0.5809 |
| sex, men (%) | 289 (51.6) | 58 (63.7) | 0.0416 |
| WHO score admission | 3 (2–4) | 4 (3–4) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwy, J.; Keller, F.; Banasik, M.; Peters, B.; Dudoignon, E.; Mebazaa, A.; Gülmez, D.; Spasovski, G.; Lazo, M.S.; Rajzer, M.W.; et al. Mortality Risk and Urinary Proteome Changes in Acute COVID-19 Survivors in the Multinational CRIT-COV-U Study. Biomedicines 2024, 12, 2090. https://doi.org/10.3390/biomedicines12092090
Siwy J, Keller F, Banasik M, Peters B, Dudoignon E, Mebazaa A, Gülmez D, Spasovski G, Lazo MS, Rajzer MW, et al. Mortality Risk and Urinary Proteome Changes in Acute COVID-19 Survivors in the Multinational CRIT-COV-U Study. Biomedicines. 2024; 12(9):2090. https://doi.org/10.3390/biomedicines12092090
Chicago/Turabian StyleSiwy, Justyna, Felix Keller, Mirosław Banasik, Björn Peters, Emmanuel Dudoignon, Alexandre Mebazaa, Dilara Gülmez, Goce Spasovski, Mercedes Salgueira Lazo, Marek W. Rajzer, and et al. 2024. "Mortality Risk and Urinary Proteome Changes in Acute COVID-19 Survivors in the Multinational CRIT-COV-U Study" Biomedicines 12, no. 9: 2090. https://doi.org/10.3390/biomedicines12092090
APA StyleSiwy, J., Keller, F., Banasik, M., Peters, B., Dudoignon, E., Mebazaa, A., Gülmez, D., Spasovski, G., Lazo, M. S., Rajzer, M. W., Fuławka, Ł., Dzitkowska-Zabielska, M., Mischak, H., Hecking, M., Beige, J., Wendt, R., & UriCoV Working Group. (2024). Mortality Risk and Urinary Proteome Changes in Acute COVID-19 Survivors in the Multinational CRIT-COV-U Study. Biomedicines, 12(9), 2090. https://doi.org/10.3390/biomedicines12092090










