Retained Lead Fragments in Superior Vena Cava and Early Post-Transplant Outcomes: A Single Center Preliminary Retrospective Study
Abstract
1. Introduction
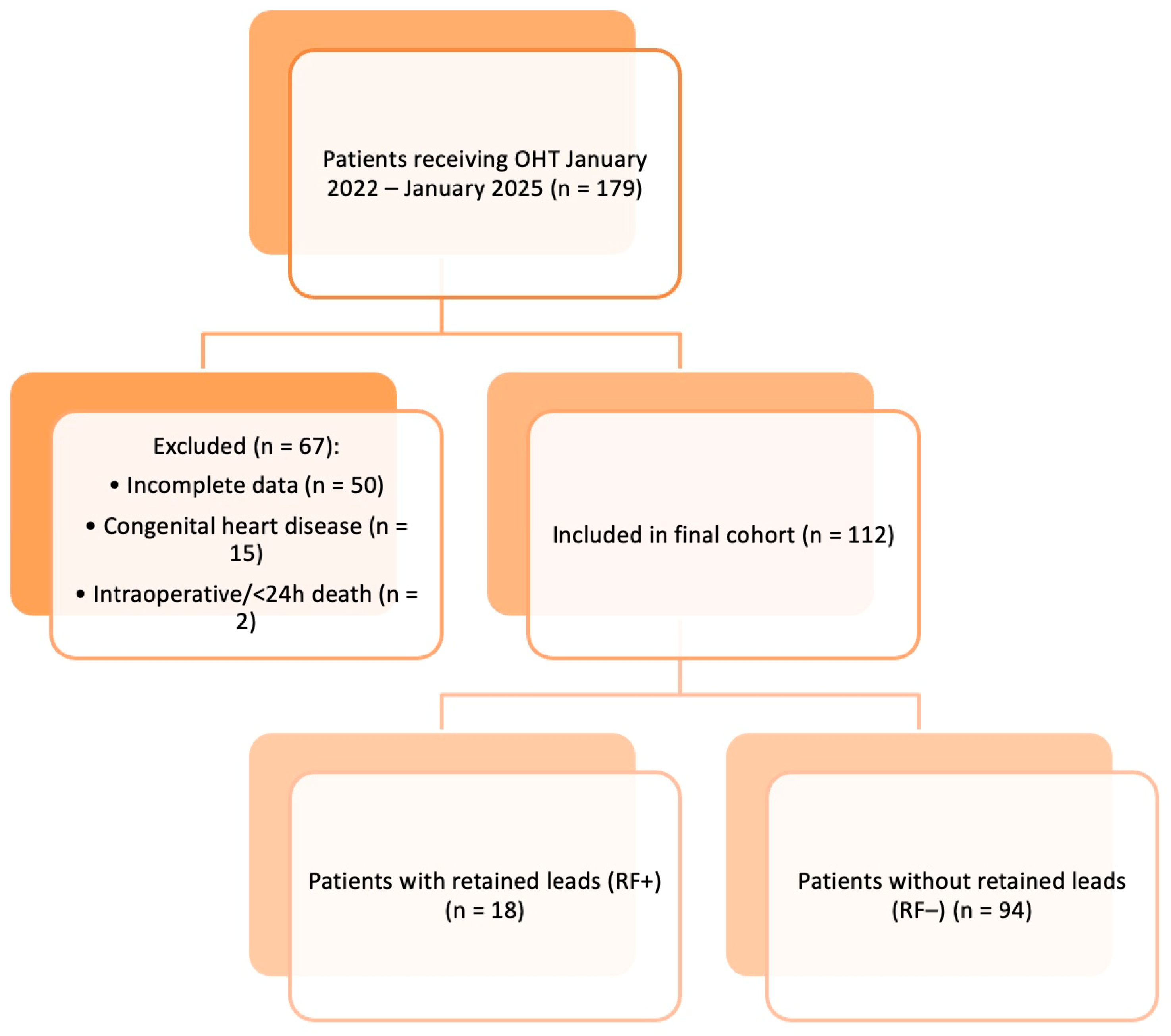
2. Subjects and Methods
- ▪
- Study Design and Population
- ▪
- Inclusion and Exclusion CriteriaPatients were eligible for inclusion if they:
- Underwent OHT during the study period;
- Had retained CIED lead fragments within the superior vena cava (SVC), as confirmed by post-transplant imaging (chest radiography or computed tomography);
- Had complete clinical data and at least 150 days of post-transplant follow-up.
Patients were excluded if they:- Died intraoperatively or within 24 h of transplantation (n = 2);
- Had incomplete clinical documentation (n = 50);
- Had congenital heart disease requiring complex vascular reconstruction (n = 15).
- ▪
- Data Collection
- ▪
- Outcomes
- ▪
- Immunosuppression Therapy
- ▪
- Lead Removal Technique
3. Statistical Analysis
4. Results
4.1. Risk Factor Analysis
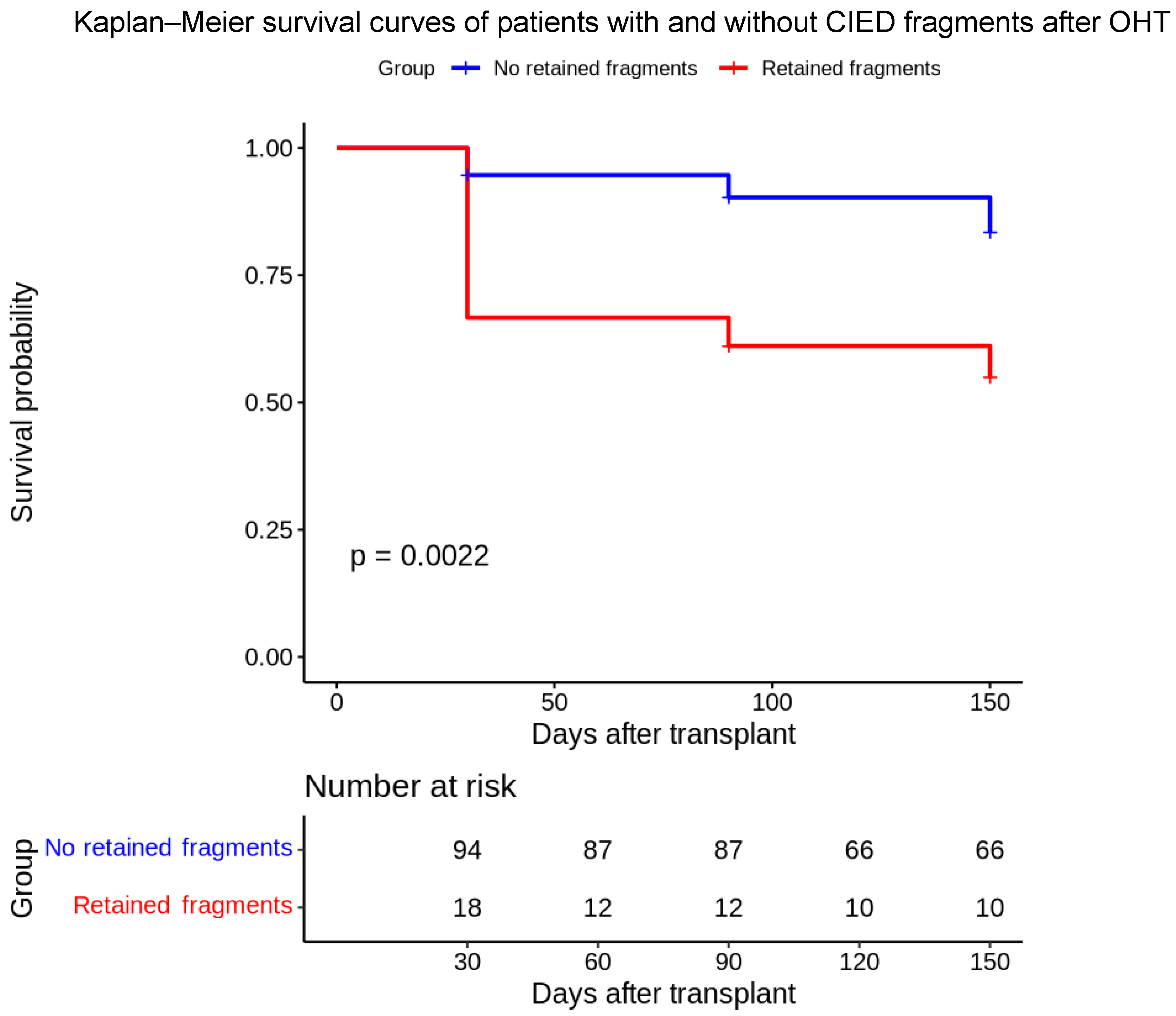
4.2. Outcome Analysis
- Hazard Ratio (HR): 3.71
- 95% Confidence Interval: 1.55–8.84
- p-value: 0.00316
5. Discussion
6. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koneru, J.N.; Jones, P.W.; Hammill, E.F.; Wold, N.; Ellenbogen, K.A. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J. Am. Heart Assoc. 2018, 7, e007691. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Voss, J.; Shannon, D.; Ruygrok, P.; Lever, N. Frequency and sequelae of retained implanted cardiac device material post heart transplantation. Pacing Clin. Electrophysiol. 2014, 37, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sticherling, C.; Burri, H.; Osswald, S. Prevalence of central venous occlusion in patients with chronic defibrillator leads. Am. Heart J. 2001, 141, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, G.; Daubert, J.P.; Huang, D.T.; Rosero, S.; Hall, B.; Francis, C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J. Interv. Card. Electrophysiol. 2005, 13, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fardman, A.; Ram, E.; Lavee, J.; Wieder, A.; Beinart, R.; Nof, E.; Peled, Y. Complications of retained pacemaker hardware in heart transplant recipients: Case series and review of the literature. Infection 2020, 48, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, J.; Choi, J.H.; Choi, H.-I.; Kim, M.-S.; Jung, S.-H.; Nam, G.-B.; Choi, K.-J.; Lee, J.W.; Kim, Y.-H.; et al. Frequency and clinical impact of retained implantable cardioverter defibrillator lead materials in heart transplant recipients. PLoS ONE 2017, 12, e0176925. [Google Scholar] [CrossRef] [PubMed]
- Holzhauser, L.; Imamura, T.; Nayak, H.M.; Sarswat, N.; Kim, G.; Raikhelkar, J.; Kalantari, S.; Patel, A.; Onsager, D.; Song, T.; et al. Consequences of retained defibrillator and pacemaker leads after heart transplantation—An underrecognized problem. J. Card. Fail. 2018, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.J.; Orzalkiewicz, M.; A Nawaz, M.; Lewis, C.; Parameshwar, J.; Tsui, S. Retained pacemaker and implantable cardioverter-defibrillator components after heart transplantation are common and may lead to adverse events. EP Eur. 2018, 20, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.O.; Landolfo, K.; Parikh, P.P.; Patel, P.C.; Venkatachalam, K.; Kusumoto, F.M. Retained cardiac implantable electronic device fragments are not associated with magnetic resonance imaging safety issues, morbidity, or mortality after orthotopic heart transplant. Am. Heart J. 2017, 190, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, E.; Choi, E.-K.; Lee, S.-R.; Oh, S.; Choi, Y.-S. Long-term outcomes of abandoned leads of cardiac implantable electronic devices. Heart Rhythm. 2023, 20, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Hear. Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in: Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, Y.; Foroutan, F.; Miller, R.J.H. Clinical outcomes and complications of heart transplantation: A systematic review and meta-analysis. Transplantation 2019, 103, 1371–1386. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm. 2009, 6, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A prospective, multicentre registry of transvenous lead extraction: Final results. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Patients with RF (N = 18) | Patients Without RF (N = 94) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 61.89 ± 5.74 years | 59.78 ± 10.29 years | 0.2197 |
| BSA (mean ± SD) | 1.82 ± 0.16 m2 | 1.85 ± 0.19 m2 | 0.5961 |
| Female Sex (%) | 31.6% | 12.9% | 0.0935 |
| Hypertension (%) | 66.0% | 66.7% | 0.954 |
| Diabetes (%) | 28.7% | 33.3% | 0.695 |
| Peripheral Vascular Disease (PVD) (%) | 22.3% | 22.2% | 0.991 |
| COPD (%) | 22.3% | 33.3% | 0.322 |
| Atrial Fibrillation (%) | 28.7% | 22.2% | 0.574 |
| ≥2 Leads (%) | 52.6% | 35.5% | 0.2536 |
| Dual Coil ICD (%) | 47.4% | 29.0% | 0.3214 |
| ICD implant >18 months (%) | 68.4% | 52.7% | 0.3155 |
| Dilated cardiomyopathy | 44.4% | 42.6% | 1.0 |
| Hypertrophic cardiomyopathy | 5.6% | 2.1% | 0.4119 |
| Ischemic heart disease | 33.3% | 24.5% | 0.6221 |
| Valvular disease | 11.1% | 4.3% | 0.2467 |
| Myocarditis | 5.6% | 0.0% | 0.1607 |
| Time (Days) | Group | Survival Probability | 95% CI (Lower) | 95% CI (Upper) | Number at Risk | Number of Events |
|---|---|---|---|---|---|---|
| 30 | 1 | 94.7% | 90.3% | 99.3% | 94 | 5 |
| 90 | 1 | 90.3% | 84.5% | 96.5% | 87 | 4 |
| 150 | 1 | 83.5% | 75.9% | 91.9% | 66 | 5 |
| 30 | 2 | 66.7% | 48.1% | 92.4% | 18 | 6 |
| 90 | 2 | 61.1% | 42.3% | 88.3% | 12 | 1 |
| 150 | 2 | 55.0% | 36.1% | 83.9% | 10 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzone, F.; Giovannico, L.; Santobuono, V.E.; Fischetti, G.; Parigino, D.; Savino, L.; Leo, C.; Cristiano, G.; Milano, A.D.; Guaricci, A.I.; et al. Retained Lead Fragments in Superior Vena Cava and Early Post-Transplant Outcomes: A Single Center Preliminary Retrospective Study. Biomedicines 2025, 13, 2509. https://doi.org/10.3390/biomedicines13102509
Mazzone F, Giovannico L, Santobuono VE, Fischetti G, Parigino D, Savino L, Leo C, Cristiano G, Milano AD, Guaricci AI, et al. Retained Lead Fragments in Superior Vena Cava and Early Post-Transplant Outcomes: A Single Center Preliminary Retrospective Study. Biomedicines. 2025; 13(10):2509. https://doi.org/10.3390/biomedicines13102509
Chicago/Turabian StyleMazzone, Federica, Lorenzo Giovannico, Vincenzo Ezio Santobuono, Giuseppe Fischetti, Domenico Parigino, Luca Savino, Claudia Leo, Giuseppe Cristiano, Aldo Domenico Milano, Andrea Igoren Guaricci, and et al. 2025. "Retained Lead Fragments in Superior Vena Cava and Early Post-Transplant Outcomes: A Single Center Preliminary Retrospective Study" Biomedicines 13, no. 10: 2509. https://doi.org/10.3390/biomedicines13102509
APA StyleMazzone, F., Giovannico, L., Santobuono, V. E., Fischetti, G., Parigino, D., Savino, L., Leo, C., Cristiano, G., Milano, A. D., Guaricci, A. I., Padalino, M., Ciccone, M. M., & Bottio, T. (2025). Retained Lead Fragments in Superior Vena Cava and Early Post-Transplant Outcomes: A Single Center Preliminary Retrospective Study. Biomedicines, 13(10), 2509. https://doi.org/10.3390/biomedicines13102509









