Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae
Abstract
:1. Introduction
2. Viruses
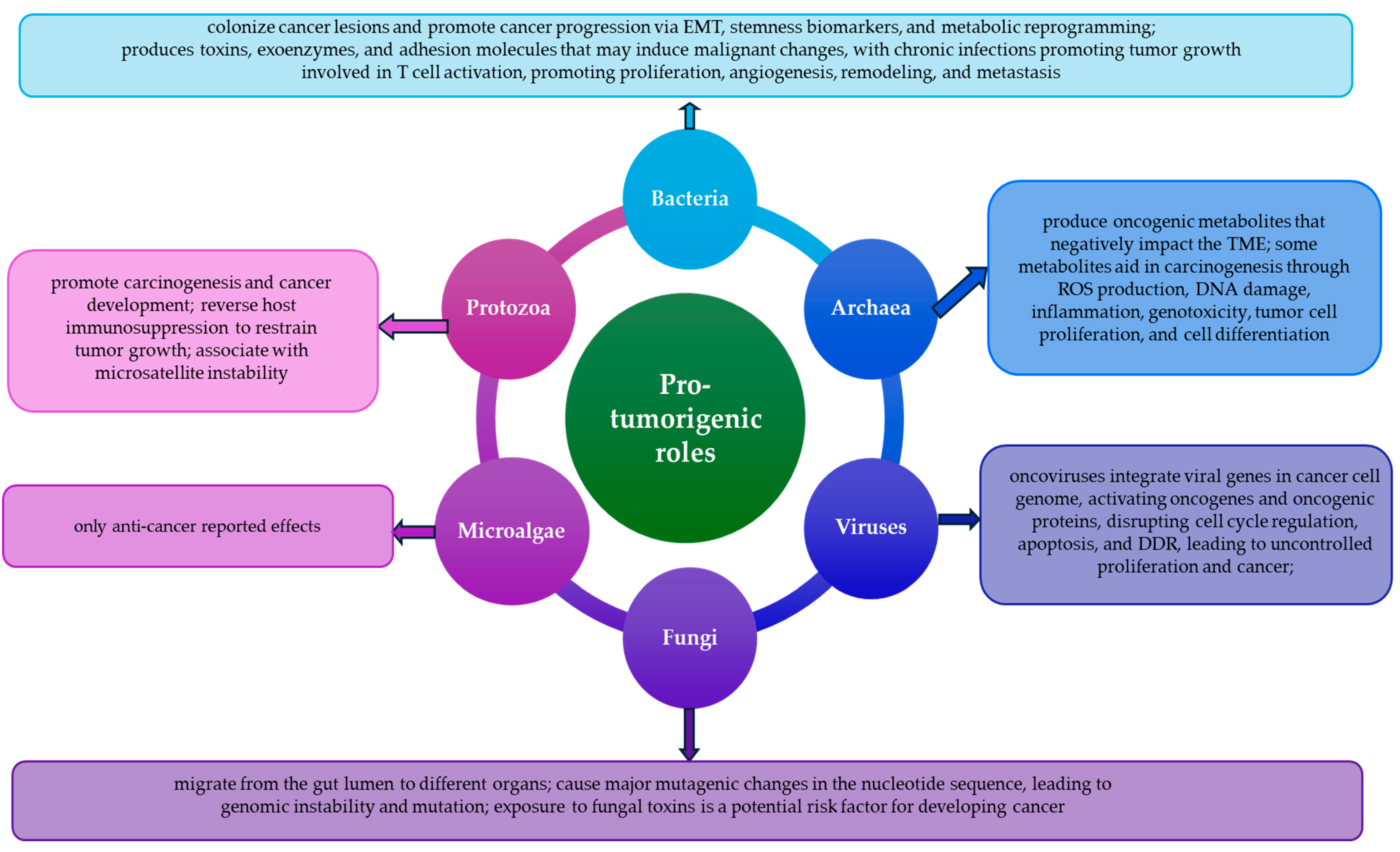
2.1. Pro-Cancer
2.2. Anti-Cancer
3. Bacteria
3.1. Anti-Cancer
3.2. Pro-Cancer
4. Archaea
5. Fungal Infections
5.1. Pro-Cancer
5.2. Anti-Cancer
6. Protozoa
6.1. Pro-Cancer
6.2. Anti-Cancer
7. Microalgae
8. Limitations and Future Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jassim, A.; Rahrmann, E.P.; Simons, B.D.; Gilbertson, R.J. Cancers make their own luck: Theories of cancer origins. Nat. Rev. Cancer 2023, 23, 710–724. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Neagu, A.-N.; Jayaweera, T.; Corrice, L.; Johnson, K.; Darie, C.C. Breast Cancer Exposomics. Life 2024, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.-N.; Josan, C.-L.; Jayaweera, T.M.; Weraduwage, K.; Nuru, N.; Darie, C.C. Double-Edged Sword Effect of Diet and Nutrition on Carcinogenic Molecular Pathways in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 11078. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Lowy, D.R. An Introduction to Virus Infections and Human Cancer. In Viruses and Human Cancer: From Basic Science to Clinical Prevention; Wu, T.C., Chang, M.-H., Jeang, K.-T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–11. [Google Scholar]
- Bezeljak, U. Cancer gene therapy goes viral: Viral vector platforms come of age. Radiol. Oncol. 2022, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Zare, M.; Aghayari, M.; Afkhami, H.; Jafari, G.A. Therapeutic bacteria and viruses to combat cancer: Double-edged sword in cancer therapy: New insights for future. Cell Commun. Signal. 2024, 22, 239. [Google Scholar] [CrossRef]
- Sedighi, M.; Zahedi Bialvaei, A.; Hamblin, M.R.; Ohadi, E.; Asadi, A.; Halajzadeh, M.; Lohrasbi, V.; Mohammadzadeh, N.; Amiriani, T.; Krutova, M.; et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019, 8, 3167–3181. [Google Scholar] [CrossRef]
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A new era of cancer therapy. J. Control. Release 2021, 338, 1–7. [Google Scholar] [CrossRef]
- Cai, M.; Kandalai, S.; Tang, X.; Zheng, Q. Contributions of Human-Associated Archaeal Metabolites to Tumor Microenvironment and Carcinogenesis. Microbiol. Spectr. 2022, 10, e02367-21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, F.; Gao, Y.; Yang, R. Fungi and tumors: The role of fungi in tumorigenesis (Review). Int. J. Oncol. 2024, 64, 52. [Google Scholar] [CrossRef]
- Hosseini, K.; Ahangari, H.; Chapeland-leclerc, F.; Ruprich-Robert, G.; Tarhriz, V.; Dilmaghani, A. Role of Fungal Infections in Carcinogenesis and Cancer Development: A Literature Review. Adv. Pharm. Bull. 2022, 12, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lu, X.; Zhou, D.; Deng, X.-F.; Liu, Q.-X.; Liu, X.-B.; Zhang, J.; Li, Y.-Q.; Zheng, H.; Dai, J.-G. A novel enemy of cancer: Recent investigations into protozoan anti-tumor properties. Front. Cell. Infect. Microbiol. 2024, 13, 1325144. [Google Scholar] [CrossRef]
- Mani, R.; Martin, C.G.; Balu, K.E.; Wang, Q.; Rychahou, P.; Izumi, T.; Evers, B.M.; Suzuki, Y. A Novel Protozoa Parasite-Derived Protein Adjuvant Is Effective in Immunization with Cancer Cells to Activate the Cancer-Specific Protective Immunity and Inhibit the Cancer Growth in a Murine Model of Colorectal Cancer. Cells 2024, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Xin, Z.; Zhang, M.; Cui, H.; Ding, X.; Zhang, T.; Wu, L.; Cui, H.; Xue, Q.; Chen, C.; Gao, J. Algae: A Robust Living Material Against Cancer. Int. J. Nanomed. 2023, 18, 5243–5264. [Google Scholar] [CrossRef]
- Wang, Z. Virotherapy in Head and Neck Cancer. Oral Oncol. Rep. 2024, 9, 100151. [Google Scholar] [CrossRef]
- Handy, C.R.; Krudy, C.; Boulis, N. Gene Therapy: A Potential Approach for Cancer Pain. Pain Res. Treat. 2011, 2011, 987597. [Google Scholar] [CrossRef]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, M.P.; Mullish, B.H.; Frampton, A.E.; Krell, J. Polymorphic microbes: A new emerging hallmark of cancer. Trends Microbiol. 2022, 30, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Ribeiro, M.; Fonseca, L.; Regis, B.; Cardozo, L.F.M.F.; Fragoso dos Santos, H.; Emiliano de Jesus, H.; Schultz, J.; Shiels, P.G.; Stenvinkel, P.; et al. Archaea from the gut microbiota of humans: Could be linked to chronic diseases? Anaerobe 2022, 77, 102629. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Biswas, P.; Pal, S.; Das, M.; Dam, S. Microbe-Induced Oxidative Stress in Cancer Development and Efficacy of Probiotics as Therapeutics in Preventing Its Onset and Progression. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 3513–3542. [Google Scholar]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human Viral Oncogenesis: A Cancer Hallmarks Analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Ye, R.; Wang, A.; Bu, B.; Luo, P.; Deng, W.; Zhang, X.; Yin, S. Viral oncogenes, viruses, and cancer: A third-generation sequencing perspective on viral integration into the human genome. Front. Oncol. 2023, 13, 1333812. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, S.; He, Y.; Wang, L.; Chen, C.; Tang, J.; Liu, W.-L.; Yu, F. Possible Oncogenic Viruses Associated with Lung Cancer. Onco Targets Ther. 2020, 13, 10651–10666. [Google Scholar] [CrossRef] [PubMed]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef]
- Chumakov, P.M.; Morozova, V.V.; Babkin, I.V.; Baikov, I.K.; Netesov, S.V.; Tikunova, N.V. Oncolytic enteroviruses. Mol. Biol. 2012, 46, 639–650. [Google Scholar] [CrossRef]
- Gazal, S.; Gazal, S.; Kaur, P.; Bhan, A.; Olagnier, D. Breaking Barriers: Animal viruses as oncolytic and immunotherapeutic agents for human cancers. Virology 2024, 600, 110238. [Google Scholar] [CrossRef]
- Mager, D.L. Bacteria and cancer: Cause, coincidence or cure? A review. J. Transl. Med. 2006, 4, 14. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, F.; Xie, T.; Du, Z.; Zhong, D.; Qi, Y.; Li, Y.; Li, W.; Lu, Z.; Rao, J.; et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Sci. Adv. 2020, 6, eaba5996. [Google Scholar] [CrossRef]
- Han, D.; Zhang, X.; Ma, Y.; Yang, X.; Li, Z. The development of live microorganism-based oxygen shuttles for enhanced hypoxic tumor therapy. Mater. Today Bio 2023, 18, 100517. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Ventegodt, S.; Morad, M.; Hyam, E.; Merrick, J. Clinical Holistic Medicine: When Biomedicine is Inadequate. Sci. World J. 2004, 4, 462719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kitahara, S.; Kusuda, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Current Landscape of Sonodynamic Therapy for Treating Cancer. Cancers 2021, 13, 6184. [Google Scholar] [CrossRef]
- Ulu, G.T.; Kiraz, Y.; Baran, Y. Chapter 22—Personalized biomedicine in cancer: From traditional therapy to sustainable healthcare. In Biodiversity and Biomedicine; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 441–457. [Google Scholar]
- Xie, L.; Han, Y.; Liu, Y.; Zhou, Y.; Yu, J.; von Brunn, A.; Lei, J. Viral vector-based cancer treatment and current clinical applications. MedComm–Oncology 2023, 2, e55. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Q.; Zhou, J.; Yang, W.; Wang, Y.; Guo, H.; Wei, W. Exploring enterovirus pathogenesis and cancer therapy potential through reverse genetics. Biosaf. Health 2025, 2, e55. [Google Scholar] [CrossRef]
- Asad, A.S.; Moreno Ayala, M.A.; Gottardo, M.F.; Zuccato, C.; Nicola Candia, A.J.; Zanetti, F.A.; Seilicovich, A.; Candolfi, M. Viral gene therapy for breast cancer: Progress and challenges. Expert Opin. Biol. Ther. 2017, 17, 945–959. [Google Scholar] [CrossRef]
- Liang, S.; Wang, C.; Shao, Y.; Wang, Y.; Xing, D.; Geng, Z. Recent advances in bacteria-mediated cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1026248. [Google Scholar] [CrossRef] [PubMed]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Sun, X.; Zhou, Q.; Yan, X. Natural algae-inspired microrobots for emerging biomedical applications and beyond. Cell Rep. Phys. Sci. 2024, 5, 101979. [Google Scholar] [CrossRef]
- Dash, S.R.; Kundu, A.; Kundu, C.N. The role of viruses in cancer progression versus cancer treatment: A dual paradigm. Life Sci. 2024, 341, 122506. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Boorjian, S.A.; Burton, J.P.; Hahn, N.M.; Ingersoll, M.A.; Maleki Vareki, S.; Pal, S.K.; Sfanos, K.S. The Microbiome and Genitourinary Cancer: A Collaborative Review. Eur. Urol. 2019, 75, 637–646. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Munger, K. Viruses associated with human cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 127–150. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, Y.; Wang, X.; Shen, L.; Liao, X.; Zhu, Y.; Yu, J.; Zhao, F.; Zhou, Y.; Shen, H.; et al. Microbiome in cancer: An exploration of carcinogenesis, immune responses and immunotherapy. Front. Immunol. 2022, 13, 877939. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, J.; Qi, F.; Hu, Z.; Sweren, E.; Reddy, S.K.; Chen, L.; Feng, X.; Grice, E.A.; Garza, L.A.; et al. Commensal microbe regulation of skin cells in disease. Cell Host Microbe 2024, 32, 1264–1279. [Google Scholar] [CrossRef]
- Becerril, S.; Corchado-Cobos, R.; García-Sancha, N.; Revelles, L.; Revilla, D.; Ugalde, T.; Román-Curto, C.; Pérez-Losada, J.; Cañueto, J. Viruses and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 5399. [Google Scholar] [CrossRef]
- Sand, L.; Jalouli, J. Viruses and oral cancer. Is there a link? Microbes Infect. 2014, 16, 371–378. [Google Scholar] [CrossRef]
- Monot, M.; Archer, F.; Gomes, M.; Mornex, J.F.; Leroux, C. Advances in the study of transmissible respiratory tumours in small ruminants. Vet. Microbiol. 2015, 181, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xu, Z.; Duan, C.; Zhang, Y.; Wu, X.; Wu, H.; Liu, K.; Mao, X.; Li, B.; Gao, Y.; et al. Role of human papillomavirus and associated viruses in bladder cancer: An updated review. J. Med. Virol. 2023, 95, e29088. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Sausen, D.G.; Shechter, O.; Gallo, E.S.; Dahari, H.; Borenstein, R. Herpes Simplex Virus, Human Papillomavirus, and Cervical Cancer: Overview, Relationship, and Treatment Implications. Cancers 2023, 15, 3692. [Google Scholar] [CrossRef]
- Marônek, M.; Link, R.; Monteleone, G.; Gardlík, R.; Stolfi, C. Viruses in Cancers of the Digestive System: Active Contributors or Idle Bystanders? Int. J. Mol. Sci. 2020, 21, 8133. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; McQuaid, T.J.; Jacobson, I.M. HBV-Induced Carcinogenesis: Mechanisms, Correlation With Viral Suppression, and Implications for Treatment. Liver Int. 2025, 45, e16202. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K. The viral origins of breast cancer. Infect. Agents Cancer 2024, 19, 39. [Google Scholar] [CrossRef]
- Sharma, A.; Grill, M.F.; Spritzer, S.; Leis, A.A.; Anderson, M.; Vig, P.; Porter, A.B. Malignant Glial Neuronal Tumors After West Nile Virus Neuroinvasive Disease: A Coincidence or a Clue? Neurohospitalist 2019, 9, 160–164. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K. Chapter 22—Feline Leukemia Virus Infection. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2014; pp. 224–238. [Google Scholar]
- Zafar, H.S.; Akbar, H.; Xu, H.; Ponnuraj, N.; Van Etten, K.; Jarosinski, K.W. Oncogenic Animal Herpesviruses. Curr. Opin. Virol. 2024, 67, 101424. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Aghi, M.K. Using viral vectors to deliver local immunotherapy to glioblastoma. Neurosurg. Focus FOC 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Mozhei, O.; Teschemacher, A.G.; Kasparov, S. Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers 2020, 12, 3724. [Google Scholar] [CrossRef]
- Lv, Y.-F.; Zhang, H.; Cui, Z.; Ma, C.-J.; Li, Y.-L.; Lu, H.; Wu, H.-Y.; Yang, J.-L.; Cao, C.-Y.; Sun, W.-Z.; et al. Gene delivery to breast cancer by incorporated EpCAM targeted DARPins into AAV2. BMC Cancer 2023, 23, 1220. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Priya, L.B.; Mahalakshmi, B.; Subbiah, S.; Hu, R.-M.; Velmurugan, B.K.; Baskaran, R. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci. 2020, 250, 117550. [Google Scholar] [CrossRef]
- Bieńkowska, A.; Kuźmicka, W.; Ciepiela, O.; Ochocki, J.; Małecki, M. Increased Temperature Facilitates Adeno-Associated Virus Vector Transduction of Colorectal Cancer Cell Lines in a Manner Dependent on Heat Shock Protein Signature. BioMed Res. Int. 2020, 2020, 9107140. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, H.H.; Musa, M. Gene Therapy Targeting p53 and KRAS for Colorectal Cancer Treatment: A Myth or the Way Forward? Int. J. Mol. Sci. 2021, 22, 11941. [Google Scholar] [CrossRef]
- Słyk, Ż.; Wrzesień, R.; Barszcz, S.; Gawrychowski, K.; Małecki, M. Adeno-associated virus vector hydrogel formulations for brain cancer gene therapy applications. Biomed. Pharmacother. 2024, 170, 116061. [Google Scholar] [CrossRef]
- Grygoryev, D.; Ekstrom, T.; Manalo, E.; Link, J.M.; Alshaikh, A.; Keith, D.; Allen-Petersen, B.L.; Sheppard, B.; Morgan, T.; Soufi, A.; et al. Sendai virus is robust and consistent in delivering genes into human pancreatic cancer cells. Heliyon 2024, 10, e27221. [Google Scholar] [CrossRef]
- Hamidi-Sofiani, V.; Rakhshi, R.; Moradi, N.; Zeynali, P.; Nakhaie, M.; Behboudi, E. Oncolytic viruses and pancreatic cancer. Cancer Treat. Res. Commun. 2022, 31, 100563. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, T.; Wei, L.; Chen, F.; Ding, Y.; Zhang, Q.; Dang, Y. Adeno-associated virus vector-mediated gene therapy for the treatment of ovarian cancer: A literature review. Ann. Transl. Med. 2022, 10, 1024. [Google Scholar] [CrossRef]
- Killock, D. Viral gene therapy active in ovarian cancer. Nat. Rev. Clin. Oncol. 2020, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Hromic-Jahjefendic, A.; Lundstrom, K. Viral Vector-Based Melanoma Gene Therapy. Biomedicines 2020, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Takagi-Kimura, M.; Kasahara, N. Efficient tumor transduction and antitumor efficacy in experimental human osteosarcoma using retroviral replicating vectors. Cancer Gene Ther. 2019, 26, 41–47. [Google Scholar] [CrossRef]
- Vardeu, A.; Davis, C.; McDonald, I.; Stahlberg, G.; Thapa, B.; Piotrowska, K.; Marshall, M.A.; Evans, T.; Wheeler, V.; Sebastian, S.; et al. Intravenous administration of viral vectors expressing prostate cancer antigens enhances the magnitude and functionality of CD8+ T cell responses. J. ImmunoTherapy Cancer 2022, 10, e005398. [Google Scholar] [CrossRef]
- de Jong, Y.P.; Herzog, R.W. Liver gene therapy and hepatocellular carcinoma: A complex web. Mol. Ther. 2021, 29, 1353–1354. [Google Scholar] [CrossRef]
- Hadi, M.; Qutaiba, B.; Allela, O.; Jabari, M.; Jasoor, A.M.; Naderloo, O.; Yasamineh, S.; Gholizadeh, O.; Kalantari, L. Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma. Virol. J. 2024, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Tholomier, C.; Mokkapati, S.; Dinney, C.P.N. Interferon gene therapy with nadofaragene firadenovec for bladder cancer: From bench to approval. Front. Immunol. 2023, 14, 1260498. [Google Scholar] [CrossRef]
- Vu, D.L.; Kaiser, L. The concept of commensal viruses almost 20 years later: Redefining borders in clinical virology. Clin. Microbiol. Infect. 2017, 23, 688–690. [Google Scholar] [CrossRef]
- Liu, L.; Gong, T.; Tao, W.; Lin, B.; Li, C.; Zheng, X.; Zhu, S.; Jiang, W.; Zhou, R. Commensal viruses maintain intestinal intraepithelial lymphocytes via noncanonical RIG-I signaling. Nat. Immunol. 2019, 20, 1681–1691. [Google Scholar] [CrossRef]
- Bosch, A.; Biesbroek, G.; Trzciński, K.; Sanders, E.; Bogaert, D. Viral and Bacterial Interactions in the Upper Respiratory Tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Javid, H.; Sharbaf Mashhad, A.; Yazdani, S.; Akbari Oryani, M.; Akbari, S.; Rezagholinejad, N.; Tajaldini, M.; Karimi-Shahri, M. The role of viruses in cancer development versus cancer therapy: An oncological perspective. Cancer Med. 2023, 12, 11127–11148. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Arenas, M.; Galán, J.C.; Palero, F.; González-Candelas, F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect. Genet. Evol. 2015, 30, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Solovyeva, V.V.; Kitaeva, K.V.; Dunham, S.P.; Khaiboullina, S.F.; Rizvanov, A.A. Recombinant Viruses for Cancer Therapy. Biomedicines 2018, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Scanlan, H.; Coffman, Z.; Bettencourt, J.; Shipley, T.; Bramblett, D.E. Herpes simplex virus 1 as an oncolytic viral therapy for refractory cancers. Front. Oncol. 2022, 12, 940019. [Google Scholar] [CrossRef]
- Shen, Y.; Nemunaitis, J. Fighting Cancer with Vaccinia Virus: Teaching New Tricks to an Old Dog. Mol. Ther. 2005, 11, 180–195. [Google Scholar] [CrossRef]
- Robert, S.; Roman Ortiz, N.I.; LaRocca, C.J.; Ostrander, J.H.; Davydova, J. Oncolytic Adenovirus for the Targeting of Paclitaxel-Resistant Breast Cancer Stem Cells. Viruses 2024, 16, 567. [Google Scholar] [CrossRef]
- Robertson, M.G.; Eidenschink, B.B.; Iguchi, E.; Zakharkin, S.O.; LaRocca, C.J.; Tolosa, E.J.; Truty, M.J.; Jacobsen, K.; Fernandez-Zapico, M.E.; Davydova, J. Cancer imaging and therapy utilizing a novel NIS-expressing adenovirus: The role of adenovirus death protein deletion. Mol. Ther.-Oncolytics 2021, 20, 659–668. [Google Scholar] [CrossRef]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic Herpes Simplex Virus-Based Therapies for Cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef]
- Ammayappan, A.; Russell, S.J.; Federspiel, M.J. Recombinant mumps virus as a cancer therapeutic agent. Mol. Ther. Oncolytics 2016, 3, 16019. [Google Scholar] [CrossRef] [PubMed]
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Kohoutová, M.; Slanař, O. Human Endogenous Retroviruses in Breast Cancer: Altered Expression Pattern Implicates Divergent Roles in Carcinogenesis. Oncology 2024, 102, 858–867. [Google Scholar] [CrossRef]
- Dhuri, K.; Gharat, S.; Fernandes, N.; Basudkar, V.; Doshi, G.; Momin, M. Viral therapy for targeted drug delivery to cancers: Recent advances, clinical and regulatory perspectives. J. Drug Deliv. Sci. Technol. 2024, 92, 105365. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Steinmetz, N.F. The pharmacology of plant virus nanoparticles. Virology 2021, 556, 39–61. [Google Scholar] [CrossRef]
- Esfandiari, N.; Arzanani, M.K.; Koohi-Habibi, M. The study of toxicity and pathogenicity risk of Potato Virus X/Herceptin nanoparticles as agents for cancer therapy. Cancer Nanotechnol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Shahgolzari, M.; Venkataraman, S.; Osano, A.; Akpa, P.A.; Hefferon, K. Plant Virus Nanoparticles Combat Cancer. Vaccines 2023, 11, 1278. [Google Scholar] [CrossRef]
- Le, D.H.T.; Lee, K.L.; Shukla, S.; Commandeur, U.; Steinmetz, N.F. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 2017, 9, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Gamper, C.; Spenlé, C.; Boscá, S.; van der Heyden, M.; Erhardt, M.; Orend, G.; Bagnard, D.; Heinlein, M. Functionalized Tobacco Mosaic Virus Coat Protein Monomers and Oligomers as Nanocarriers for Anti-Cancer Peptides. Cancers 2019, 11, 1609. [Google Scholar] [CrossRef]
- Czapar, A.E.; Zheng, Y.-R.; Riddell, I.A.; Shukla, S.; Awuah, S.G.; Lippard, S.J.; Steinmetz, N.F. Tobacco Mosaic Virus Delivery of Phenanthriplatin for Cancer therapy. ACS Nano 2016, 10, 4119–4126. [Google Scholar] [CrossRef]
- Lin, R.D.; Steinmetz, N.F. Tobacco mosaic virus delivery of mitoxantrone for cancer therapy. Nanoscale 2018, 10, 16307–16313. [Google Scholar] [CrossRef]
- Shukla, S.; Roe, A.J.; Liu, R.; Veliz, F.A.; Commandeur, U.; Wald, D.N.; Steinmetz, N.F. Affinity of plant viral nanoparticle potato virus X (PVX) towards malignant B cells enables cancer drug delivery. Biomater. Sci. 2020, 8, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fiering, S.N.; Steinmetz, N.F. Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Adv. Ther. 2019, 2, 1900003. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Perillo, F.; Amoroso, C.; Strati, F.; Giuffrè, M.R.; Díaz-Basabe, A.; Lattanzi, G.; Facciotti, F. Gut Microbiota Manipulation as a Tool for Colorectal Cancer Management: Recent Advances in Its Use for Therapeutic Purposes. Int. J. Mol. Sci. 2020, 21, 5389. [Google Scholar] [CrossRef]
- Yadav, A.; Kaushik, M.; Tiwari, P.; Dada, R. From microbes to medicine: Harnessing the gut microbiota to combat prostate cancer. Microb. Cell 2024, 11, 187–197. [Google Scholar] [CrossRef]
- Soe Thu, M.; Ondee, T.; Nopsopon, T.; Amayazifun, I.; Fothergill, J.; Hirankarn, N.; Campbell, B.; Pongpirul, K. Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis. Biology 2023, 12, 280. [Google Scholar] [CrossRef]
- Supriya, Y.; Sivamalar, S.; Nallusamy, D.; Sureka, V.; Arunagirinathan, N.; Saravanan, S.; Balakrishnan, P.; Viswanathan, D.; Rajakumar, G. Application of probiotics in cervical cancer infections to enhance the immune response. Microb. Pathog. 2024, 193, 106764. [Google Scholar] [CrossRef]
- Yang, Y.; An, Y.; Dong, Y.; Chu, Q.; Wei, J.; Wang, B.; Cao, H. Fecal microbiota transplantation: No longer cinderella in tumour immunotherapy. EBioMedicine 2024, 100, 104967. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal microbiota transplantation is a truly opulent and promising edge: Fully grasp its potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Chen, T. Potential Role of Vaginal Microbiota in Ovarian Cancer Carcinogenesis, Progression and Treatment. Pharmaceutics 2023, 15, 948. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fan, Y.; Li, Y.; Dong, J.; Zhang, S.; Wang, B.; Liu, J.; Liu, X.; Fan, S.; Guan, J.; et al. Oral microbiota transplantation fights against head and neck radiotherapy-induced oral mucositis in mice. Comput. Struct. Biotechnol. J. 2021, 19, 5898–5910. [Google Scholar] [CrossRef]
- Wei, H.; Chen, L.; Lian, G.; Yang, J.; Li, F.; Zou, Y.; Lu, F.; Yin, Y. Antitumor mechanisms of bifidobacteria (Review). Oncol. Lett. 2018, 16, 3–8. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, S.-Y.; Yoon, Y.; Park, C.; Sohn, J.; Jeong, J.-J.; Jeon, B.-N.; Jang, M.; An, C.; Lee, S.; et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021, 6, 277–288. [Google Scholar] [CrossRef]
- Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells 2023, 12, 1945. [Google Scholar] [CrossRef]
- Nguyen, D.-H.; Chong, A.; Hong, Y.; Min, J.-J. Bioengineering of bacteria for cancer immunotherapy. Nat. Commun. 2023, 14, 3553. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.; Shao, Y.; Yue, X.; Chu, Y.; Chen, D. Attenuated Salmonella typhimurium L forms suppress tumor growth and promote apoptosis in murine ovarian tumors. Sci. Rep. 2024, 14, 16045. [Google Scholar] [CrossRef]
- Jazeela, K.; Chakraborty, A.; Karunasagar, I.; Deekshit, V.K. Nontyphoidal Salmonella: A potential anticancer agent. J. Appl. Microbiol. 2020, 128, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Aganja, R.P.; Sivasankar, C.; Senevirathne, A.; Lee, J.H. Salmonella as a Promising Curative Tool against Cancer. Pharmaceutics 2022, 14, 2100. [Google Scholar] [CrossRef] [PubMed]
- Trivanović, D.; Pavelić, K.; Peršurić, Ž. Fighting Cancer with Bacteria and Their Toxins. Int. J. Mol. Sci. 2021, 22, 12980. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.R.; Odiase, P.; Rachakonda, K.; Garg, A.P.; Adunyah, S.E.; Rachakonda, G. Application of C-Terminal Clostridium Perfringens Enterotoxin in Treatment of Brain Metastasis from Breast Cancer. Cancers 2022, 14, 4309. [Google Scholar] [CrossRef]
- Morgan, R.N.; Saleh, S.E.; Farrag, H.A.; Aboshanab, K.M. New Insights on Pseudomonas Aeruginosa Exotoxin A-based Immunotoxins in Targeted Cancer Therapeutic Delivery. Ther. Deliv. 2023, 14, 31–60. [Google Scholar] [CrossRef]
- Hulst, M.B.; Zhang, L.; van der Heul, H.U.; Du, C.; Elsayed, S.S.; Koroleva, A.; Grocholski, T.; Wander, D.P.A.; Metsä-Ketelä, M.; Neefjes, J.J.C.; et al. Metabolic engineering of Streptomyces peucetius for biosynthesis of N,N-dimethylated anthracyclines. Front. Bioeng. Biotechnol. 2024, 12, 1363803. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Tang, Y.; Wang, L.; Jiang, F.; Luo, Y.; Gao, X.; Li, P.; Zou, J. Bifidobacterium-mediated high-intensity focused ultrasound for solid tumor therapy: Comparison of two nanoparticle delivery methods. Int. J. Hyperth. 2020, 37, 870–878. [Google Scholar] [CrossRef]
- Zeng, F.; Du, M.; Yang, Y.; Fang, J.; Wang, Y.; Goh, M.; Lin, Y.; Wang, H.; Yan, F.; Chen, Z. Enhancing photothermal therapy of tumors with image-guided thermal control of gene-expressing bacteria. Theranostics 2024, 14, 5945–5964. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.; Liu, H.; Fu, K.; Ji, Y.; Ji, W.; Li, Y.; Yan, Q.; Yang, G. Escherichia coli Nissle 1917-driven microrobots for effective tumor targeted drug delivery and tumor regression. Acta Biomater. 2023, 169, 477–488. [Google Scholar] [CrossRef]
- Yang, X.; Komatsu, S.; Reghu, S.; Miyako, E. Optically activatable photosynthetic bacteria-based highly tumor specific immunotheranostics. Nano Today 2021, 37, 101100. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, G.; Lin, R.; Chen, W.; Chen, R.; Sun, Y.; Chen, L.; Hong, D.; Chen, L. Magnetotactic Bacteria AMB-1 with Active Deep Tumor Penetrability for NIR-II Photothermal Tumor Therapy. ACS Omega 2024, 9, 23060–23068. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Raouf, R.; Ouf, S.A.; Gabr, M.M.; Zakaria, M.M.; El-Yasergy, K.F.; Ali-El-Dein, B. Escherichia coli foster bladder cancer cell line progression via epithelial mesenchymal transition, stemness and metabolic reprogramming. Sci. Rep. 2020, 10, 18024. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Cancerous patients and outbreak of Escherichia coli: An important issue in oncology. Asian Pac. J. Trop. Dis. 2014, 4, 204–206. [Google Scholar] [CrossRef]
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 2018, 44, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xu, Y.; Dou, Y.; Xu, D. Helicobacter pylori and gastric cancer: Mechanisms and new perspectives. J. Hematol. Oncol. 2025, 18, 10. [Google Scholar] [CrossRef]
- Li, M.-L.; Hong, X.-X.; Zhang, W.-J.; Liang, Y.-Z.; Cai, T.-T.; Xu, Y.; Pan, H.-F.; Kang, J.-Y.; Guo, S.-J.; Li, H.-W. Helicobacter pylori plays a key role in gastric adenocarcinoma induced by spasmolytic polypeptide-expressing metaplasia. World J. Clin. Cases 2023, 11, 3714–3724. [Google Scholar] [CrossRef]
- Park, J.; Koo, J. Helicobacter pylori infection in gastric mucosa-associated lymphoid tissue lymphoma. World J. Gastroenterol. WJG 2014, 20, 2751–2759. [Google Scholar] [CrossRef]
- Enroth, H.; Kraaz, W.; Engstrand, L.; Nyrén, O.; Rohan, T. Helicobacter pylori Strain Types and Risk of Gastric Cancer: A Case-Control Study1. Cancer Epidemiol. Biomark. Prev. 2000, 9, 981–985. [Google Scholar]
- Fol, M.; Koziński, P.; Kulesza, J.; Białecki, P.; Druszczyńska, M. Dual Nature of Relationship between Mycobacteria and Cancer. Int. J. Mol. Sci. 2021, 22, 8332. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontology 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- Wei, Y.; Sandhu, E.; Yang, X.; Yang, J.; Ren, Y.; Gao, X. Bidirectional Functional Effects of Staphylococcus on Carcinogenesis. Microorganisms 2022, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Odunitan, T.T.; Apanisile, B.T.; Akinboade, M.W.; Abdulazeez, W.O.; Oyaronbi, A.O.; Ajayi, T.M.; Oyekola, S.A.; Ibrahim, N.O.; Nafiu, T.; Afolabi, H.O.; et al. Microbial mysteries: Staphylococcus aureus and the enigma of carcinogenesis. Microb. Pathog. 2024, 194, 106831. [Google Scholar] [CrossRef]
- Krueger, A.; Mohamed, A.; Kolka, C.; Stoll, T.; Zaugg, J.; Linedale, R.; Morrison, M.; Soyer, P.; Philip, H.; Frazer, I.; et al. Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress. Cancers 2022, 14, 2143. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and Downsides of Reactive Oxygen Species for Cancer: The Roles of Reactive Oxygen Species in Tumorigenesis, Prevention, and Therapy. Antioxid. Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Oehmcke-Hecht, S.; Mandl, V.; Naatz, L.T.; Dühring, L.; Köhler, J.; Kreikemeyer, B.; Maletzki, C. Streptococcus gallolyticus abrogates anti-carcinogenic properties of tannic acid on low-passage colorectal carcinomas. Sci. Rep. 2020, 10, 4714. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013.e1016. [Google Scholar] [CrossRef]
- Scott, N.; Whittle, E.; Jeraldo, P.; Chia, N. A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia 2022, 29, 100797. [Google Scholar] [CrossRef]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. MJMS 2020, 27, 9–21. [Google Scholar] [CrossRef]
- Chung, L.; Thiele Orberg, E.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 203–214.e205. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N. Symbiotic Interactions of Archaea in Animal and Human Microbiomes. Curr. Clin. Microbiol. Rep. 2023, 10, 161–173. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J. Archaeosomes: New Generation of Liposomes Based on Archaeal Lipids for Drug Delivery and Biomedical Applications. ACS Omega 2023, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, F.; Akbari, B.; Tayebi, L. Chapter 9—Functionalized Archaeosomes for Cancer Therapy. In Functionalized Nanomaterials for Cancer Research; Barabadi, H., Mostafavi, E., Mustansar Hussain, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 181–195. [Google Scholar]
- Alavi, S.E.; Mansouri, H.; Esfahani, M.K.M.; Movahedi, F.; Akbarzadeh, A.; Chiani, M. Archaeosome: As New Drug Carrier for Delivery of Paclitaxel to Breast Cancer. Indian J. Clin. Biochem. 2014, 29, 150–153. [Google Scholar] [CrossRef]
- Mazloumi Tabrizi, M.; Rastin, S.; Kabiri, N.; Akbarzadeh, A. Synthesis of Paclitaxel Loaded on Nanoparticles of Archaeosomes and Investigation of Its Anti-cancer Properties. Asian Pac. J. Cancer Biol. 2017, 2, 67–70. [Google Scholar] [CrossRef]
- Babunagappan, K.; Seetharaman, A.; Ariraman, S.; Santhosh, P.; Ulrih, N.; Genova, J.; Sudhakar, S. Doxorubicin loaded thermostable nanoarchaeosomes: A next-generation drug carrier for breast cancer therapeutics. Nanoscale Adv. 2024, 6, 2026–2037. [Google Scholar] [CrossRef]
- Li, F.; Gao, Y.; Cheng, W.; Su, X.; Yang, R. Gut fungal mycobiome: A significant factor of tumor occurrence and development. Cancer Lett. 2023, 569, 216302. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Szóstak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Schmidt, M.; Pruss, Ł.; Milanowska-Zabel, K.; Kozlowski, P.; Philips, A. Host Factors Associated with Gut Mycobiome Structure. mSystems 2023, 8, e00986-22. [Google Scholar] [CrossRef] [PubMed]
- Witchley, J.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 2019, 25, 432–443.e436. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Kalan, L.R. Forgotten fungi: The importance of the skin mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef]
- White, T.C.; Findley, K.; Dawson, T.L.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Hemmerling, A.; Miller, S.; Huibner, S.; Kulikova, M.; Liu, R.; Crawford, E.; Castañeda, G.R.; Coburn, B.; Cohen, C.R.; et al. Vaginal fungi are associated with treatment-induced shifts in the vaginal microbiota and with a distinct genital immune profile. Microbiol. Spectr. 2024, 12, e03501-23. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guan, Y.-X.; Li, C.-H.; Zheng, Q.; Yin, Z.-J.; Wang, H.; Liu, N.-N. “Nutrient–fungi–host” tripartite interaction in cancer progression. iMeta 2024, 3, e170. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wu, W.; Wu, S.; Young, A.; Yan, Z. Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol. Res. 2023, 272, 127370. [Google Scholar] [CrossRef]
- Wang, H.; Capula, M.; Krom, B.P.; Yee, D.; Giovannetti, E.; Deng, D. Of fungi and men: Role of fungi in pancreatic cancer carcinogenesis. Ann. Transl. Med. 2020, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e3812. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Chai, D.; Li, C.; Qiu, Z.; Kuang, T.; Liu, L.; Deng, W.; Wang, W. Characterization of the intestinal fungal microbiome in patients with hepatocellular carcinoma. J. Transl. Med. 2023, 21, 126. [Google Scholar] [CrossRef]
- Ashrafi, S.; Amini, A.A.; Karimi, P.; Bagherian, M.; Adibzadeh Sereshgi, M.M.; Asgarhalvaei, F.; Ahmadi, K.; Yazdi, M.H.; Jahantigh, H.; Mahdavi, M.; et al. Candidiasis in breast cancer: Tumor progression or not? Iran. J. Basic Med. Sci. 2024, 27, 1346–1356. [Google Scholar]
- Zhong, M.; Xiong, Y.; Zhao, J.; Gao, Z.; Ma, J.; Wu, Z.; Song, Y.; Hong, X. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [PubMed]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021, 268, 115713. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Zheng, H.; She, J.; Feng, N.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Molecular Mechanism of Aflatoxin-Induced Hepatocellular Carcinoma Derived from a Bioinformatics Analysis. Toxins 2020, 12, 203. [Google Scholar] [CrossRef]
- Adam, M.A.A.; Kamal, L.Z.M.; Kanakal, M.; Babu, D.; Dahham, S.S.; Tabana, Y.; Lok, B.; Bermoy, B.M.; Yunus, M.A.; Than, L.T.L.; et al. The Effect of Aflatoxin B1 on Tumor-Related Genes and Phenotypic Characters of MCF7 and MCF10A Cells. Int. J. Mol. Sci. 2022, 23, 11856. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ejileugha, C.; Augustine, J.; Okolo, C.A.; Onyeaka, H. A Review of the Mycotoxin Family of Fumonisins, Their Biosynthesis, Metabolism, Methods of Detection and Effects on Humans and Animals. Int. J. Mol. Sci. 2025, 26, 184. [Google Scholar] [CrossRef]
- Ferreira, R.; Limeta, A.; Nielsen, J. Tackling Cancer with Yeast-Based Technologies. Trends Biotechnol. 2019, 37, 592–603. [Google Scholar] [CrossRef]
- Hu, F.; Wu, Y.; Liu, C.; Zhu, Y.; Ye, S.; Xi, Y.; Cui, W.; Bu, S. Penicillin disrupts mitochondrial function and induces autophagy in colorectal cancer cell lines. Oncol. Lett. 2021, 22, 691. [Google Scholar] [CrossRef]
- He, X.; Yao, Q.; Fan, D.; Duan, L.; You, Y.; Liang, W.; Zhou, Z.; Teng, S.; Liang, Z.; Hall, D.D.; et al. Cephalosporin antibiotics specifically and selectively target nasopharyngeal carcinoma through HMOX1-induced ferroptosis. Life Sci. 2021, 277, 119457. [Google Scholar] [CrossRef]
- Mousazadeh, R.; Hesaraki, S.; Bayat, M.; Jahandideh, A.; Hashemi, J. Anticancer tendency of aflatoxin B1 in 4T1 breast cancer cell line. Gene Rep. 2019, 16, 100442. [Google Scholar] [CrossRef]
- Perlatti, B.; Lan, N.; Earp, C.E.; AghaAmiri, S.; Vargas, S.H.; Azhdarinia, A.; Bills, G.F.; Gloer, J.B. Arenicolins: C-Glycosylated Depsides from Penicillium arenicola. J. Nat. Prod. 2020, 83, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Abdolalizadeh, J.; Sambrani, R.; Kohan, L.; Jafari, B. Effect of Heat-killed Saccharomyces cerevisiae on Growth Rate and Apoptosis in Colorectal Cancer Cells. J. Maz. Univ. Med. Sci. 2020, 30, 133–139. [Google Scholar]
- Elwakkad, A.; Ghoneum, M.; El-sawi, M.; Mohamed, S.I.; Gamal el Din, A.A.; Pan, D.; Elqattan, G.M. Baker’s yeast induces apoptotic effects and histopathological changes on skin tumors in mice. Cogent Med. 2018, 5, 1437673. [Google Scholar] [CrossRef]
- Wansley, E.K.; Chakraborty, M.; Hance, K.W.; Bernstein, M.B.; Boehm, A.L.; Guo, Z.; Quick, D.; Franzusoff, A.; Greiner, J.W.; Schlom, J.; et al. Vaccination with a Recombinant Saccharomyces cerevisiae Expressing a Tumor Antigen Breaks Immune Tolerance and Elicits Therapeutic Antitumor Responses. Clin. Cancer Res. 2008, 14, 4316–4325. [Google Scholar] [CrossRef]
- Jadid, M.F.S.; Jafari-Gharabaghlou, D.; Bahrami, M.K.; Bonabi, E.; Zarghami, N. Enhanced anti-cancer effect of curcumin loaded-niosomal nanoparticles in combination with heat-killed Saccharomyces cerevisiae against human colon cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104167. [Google Scholar] [CrossRef]
- Varghese, S.; Jisha, M.S.; Rajeshkumar, K.C.; Gajbhiye, V.; Alrefaei, A.F.; Jeewon, R. Endophytic fungi: A future prospect for breast cancer therapeutics and drug development. Heliyon 2024, 10, e33995. [Google Scholar] [CrossRef]
- Kaliaperumal, K.; Salendra, L.; Liu, Y.; Ju, Z.; Sahu, S.K.; Elumalai, S.; Subramanian, K.M.; Alotaibi, N.; Alshammari, N.; Saeed, M.; et al. Isolation of anticancer bioactive secondary metabolites from the sponge-derived endophytic fungi Penicillium sp. and in-silico computational docking approach. Front. Microbiol. 2023, 14, 1216928. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Elmaidomy, A.H.; Hisham, M.; Glaeser, S.P.; Kämpfer, P.; Wu, J.; Abdelmohsen, U.R. Hyrtios sp.-associated Cladosporium sp. UR3 as a potential source of antiproliferative metabolites. BMC Microbiol. 2024, 24, 445. [Google Scholar] [CrossRef]
- Anandan, S.; Gowtham, H.G.; Shivakumara, C.S.; Thampy, A.; Singh, S.B.; Murali, M.; Shivamallu, C.; Pradeep, S.; Shilpa, N.; Shati, A.A.; et al. Integrated approach for studying bioactive compounds from Cladosporium spp. against estrogen receptor alpha as breast cancer drug target. Sci. Rep. 2022, 12, 22446. [Google Scholar] [CrossRef]
- Adorisio, S.; Fierabracci, A.; Muscari, I.; Liberati, A.M.; Cannarile, L.; Thuy, T.T.; Sung, T.V.; Sohrab, H.; Hasan, C.M.; Ayroldi, E.; et al. Fusarubin and Anhydrofusarubin Isolated from A Cladosporium Species Inhibit Cell Growth in Human Cancer Cell Lines. Toxins 2019, 11, 503. [Google Scholar] [CrossRef]
- El Skhawy, N.; Eissa, M.M. Shedding light on a mysterious link between Toxoplasma gondii and cancer: A review. Exp. Parasitol. 2023, 250, 108544. [Google Scholar] [CrossRef] [PubMed]
- Grandes Blanco, A.I.; Sánchez Minutti, L.; García Barrientos, R.; Toledo Rueda, W. Toxoplasma gondii and Its Relation to Cancer. In Pathogens Associated with the Development of Cancer in Humans: OMICs, Immunological, and Pathophysiological Studies; Velázquez-Márquez, N., Paredes-Juárez, G.A., Vallejo-Ruiz, V., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 225–239. [Google Scholar]
- Caner, A. Toxoplasma gondii could have a possible role in the cancer mechanism by modulating the host's cell response. Acta Trop. 2021, 220, 105966. [Google Scholar] [CrossRef]
- Chen, X.; Qin, L.; Hu, W.; Adah, D. The mechanisms of action of Plasmodium infection against cancer. Cell Commun. Signal. 2021, 19, 74. [Google Scholar] [CrossRef]
- Kaufman, C.D.; Farré, C.; Biscari, L.; Pérez, A.R.; Alloatti, A. Trypanosoma cruzi, Chagas disease and cancer: Putting together the pieces of a complex puzzle. Front. Cell Dev. Biol. 2023, 11, 1260423. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, W.; Wang, H.; Wang, Y.; Li, J.; Wu, X. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 166–173. [Google Scholar] [CrossRef]
- Mahdavi, F.; Sadrebazzaz, A.; Chahardehi, A.M.; Badali, R.; Omidian, M.; Hassanipour, S.; Asghari, A. Global epidemiology of Giardia duodenalis infection in cancer patients: A systematic review and meta-analysis. Int. Health 2021, 14, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, L.; Razmjou, E.; Rafiei-Sefiddashti, R.; Meamar, A.R.; Akhlaghi, L. Entamoeba histolytica and Probable Effect on Production Microsatellite Instability in Colorectal Cancer. Curr. Microbiol. 2022, 79, 111. [Google Scholar] [CrossRef]
- Al-Kamel, M.A.N. Leishmaniasis and Malignancy: A Review and Perspective. Clin. Ski. Cancer 2017, 2, 54–58. [Google Scholar] [CrossRef]
- Lantier, L.; Poupée-Beaugé, A.; Tommaso, A.; Ducournau, C.; Epardaud, M.; Lakhrif, Z.; Germon, S.; Debierre-Grockiego, F.; Mévélec, M.-N.; Battistoni, A.; et al. Neospora caninum: A new class of biopharmaceuticals in the therapeutic arsenal against cancer. J. Immunother. Cancer 2020, 8, e001242. [Google Scholar] [CrossRef]
- Lu, J.; Wei, N.; Zhu, S.; Chen, X.; Gong, H.; Mi, R.; Huang, Y.; Chen, Z.; Li, G. Exosomes Derived From Dendritic Cells Infected With Toxoplasma gondii Show Antitumoral Activity in a Mouse Model of Colorectal Cancer. Front. Oncol. 2022, 12, 899737. [Google Scholar] [CrossRef]
- Eissa, M.M.; Salem, A.E.; El Skhawy, N. Parasites revive hope for cancer therapy. Eur. J. Med. Res. 2024, 29, 489. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, A.; Debnath, B.; Rajkhowa, A.; Paul, K.; Majumder, R.; Manna, K. Amoebiasis: An Infectious Disease Caused by Entamoeba histolytica. Asian J. Basic Sci. Res. 2022, 4, 32–40. [Google Scholar] [CrossRef]
- Zaki, L.; Olfatifar, M.; Ghaffarifar, F.; Eslahi, A.V.; KarimiPourSaryazdi, A.; Taghipour, A.; Hamidianfar, N.; Badri, M.; Jokelainen, P. Global prevalence of Toxoplasma gondii in birds: A systematic review and meta-analysis. Parasite Epidemiol. Control 2024, 25, e00350. [Google Scholar] [CrossRef]
- Yu, C.-P.; Chen, B.-C.; Chou, Y.-C.; Hsieh, C.-J.; Lin, F.-H. The epidemiology of patients with toxoplasmosis and its associated risk factors in Taiwan during the 2007–2020 period. PLoS ONE 2023, 18, e0290769. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, W.; Peng, H. Toxoplasma gondii infection possibly reverses host immunosuppression to restrain tumor growth. Front. Cell. Infect. Microbiol. 2022, 12, 959300. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, O.T.; Opsteen, S.A.; Graves, K.J.; Muzny, C.A. Trichomoniasis. Infect. Dis. Clin. N. Am. 2023, 37, 245–265. [Google Scholar] [CrossRef]
- Hamar, B.; Teutsch, B.; Eszter, H.; Hegyi, P.; Váradi, A.; Nyirády, P.; Hunka, Z.; Acs, N.; Lintner, B.; Hermánné, R.; et al. Trichomonas vaginalis infection is associated with increased risk of cervical carcinogenesis: A systematic review and meta-analysis of 470,000 patients. Int. J. Gynecol. Obstet. 2023, 163, 31–43. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Su, R.-Y.; Chung, C.-H.; Huang, K.-Y.; Lin, H.-A.; Wang, J.-Y.; Chen, C.-C.; Chien, W.-C.; Lin, H.-C. Association between trichomoniasis and prostate and bladder diseases: A population-based case–control study. Sci. Rep. 2022, 12, 15358. [Google Scholar] [CrossRef]
- Mannan, S.B.; Elhadad, H.; Loc, T.T.H.; Sadik, M.; Mohamed, M.Y.F.; Nam, N.H.; Thuong, N.D.; Hoang-Trong, B.-L.; Duc, N.T.M.; Hoang, A.N.; et al. Prevalence and associated factors of asymptomatic leishmaniasis: A systematic review and meta-analysis. Parasitol. Int. 2021, 81, 102229. [Google Scholar] [CrossRef]
- Ding, H.; Wu, S.; Jin, Z.; Zheng, B.; Hu, Y.; He, K.; Lu, S.; Zhuo, X. Anti-Tumor Effect of Parasitic Protozoans. Bioengineering 2022, 9, 395. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, F.; Chen, H.; Tang, Y.; Xing, T.; Luo, Q.; Yu, L.; Du, J.; Shen, J.; Zhang, L. Toxoplasma gondii dense granule protein 15 induces apoptosis in choriocarcinoma JEG-3 cells through endoplasmic reticulum stress. Parasites Vectors 2018, 11, 251. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, X.; Zhu, B.; Xiong, T.; Huang, W.; He, F.; Li, H.; Chen, L.; Tang, L.; Ren, Z. Toxoplasma gondii suppresses proliferation and migration of breast cancer cells by regulating their transcriptome. Cancer Cell Int. 2024, 24, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Tan, X.; Tao, Z.; Adah, D.; Yu, S.; Lu, J.; Zhao, S.; Qin, L.; Qin, L.; et al. Plasmodium parasite as an effective hepatocellular carcinoma antigen glypican-3 delivery vector. Oncotarget 2017, 8, 24785–24796. [Google Scholar] [CrossRef]
- Irish, A.; Whitman, J.; Clark, E.; Marcus, R.; Bern, C. Updated Estimates and Mapping for Prevalence of Chagas Disease among Adults, United States. Emerg. Infect. Dis. 2022, 28, 1313–1320. [Google Scholar] [CrossRef]
- Gómez Ochoa, S.; Rojas, L.; Echeverría, L.; Muka, T.; Franco, O. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob. Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.; Sosoniuk-Roche, E.; Maldonado, I.; Torres, C.G.; Ferreira, A. Trypanosoma cruzi calreticulin: In vitro modulation of key immunogenic markers of both canine tumors and relevant immune competent cells. Immunobiology 2020, 225, 151892. [Google Scholar] [CrossRef] [PubMed]
- Abello-Cáceres, P.; Pizarro-Bauerle, J.; Rosas, C.; Maldonado, I.; Aguilar-Guzmán, L.; González, C.; Ramírez, G.; Ferreira, J.; Ferreira, A. Does native Trypanosoma cruzi calreticulin mediate growth inhibition of a mammary tumor during infection? BMC Cancer 2016, 16, 731. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Horcajo, P.; Hernández, M.; Mora, L.; Fuentes, I. Absence of Neospora caninum DNA in Human Clinical Samples, Spain. Emerg. Infect. Dis. 2019, 25, 1226. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; He, K.; Liu, H.; Yan, W.; Zhao, L.; Jia, Y.; He, L.; Lv, C.; Zhang, M.Z.; et al. Neospora caninum inhibits tumor development by activating the immune response and destroying tumor cells in a B16F10 melanoma model. Parasites Vectors 2022, 15, 332. [Google Scholar] [CrossRef]
- Vicier, C.; Werner, L.; Chipman, J.; Harshman, L.C.; Patil, D.H.; Fichorova, R.N.; Rider, J.R.; Sanda, M.G.; Mucci, L.A.; Sweeney, C.J. Elevated Serum Cytokines and Trichomonas vaginalis Serology at Diagnosis Are Not Associated With Higher Gleason Grade or Lethal Prostate Cancer. Clin. Genitourin. Cancer 2019, 17, 32–37. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, N.; Bose, D.; Banerjee, S.; Jha, T.; Saha, K.D. Leishmanial sphingolipid induces apoptosis in Sarcoma 180 cancer cells through regulation of tumour growth via angiogenic switchover. Tumor Biol. 2015, 36, 3109–3118. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, N.; Bose, D.; Banerjee, S.; Jha, T.; Das Saha, K. Antineoplastic impact of leishmanial sphingolipid in tumour growth with regulation of angiogenic event and inflammatory response. Apoptosis 2015, 20, 869–882. [Google Scholar] [CrossRef]
- Rashidi, S.; Fernández-Rubio, C.; Manzano-Román, R.; Mansouri, R.; Shafiei, R.; Ali-Hassanzadeh, M.; Barazesh, A.; Karimazar, M.; Hatam, G.; Nguewa, P. Potential therapeutic targets shared between leishmaniasis and cancer. Parasitology 2021, 148, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Vega-Benedetti, A.F.; Loi, E.; Zavattari, P. DNA methylation alterations caused by Leishmania infection may generate a microenvironment prone to tumour development. Front. Cell. Infect. Microbiol. 2022, 12, 984134. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, L.; Zhang, B.; Nie, G.; Xie, Z.; Zhang, H.; Ågren, H. Material-based engineering of bacteria for cancer diagnosis and therapy. Appl. Mater. Today 2021, 25, 101212. [Google Scholar] [CrossRef]
- Adnane, F.; Soliman, S.M.A.; ElZayat, E.; Abdelsalam, E.M.; Fahmy, H.M. Evaluation of chlorophyll-loaded mesoporous silica nanoparticles for photodynamic therapy on cancer cell lines. Lasers Med. Sci. 2024, 39, 45. [Google Scholar] [CrossRef]
- Vaňková, K.; Marková, I.; Jašprová, J.; Dvořák, A.; Subhanová, I.; Zelenka, J.; Novosádová, I.; Rasl, J.; Vomastek, T.; Sobotka, R.; et al. Chlorophyll-Mediated Changes in the Redox Status of Pancreatic Cancer Cells Are Associated with Its Anticancer Effects. Oxidative Med. Cell. Longev. 2018, 2018, 4069167. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, Z.; Li, Z.; Luan, H.; Yu, Y.; Zhu, A.T.; Ding, S.; Gao, W.; Fang, R.H.; Zhang, L.; et al. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci. Adv. 2024, 10, eadn6157. [Google Scholar] [CrossRef]
- Meng, F.; Lin, Z.; Ma, Y.; Che, R.; Zhang, C.; Wei, Y.; Song, X.; Liang, X.; Zhang, X. Engineered algae microrobots as photosynthetic living materials promote T cells’ anti-tumor immunity. Cell Rep. Phys. Sci. 2024, 5, 102023. [Google Scholar] [CrossRef]
- Liang, F.; An, X.; Wang, R.; Wu, W.; Yang, L.; Zheng, Y.; Jiang, Q.; Xu, X.; Zhong, D.; Zhou, M. Microalgae-based drug delivery system for tumor microenvironment photo-modulating and synergistic chemo-photodynamic therapy of osteosarcoma. Eng. Regen. 2024, 5, 199–209. [Google Scholar] [CrossRef]
- Xu, C.; Dong, J.; Shi, X.; Rui, J.; Chen, M.; Lu, W.; Zhang, A.; Wang, S.; Teng, Z.; Ye, X. Engineered microalgae for photo-sonodynamic synergistic therapy in breast cancer treatment. Acta Biomater. 2024, 193, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Liu, C.; Li, Z. The mycobiome in human cancer: Analytical challenges, molecular mechanisms, and therapeutic implications. Mol. Cancer 2025, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, T.; Wang, N.; Zhang, H.; Zha, Y.; Ji, L.; Chu, Y.; Ning, K. Artificial intelligence-enabled microbiome-based diagnosis models for a broad spectrum of cancer types. Brief. Bioinform. 2023, 24, bbad178. [Google Scholar] [CrossRef]
- Aurelian, L. Oncolytic virotherapy: The questions and the promise. Oncolytic Virotherapy 2013, 2013, 19. [Google Scholar] [CrossRef]
- Soleimani, N.; Javadi, M.M. Future prospects of bacteria-mediated cancer therapies: Affliction or opportunity? Microb. Pathog. 2022, 172, 105795. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]

| Viruses | Anti-Cancer Effects |
|---|---|
| Engineered adenoviruses (OAd) | deliver transgenes specifically to cancer cells while sparing normal cells, making them take up radioactive iodine, which can be exploited for noninvasive imaging and radiotherapy [92]; become promising options for invasive BC [92]; target paclitaxel resistant ER+ BCSCs [92] and PDAC cells [93] |
| Engineered adeno-associated viruses (AAVs) | used as gene delivery vectors, selectively target BC cells [67] |
| Genetically engineered Herpes simplex virus (oHSV) | selectively targets cancer cells while sparing normal cells, kills cancer cells mainly by boosting host innate or/and adaptive immunity [94] |
| Recombinant mumps virus | acts as a promising oncolytic agent, emphasizing anti-cancer activity against various cancers, including advanced gynecological cancer [95] |
| Engineered Variola virus | oncolytic agent, selectively kills cancer cells, delivery vehicle for anti-cancer transgenes as well as a vaccine carrier for tumor-associated antigens and immunoregulatory molecules in cancer immunotherapy [91] |
| Human endogenous retroviruses (HERs) | play divergent roles in BC carcinogenesis [96] |
| Tobacco mosaic virus (TMV) NPs | component of delivery platforms for pheanthriplatin, which can be released in acidic TME [103]; loaded with MTO, act on cancer cell lines and mouse model of TNBC [104] |
| Potato virus X (PVX) NPs | emphasize good tumor penetration [101]; PVX-NPs-DOX showed efficacy on OC, BC, and cervical cancer cell lines, reducing tumor growth [101] |
| Cowpea mosaic virus (CPMV) NPs | strong immunostimulatory properties, reshape the immunosuppressive TME in murine orthotopic ovarian cancer model by modulating cytokine secretion [106] |
| Bacteria | Anti-Cancer Effects |
|---|---|
| Lactobacillus spp. and Bifidobacterium bifidum strains (probiotics) | involved in cell cycle regulation, inhibit cell proliferation and activate pro-caspases and BAX, downregulate BCL-2 [120] |
| Bifidobacterium longum | engineered, targets tumor hypoxia, enhances the imaging of solid tumors and improves the efficiency of HIFU treatment by enhancing NP targeting ability and increasing their retention time and effects of engineered HIFU synergists [131] |
| Salmonella typhimurium | aggregates and proliferates inside TME, stimulating inflammation and promoting anti-tumor immunity [124,125]; modified or less toxic/attenuated are used as DDSs, inhibiting tumor growth and metastasis or promoting apoptosis in ovarian murine tumors [124], delivers toxins that induce apoptosis in cancer cells [126] |
| Clostridium perfringens | produces enterotoxin, induces disruption of membrane permeability, influx of calcium ions, and cancer cell death [128] |
| Streptomyces peucetius var. caesius | produces DNR and DOX used for anti-cancer activities in acute leukemia and solid tumors, triggers both DNA double-strand breaks and histone eviction [130] |
| Escherichia coli (non-pathogenic) | engineered, selectively targets, colonizes, and proliferates within solid tumors, especially in hypoxic regions [132]; component of DDSs [133] |
| MTB strains | synthesize and contain magnetosomes for targeted therapy, enhance the effects of PTT [45,135] |
| PPSB strains | immunotheranostics [134] |
| Fungal Infections | Associated with | Pro-Cancer Effects | Anti-Cancer Effects |
|---|---|---|---|
| Candida albicans/ candidiasis | human gut opportunistic inhabitant, oral cancer [169], skin cancers [14], GI tumors (GC), HCC [173], BC [174] | carcinogenic metabolites production, chronic inflammation, immune environment remodeling, activation of tumor-pathways, and fungal–bacterial interaction | - |
| Malassezia spp. | human gut opportunistic inhabitant, PDAC [170], BC [172], GI tumors, HCC [173] | - | |
| Aspergillus flavus A. parasiticus, Fusarium spp. | potential risk for HCC [179] and BC [180] | produce exogenous toxins (aflatoxins, fumonisins) cause DNA mutations and genomic instability [177,178]; induce OS, protein and lipid peroxidation, epigenetic modifications, affect cellular signaling, membrane integrity, and apoptosis | aflatoxins can also induce cancer cells apoptosis in 4T1 mouse mammary invasive carcinoma cell line [185] |
| Penicillium chrisogenum P. rubens | many cancers | - | produce penicillin that disrupts mitochondrial function and energy metabolism in colon cancer cells, leading to autophagic apoptosis and inhibition of cancer cell growth and metastasis [183] |
| Penicillium arenicola (endophytic fungi) | CRC, neuroblastoma, BC [186] | - | produces arenicolins that exhibit cytotoxicity on cancer cell lines [186] |
| Penicillium verruculosum (endophytic fungi) | myeloid leukemia [192] | - | averufin has good pharmacokinetic properties [192] |
| Cephalosporium spp. | many cancers | - | produces cephalosporins that increase the effects of radiotherapy by increasing DNA, proteins and membrane lipids oxidative damage, possible through ROS overproduction, leading to toxic effects on cancer cells, selectively and specifically target nasopharyngeal carcinoma cells via HMOX1-induced ferroptosis [184] |
| Cladosporium spp. (endophytic fungi) | CRC, BC, HCC cell lines [193] | - | contains metabolites with AKT1, ESR1, and EGFR tyrosine kinase inhibitory potential and powerful activity against cancer cell lines [193]; FUS and AFU inhibit cancer cell proliferation and increase apoptosis in human acute myeloid leukemia and other hematologic cancer cell lines, FUS upregulates p21 expression and stability in a p53-dependent manner, decreasing ERK and AKT phosphorylation [195] |
| Saccharomyces cerevisiae (Brewer’s yeast) | human gut opportunistic inhabitant, CRC [187], skin cancer [188] | heat-killed form induces apoptosis and reduces cancer cell proliferation, PTEN overexpression [187]; BCL2 downregulation, BAX and caspases 3/8/9 upregulation [188]; vaccination reduces tumor burden and increases survival in CEA-transgenic mice [189]; in different nano-combinations, downregulates genes involved in CRC metastasis, including MMP2/9 and COL10A1, inducing apoptosis and cancer cell cycle arrest [190] |
| Protozoa/Infection | Associated with | Pro-Cancer Effects | Anti-Cancer Effects |
|---|---|---|---|
| many cancers | modification in cell cycle, metabolism, glycosylation, DNA mutations, apoptosis, cell senescence, metastatic cascade, angiogenesis [200] | activation of host immune system, inhibition of tumor growth, angiogenesis, and metastasis [216] | |
| Entamoeba histolytica/ amoebiasis | CRC [203] | microsatellite instability [203] | - |
| Toxoplasma gondii/ toxoplasmosis | solid tumors (anti-cancer activity on MCF7 and MDA-MB-231 BC cells [218]) | regulates many signaling pathways (energetic metabolism, immune response and inflammation), involved in carcinogenesis and cancer development [198] | inhibits hematologic cancers, reverses host immunosuppression to restrain tumor growth, upregulating IL-12 and IFN-γ [211]; exosomes of infected DCs showed anti-cancer activity, by inhibition of STAT3 signaling in MDSCs, leading to tumor growth inhibition [206] |
| Trichomonas vaginalis/ trichomoniasis | cervical and prostate cancer [213,214] | causes trichomoniasis co-infected with HPV [213,214] | however, high level of serum cytokines and T. vaginalis seropositivity at diagnosis were not associated with high-grade lethal prostate cancer [226] |
| Leishmania spp./ leishmaniasis | several cancers (skin, lymphoma, HCC) have been diagnosed in patients with a history of leishmaniasis [204,230] | chronic inflammation, epigenetic alterations (DNA methylation in macrophages), metabolic and OS, apoptosis inhibition, inhibition of tumor suppressors, tumorigenesis [204,230] | LSPL1 from Leishmania donovani has anti-neoplastic potential through cellular growth modulation, apoptosis induction and angiogenesis silencing in sarcoma 180 cell-associated cancer, also exerting anti-neoplastic effects in B16F10 melanoma cells by regulation of angiogenesis and inflammatory response [227,228] |
| Plasmodium spp. (P. falciparum, P. vivax)/ malaria | HCC [219] | - | vector vaccine for HCC immunotherapy [219]; activates the immune system of the host (induces IFN-γ and TNF-α and activated immune cells NK and DCs), inhibits angiogenesis, tumor growth, and metastasis, increases survival time mice models [199] |
| Trypanosoma cruzi /trypanosomiasis/Chagas disease | in patients with Chagas’s disease, cancer is a really rare disease [200] | toxins can have a pro-tumoral role [200] | stimulates the immune system through lysates and infection, produces toxins that kill cancer cells or modulates cellular energetic metabolism [200] |
| Neospora caninum | B16F10 murine melanoma model [225] | - | oncolytic protozoan in human oncology, strongly inhibits or even eradicates tumor development; destroys infected malignant cells, reactivates immune cells, and generates an anti-tumor response dependent of NK cells and CD8+ T-cells, in association with IFN-γ secretion in TME [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, P.S.; Biggers, P.; Nuru, N.; Versaci, N.; Chirila, M.I.; Darie, C.C.; Neagu, A.-N. Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae. Biomedicines 2025, 13, 665. https://doi.org/10.3390/biomedicines13030665
Bruno PS, Biggers P, Nuru N, Versaci N, Chirila MI, Darie CC, Neagu A-N. Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae. Biomedicines. 2025; 13(3):665. https://doi.org/10.3390/biomedicines13030665
Chicago/Turabian StyleBruno, Pathea Shawnae, Peter Biggers, Niyogushima Nuru, Nicholas Versaci, Miruna Ioana Chirila, Costel C. Darie, and Anca-Narcisa Neagu. 2025. "Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae" Biomedicines 13, no. 3: 665. https://doi.org/10.3390/biomedicines13030665
APA StyleBruno, P. S., Biggers, P., Nuru, N., Versaci, N., Chirila, M. I., Darie, C. C., & Neagu, A.-N. (2025). Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae. Biomedicines, 13(3), 665. https://doi.org/10.3390/biomedicines13030665







