Nanosurgery and Bioengineered Regenerative Protocols for the Treatment of Hip Osteoarthritis: A Double-Blind Randomized Controlled Trial as an Alternative to Surgical Hip Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Ultrasound assessment of hip morphology, and radiologic imaging with MRI where necessary.
- Assessment of the joint range of motion before and after treatment (flexion, internal rotation in 90 degrees flexion, and external rotation in degrees flexion and abduction).
- Clinical and ultrasound assessment to identify local pathological mechanisms underlying symptomatic HOA.
- Pain assessment and evaluation of physical function in the activities of daily living during and after treatment, using the Visual Analog Scale (VAS), Western Ontario and McMaster Universities Arthritis Index (WOMAC), and the Harris Hip Score (HHS).
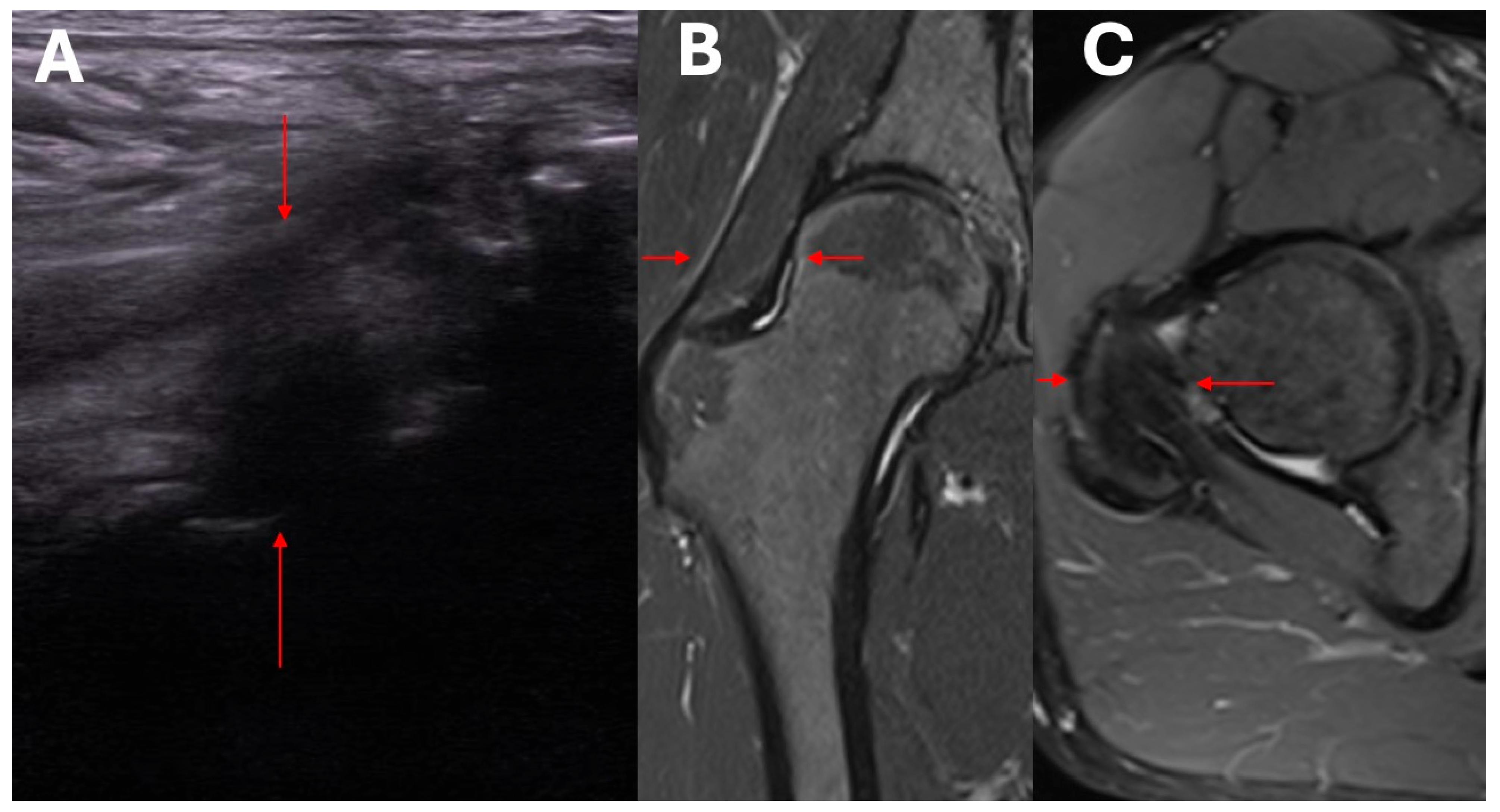
- Assessment of structural changes using imaging studies.
- Exclusion/confirmation of intracapsular and extracapsular inflammation based on ultrasound imaging.
- Clinical and ultrasound assessment of the intracapsular, extracapsular, anterior, anteromedial, lateral, and posterolateral aspect of the hip.
2.2. Intervention
2.3. Methodology of Nanosurgical and Bioengineering Procedures (NSBT)
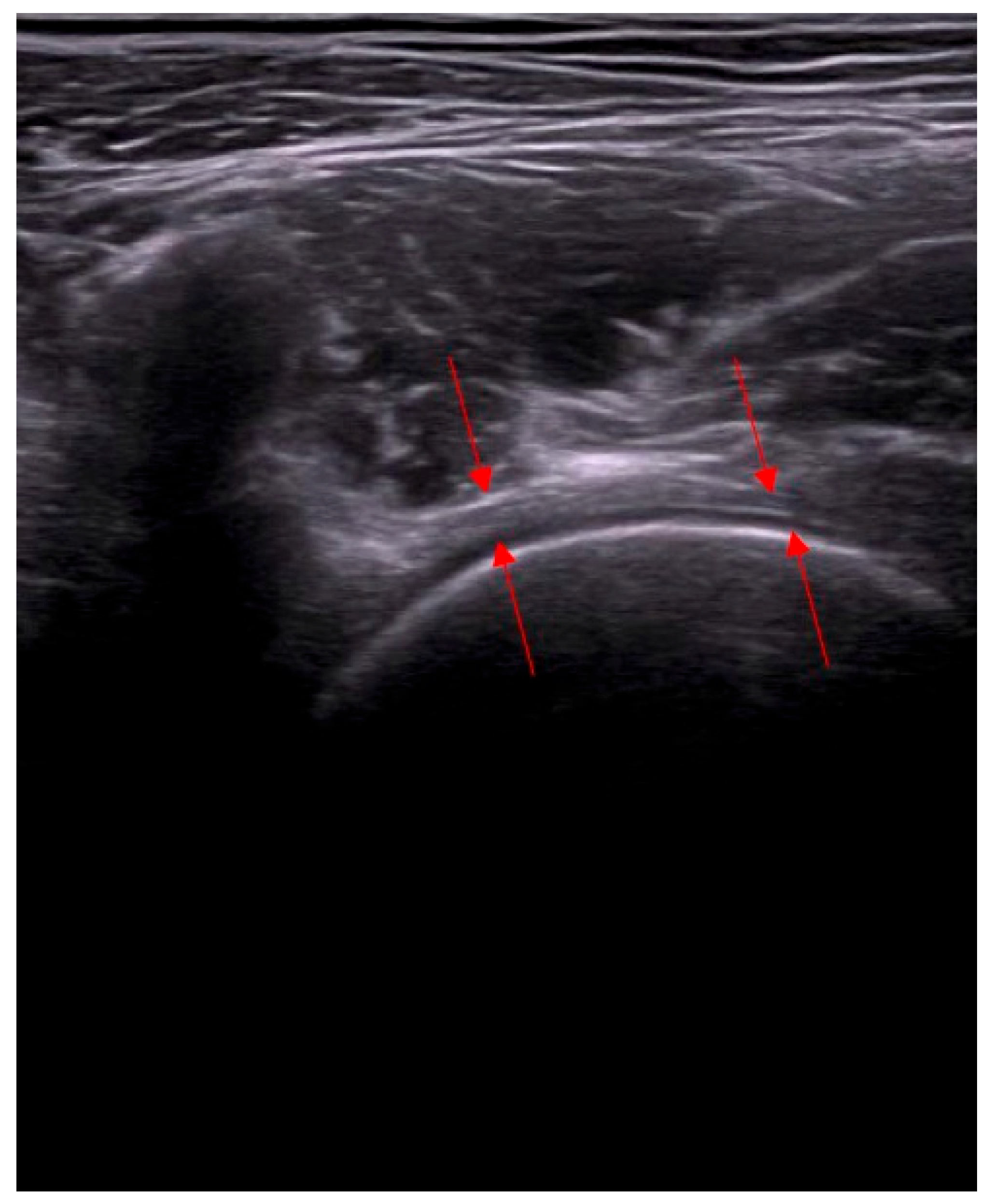
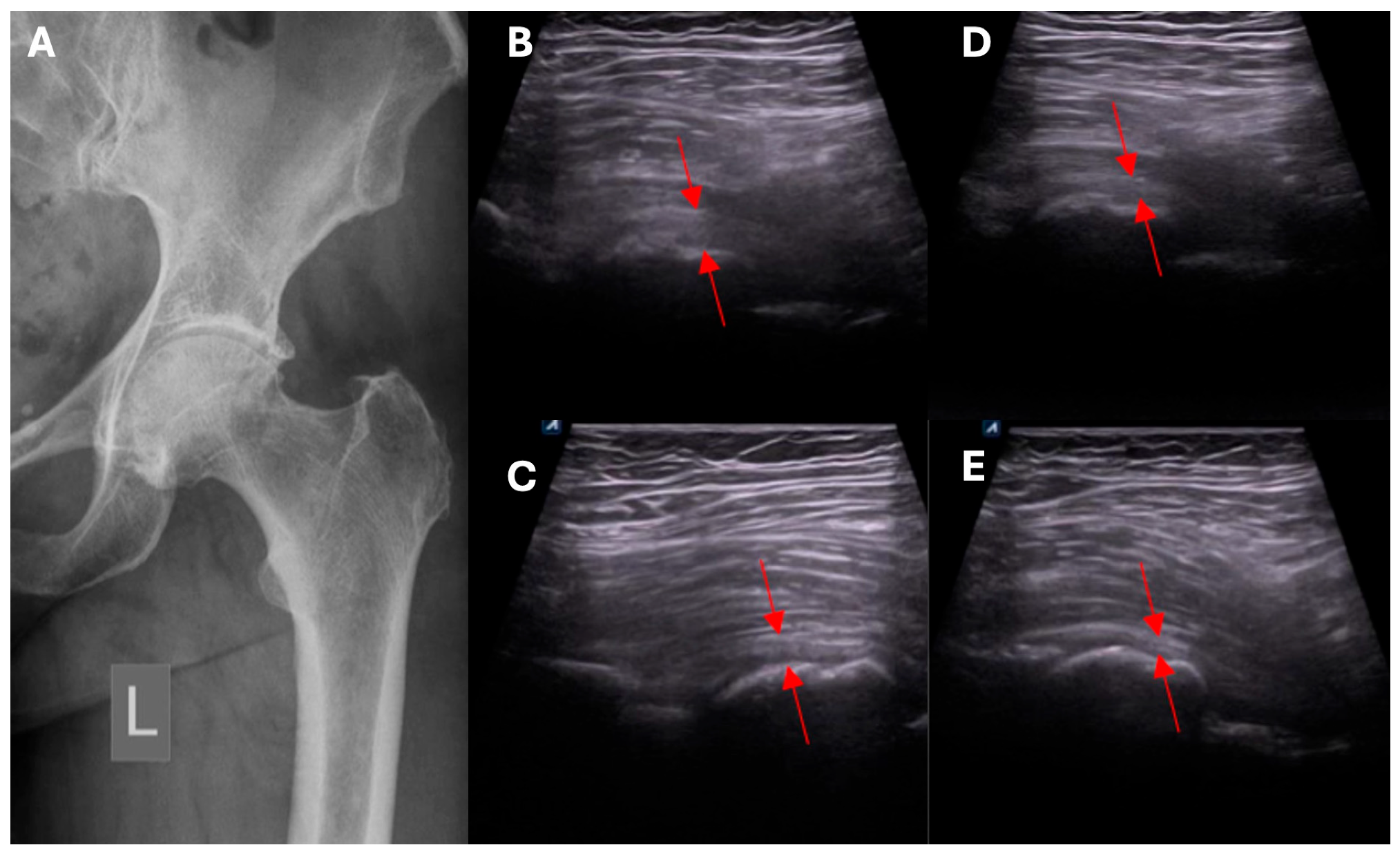
- Assessment of capsular adhesion thickness—ultrasonographic evaluation was performed with the probe positioned transversely, allowing for precise visualization of adhesions. The most common location of adhesions was in the anterolateral complex, with less frequent occurrences in the medial complex.
- Number and distribution of surgical portals—capsulotomy was performed using at least two portals, and in some cases, three or four, depending on the adhesion locations. Standardly, two access points were located in the anterolateral compartment—medially and laterally to the iliopsoas tendon.
- Access to the medial capsular complex—in selected cases, the procedure was performed via a medial approach relative to the neurovascular bundle, which is a limitation of arthroscopic methods.
- Anterior capsulotomy location—the procedure could include a capsulotomy at the equator of the femoral head in the transverse ultrasound view. Optional additional approaches included a medial approach relative to the neurovascular bundle.
2.4. Statistical Analysis
3. Results
Safety
- Transient joint stiffness and inflammatory response occurred in some cases:
- ⚬
- in one patient in the study group;
- ⚬
- in two patients in the control group.All resolved spontaneously within 48 h.
- Mild pain at the injection site, lasting until the following day, was reported by three patients in each group.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, M.; Zhou, H.; Li, Y.; Jin, H.; Liu, X. Global, regional, and national burdens of hip osteoarthritis from 1990 to 2019: Estimates from the 2019 Global Burden of Disease Study. Arthritis Res. Ther. 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- PR Newswire. Projected Volume of Primary and Revision Total Joint Replacement in the US 2030 to 2060. American Academy of Orthopaedic Surgeons. Available online: https://www.prnewswire.com/news-releases/projected-volume-of-primary-and-revision-total-joint-replacement-in-the-us-2030-to-2060-300608386.html (accessed on 14 January 2024).
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Marshall, A.; Ries, M.D.; Paprosky, W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J. Am. Acad. Orthop. Surg. 2008, 16 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- The Health of America. A Study of Cost Variations for Knee and Hip Replacement Surgeries in the U.S. Available online: https://www.bcbs.com/the-health-of-america/reports/study-of-cost-variations-knee-and-hip-replacement-surgeries-the-us (accessed on 14 January 2024).
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Berry, D.J. The epidemiology of revision total hip arthroplasty in the United States. J. Bone Jt. Surg. Am. 2009, 91, 128–133. [Google Scholar] [CrossRef]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Murphy, L.B.; Cisternas, M.G.; Pasta, D.J.; Helmick, C.G.; Yelin, E.H. Medical Expenditures and Earnings Losses Among US Adults with Arthritis in 2013. Arthritis Care. Res. 2018, 70, 869–876. [Google Scholar] [CrossRef]
- Xie, F.; Kovic, B.; Jin, X.; He, X.; Wang, M.; Silvestre, C. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. Pharmacoeconomics 2016, 34, 1087–1100. [Google Scholar] [CrossRef]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip Osteoarthritis: A Primer. Perm. J. 2018, 22, 17–084. [Google Scholar] [CrossRef]
- Aresti, N.; Kassam, J.; Nicholas, N.; Achan, P. Hip osteoarthritis. BMJ 2016, 354, i3405. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020-2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Neuprez, A.; Delcour, J.P.; Fatemi, F.; Gillet, P.; Crielaard, J.M.; Bruyère, O.; Reginster, J.Y. Patients’ Expectations Impact Their Satisfaction following Total Hip or Knee Arthroplasty. PLoS ONE 2016, 11, e0167911. [Google Scholar] [CrossRef] [PubMed]
- Halawi, M.J.; Jongbloed, W.; Baron, S.; Savoy, L.; Williams, V.J.; Cote, M.P. Patient Dissatisfaction After Primary Total Joint Arthroplasty: The Patient Perspective. J. Arthroplast. 2019, 34, 1093–1096. [Google Scholar] [CrossRef]
- Tilbury, C.; Haanstra, T.M.; Leichtenberg, C.S.; Verdegaal, S.H.; Ostelo, R.W.; de Vet, H.C.; Nelissen, R.G.; Vliet Vlieland, T.P. Unfulfilled Expectations After Total Hip and Knee Arthroplasty Surgery: There Is a Need for Better Preoperative Patient Information and Education. J. Arthroplast. 2016, 31, 2139–2145. [Google Scholar] [CrossRef]
- Goodman, S.M.; Mehta, B.Y.; Kahlenberg, C.A.; Krell, E.C.; Nguyen, J.; Finik, J.; Figgie, M.P.; Parks, M.L.; Padgett, D.E.; Antao, V.C.; et al. Assessment of a Satisfaction Measure for Use After Primary Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 1792–1799. [Google Scholar] [CrossRef]
- Mainard, D.; Guillemin, F.; Cuny, C.; Mejat-Adler, E.; Galois, L.; Delagoutte, J. Quality of life assessment one year after total hip or knee arthroplasty. Rev. Chir. Orthop. Réparatrice Appar. Mot. 2000, 86, 464–473. [Google Scholar]
- Kiebzak, G.M.; Vain, P.A.; Gregory, A.M.; Mokris, J.G.; Mauerhan, D.R. SF-36 general health status survey to determine patient satisfaction at short-term follow-up after total hip and knee arthroplasty. J. South Orthop. Assoc. 1997, 6, 169–172. [Google Scholar]
- Kawai, T.; Kataoka, M.; Goto, K.; Kuroda, Y.; So, K.; Matsuda, S. Patient- and Surgery-Related Factors that Affect Patient-Reported Outcomes after Total Hip Arthroplasty. J. Clin. Med. 2018, 7, 358. [Google Scholar] [CrossRef]
- Dong, N.; Yang, C.; Li, S.Q.; Gao, Y.H.; Liu, J.G.; Qi, X. Effect of Preoperative Leg Length Discrepancy on Functional Outcome and Patient Satisfaction After Total Hip Arthroplasty in Cases of Osteonecrosis of the Femoral Head. J. Arthroplast. 2016, 31, 2789–2794. [Google Scholar] [CrossRef]
- Osawa, Y.; Hasegawa, Y.; Seki, T.; Takegami, Y.; Amano, T.; Ishiguro, N. Patient-reported outcomes in patients who undergo total hip arthroplasty after periacetabular osteotomy. J. Orthop. Sci. 2018, 23, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Brokelman, R.B.; van Loon, C.J.; Rijnberg, W.J. Patient versus surgeon satisfaction after total hip arthroplasty. J. Bone Jt. Surg. Br. 2003, 85, 495–498. [Google Scholar] [CrossRef]
- Varacallo, M.; Chakravarty, R.; Denehy, K.; Star, A. Joint perception and patient perceived satisfaction after total hip and knee arthroplasty in the American population. J. Orthop. 2018, 15, 495–499. [Google Scholar] [CrossRef]
- Ortmaier, R.; Pichler, H.; Hitzl, W.; Emmanuel, K.; Mattiassich, G.; Plachel, F.; Hochreiter, J. Return to Sport After Short-Stem Total Hip Arthroplasty. Clin. J. Sport Med. 2019, 29, 451–458. [Google Scholar] [CrossRef]
- McLawhorn, A.S.; Bjerke-Kroll, B.T.; Blevins, J.L.; Sculco, P.K.; Lee, Y.Y.; Jerabek, S.A. Patient-Reported Allergies Are Associated with Poorer Patient Satisfaction and Outcomes After Lower Extremity Arthroplasty: A Retrospective Cohort Study. J. Arthroplast. 2015, 30, 1132–1136. [Google Scholar] [CrossRef]
- Jolbäck, P.; Rolfson, O.; Mohaddes, M.; Nemes, S.; Kärrholm, J.; Garellick, G.; Lindahl, H. Does surgeon experience affect patient-reported outcomes 1 year after primary total hip arthroplasty? Acta Orthop. 2018, 89, 265–271. [Google Scholar] [CrossRef]
- Klit, J. Results of total joint arthroplasty and joint preserving surgery in younger patients evaluated by alternative outcome measures. Dan. Med. J. 2014, 61, B4836. [Google Scholar]
- Pijls, B.G.; Meessen, J.M.; Schoones, J.W.; Fiocco, M.; van der Heide, H.J.; Sedrakyan, A.; Nelissen, R.G. Increased Mortality in Metal-on-Metal versus Non-Metal-on-Metal Primary Total Hip Arthroplasty at 10 Years and Longer Follow-Up: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0156051. [Google Scholar] [CrossRef]
- Rahm, S.; Tondelli, T.; Steinmetz, S.; Schenk, P.; Dora, C.; Zingg, P.O. Uncemented Total Hip Arthroplasty Through the Direct Anterior Approach: Analysis of a Consecutive Series of 275 Hips with a Minimum Follow-Up of 10 Years. J. Arthroplast. 2019, 34, 1132–1138. [Google Scholar] [CrossRef]
- Cho, M.R.; Jun, C.M.; Kim, K.T.; Song, S.K.; Choi, W.K. Results of primary THA using 36 mm femoral heads on first-generation highly cross-linked polyethylene in patients less than 60 years of age: Minimum 10-year follow-ups. J. Orthop. Surg. 2020, 28, 2309499019896448. [Google Scholar] [CrossRef]
- Heijnens, L.J.; Schotanus, M.G.; Kort, N.P.; Verburg, A.D.; van Haaren, E.H. Results of Cemented Anatomically Adapted Total Hip Arthroplasty: A Follow-Up Longer Than 10 years. J. Arthroplast. 2016, 31, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, A.K.; Barsoum, W.K.; Krebs, V.; Hurbanek, J.G.; Morra, N.; Brotman, D.J. Duration of anesthesia and venous thromboembolism after hip and knee arthroplasty. Mayo Clin. Proc. 2005, 80, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M. Infection in total knee replacement: A retrospective review of 6489 total knee replacements. Clin. Orthop. Relat. Res. 2001, 392, 15–23. [Google Scholar] [CrossRef]
- Khatod, M.; Barber, T.; Paxton, E.; Namba, R.; Fithian, D. An analysis of the risk of hip dislocation with a contemporary total joint registry. Clin. Orthop. Relat. Res. 2006, 447, 19–23. [Google Scholar] [CrossRef]
- Mahomed, N.N.; Barrett, J.A.; Katz, J.N.; Phillips, C.B.; Losina, E.; Lew, R.A.; Guadagnoli, E.; Harris, W.H.; Poss, R.; Baron, J.A. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J. Bone Jt. Surg. Am. 2003, 85, 27–32. [Google Scholar] [CrossRef]
- Pulido, L.; Parvizi, J.; Macgibeny, M.; Sharkey, P.F.; Purtill, J.J.; Rothman, R.H.; Hozack, W.J. In hospital complications after total joint arthroplasty. J. Arthroplast. 2008, 23, 139–145. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Boffa, A.; Andriolo, L.; Raggi, F.; Zaffagnini, S.; Filardo, G. Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review. J. Clin. Med. 2022, 11, 6663. [Google Scholar] [CrossRef]
- Hintz, R.L. Growth hormone: Uses and abuses. BMJ 2004, 328, 907–908. [Google Scholar] [CrossRef]
- Ainembabazi, D.; Zhang, Y.; Turchi, J.J. The mechanistic role of cardiac glycosides in DNA damage response and repair signaling. Cell. Mol. Life Sci. 2023, 80, 250. [Google Scholar] [CrossRef]
- Ren, Y.; Anderson, A.T.; Meyer, G.; Lauber, K.M.; Gallucci, J.C.; Douglas Kinghorn, A. Digoxin and its Na(+)/K(+)-ATPase-targeted actions on cardiovascular diseases and cancer. Bioorg. Med. Chem. 2024, 114, 117939. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs 2007, 7, 173–189. [Google Scholar] [CrossRef]
- Bolamperti, S.; Guidobono, F.; Rubinacci, A.; Villa, I. The Role of Growth Hormone in Mesenchymal Stem Cell Commitment. Int. J. Mol. Sci. 2019, 20, 5264. [Google Scholar] [CrossRef]
- Sotiropoulos, A.; Ohanna, M.; Kedzia, C.; Menon, R.K.; Kopchick, J.J.; Kelly, P.A.; Pende, M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.L.; Losina, E.; Nti, A.A.; Prokopetz, J.J.; Katz, J.N. Population-based rates of revision of primary total hip arthroplasty: A systematic review. PLoS ONE 2010, 5, e13520. [Google Scholar] [CrossRef] [PubMed]
- Gwam, C.U.; Mistry, J.B.; Mohamed, N.S.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J. Arthroplast. 2017, 32, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Hevesi, M.; Wyles, C.C.; Yao, J.J.; Maradit-Kremers, H.; Habermann, E.B.; Glasgow, A.E.; Bews, K.A.; Ransom, J.E.; Visscher, S.L.; Lewallen, D.G.; et al. Revision Total Hip Arthroplasty for the Treatment of Fracture: More Expensive, More Complications, Same Diagnosis-Related Groups: A Local and National Cohort Study. J. Bone Jt. Surg. Am. 2019, 101, 912–919. [Google Scholar] [CrossRef]
- Kelmer, G.; Stone, A.H.; Turcotte, J.; King, P.J. Reasons for Revision: Primary Total Hip Arthroplasty Mechanisms of Failure. J. Am. Acad. Orthop. Surg. 2021, 29, 78–87. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Widmer, M.; Maravic, M.; Gómez-Barrena, E.; de Pina Mde, F.; Manno, V.; Torre, M.; Walter, W.L.; et al. International survey of primary and revision total knee replacement. Int. Orthop. 2011, 35, 1783–1789. [Google Scholar] [CrossRef]
- Pan, T.; Bierowski, M.J.; King, T.S.; Mason, M.W. Outcomes of Revision Hip Arthroplasty Using the Supine Anterior-Based Muscle Sparing Approach. Arthroplast. Today 2022, 14, 199–203. [Google Scholar] [CrossRef]
- Söderman, P.; Malchau, H.; Herberts, P. Outcome after total hip arthroplasty: Part I. General health evaluation in relation to definition of failure in the Swedish National Total Hip Arthoplasty register. Acta Orthop. Scand. 2000, 71, 354–359. [Google Scholar] [CrossRef]
- Tran, J.; Yu, H.; Paprosky, W.G.; Sheth, N.P. Systematic Exposure in Revision Total Hip Arthroplasty: The Posterior Approach. J. Am. Acad. Orthop. Surg. 2023, 31, e736–e745. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Renkawitz, T.; Voellner, F.; Craiovan, B.; Greimel, F.; Worlicek, M.; Grifka, J.; Benditz, A. Revision Surgery in Total Joint Replacement Is Cost-Intensive. BioMed Res. Int. 2018, 2018, 8987104. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Wolford, M.L.; Palso, K.; Bercovitz, A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000–2010. NCHS Data Brief 2015, 186, 1–8. [Google Scholar]
- Ali Khan, M.A.; Brakenbury, P.H.; Reynolds, I.S. Dislocation following total hip replacement. J. Bone Jt. Surg. Br. 1981, 63, 214–218. [Google Scholar] [CrossRef]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kamath, A.F.; Ong, K.; Lau, E.; Kurtz, S.; Chan, V.; Vail, T.P.; Rubash, H.; Berry, D.J. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin. Orthop. Relat. Res. 2015, 473, 2131–2138. [Google Scholar] [CrossRef]
- Dargel, J.; Oppermann, J.; Brüggemann, G.P.; Eysel, P. Dislocation following total hip replacement. Dtsch. Arztebl. Int. 2014, 111, 884–890. [Google Scholar] [CrossRef]
- Hedlundh, U.; Ahnfelt, L.; Hybbinette, C.H.; Weckstrom, J.; Fredin, H. Surgical experience related to dislocations after total hip arthroplasty. J. Bone Jt. Surg. Br. 1996, 78, 206–209. [Google Scholar] [CrossRef]
- Hedlundh, U.; Fredin, H. Patient characteristics in dislocations after primary total hip arthroplasty. 60 patients compared with a control group. Acta Orthop. Scand. 1995, 66, 225–228. [Google Scholar] [CrossRef]
- Kuijpers, M.F.L.; Hannink, G.; van Steenbergen, L.N.; Schreurs, B.W. Outcome of revision hip arthroplasty in patients younger than 55 years: An analysis of 1037 revisions in the Dutch Arthroplasty Register. Acta Orthop. 2020, 91, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, H.O.; Carlsson, A.S.; Gentz, C.F.; Pettersson, H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop. Scand. 1982, 53, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Morrey, B.F. Difficult complications after hip joint replacement. Dislocation. Clin. Orthop. Relat. Res. 1997, 344, 179–187. [Google Scholar] [CrossRef]
- Ong, K.L.; Lau, E.; Suggs, J.; Kurtz, S.M.; Manley, M.T. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 3070–3076. [Google Scholar] [CrossRef]
- Haynes, D.R.; Crotti, T.N.; Zreiqat, H. Regulation of osteoclast activity in peri-implant tissues. Biomaterials 2004, 25, 4877–4885. [Google Scholar] [CrossRef]
- Valen, B. Dislocation of hip prostheses. Tidsskr. Nor. Laegeforen. 2013, 133, 1197–1199. [Google Scholar] [CrossRef]
- Tian, X.; Qu, Z.; Cao, Y.; Zhang, B. Relative efficacy and safety of mesenchymal stem cells for osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2024, 15, 1366297. [Google Scholar] [CrossRef]
- Lim, A.; Zhu, J.B.; Khanduja, V. The Use of Intra-articular Platelet-Rich Plasma as a Therapeutic Intervention for Hip Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2023, 51, 2487–2497. [Google Scholar] [CrossRef]
- McInnis, K.C.; Chen, E.T.; Finnoff, J.T.; Roh, E.Y.; Borg Stein, J. Orthobiologics for the Hip Region: A Narrative Review. PM&R 2020, 12, 1045–1054. [Google Scholar] [CrossRef]
- Villanova-López, M.M.; Núñez-Núñez, M.; Fernández-Prieto, D.; González-López, C.; García-Donaire, J.; Pérez-Pérez, A.; Sandoval Fernández Del Castillo, S.; Murillo-Izquierdo, M.; Camean-Fernández, M.; Gutiérrez-Pizarraya, A.; et al. Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 134–142. [Google Scholar] [CrossRef]
- Sánchez, M.; Guadilla, J.; Fiz, N.; Andia, I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology 2012, 51, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dallari, D.; Stagni, C.; Rani, N.; Sabbioni, G.; Pelotti, P.; Torricelli, P.; Tschon, M.; Giavaresi, G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am. J. Sports Med. 2016, 44, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Guaraldi, F.; Vannini, F.; Rossi, G.; Timoncini, A.; Buda, R.; Giannini, S. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics 2013, 36, e1501–e1508. [Google Scholar] [CrossRef]
- Fiz, N.; Pérez, J.C.; Guadilla, J.; Garate, A.; Sánchez, P.; Padilla, S.; Delgado, D.; Sánchez, M. Intraosseous Infiltration of Platelet-Rich Plasma for Severe Hip Osteoarthritis. Arthrosc. Tech. 2017, 6, e821–e825. [Google Scholar] [CrossRef]
- Dallaudière, B.; Pesquer, L.; Meyer, P.; Silvestre, A.; Perozziello, A.; Peuchant, A.; Durieux, M.H.; Loriaut, P.; Hummel, V.; Boyer, P.; et al. Intratendinous injection of platelet-rich plasma under US guidance to treat tendinopathy: A long-term pilot study. J. Vasc. Interv. Radiol. 2014, 25, 717–723. [Google Scholar] [CrossRef]
- Fader, R.R.; Mitchell, J.J.; Traub, S.; Nichols, R.; Roper, M.; Mei Dan, O.; McCarty, E.C. Platelet-rich plasma treatment improves outcomes for chronic proximal hamstring injuries in an athletic population. Muscles Ligaments Tendons J. 2014, 4, 461–466. [Google Scholar] [CrossRef]
- Wetzel, R.J.; Patel, R.M.; Terry, M.A. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics 2013, 36, e64–e70. [Google Scholar] [CrossRef]
- Boettcher, B.J.; Hollman, J.H.; Stuart, M.J.; Finnoff, J.T. Ultrasound-Guided Cutting Wire Release of the Proximal Adductor Longus Tendon: A Feasibility Study. Orthop. J. Sports Med. 2019, 7, 2325967119866010. [Google Scholar] [CrossRef]
- Begkas, D.; Chatzopoulos, S.T.; Touzopoulos, P.; Balanika, A.; Pastroudis, A. Ultrasound-guided Platelet-rich Plasma Application Versus Corticosteroid Injections for the Treatment of Greater Trochanteric Pain Syndrome: A Prospective Controlled Randomized Comparative Clinical Study. Cureus 2020, 12, e6583. [Google Scholar] [CrossRef]
- Ladurner, A.; Fitzpatrick, J.; O’Donnell, J.M. Treatment of Gluteal Tendinopathy: A Systematic Review and Stage-Adjusted Treatment Recommendation. Orthop. J. Sports Med. 2021, 9, 23259671211016850. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Bulsara, M.K.; O’Donnell, J.; McCrory, P.R.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection with a Single Corticosteroid Injection. Am. J. Sports Med. 2018, 46, 933–939. [Google Scholar] [CrossRef] [PubMed]
| Study Group (n = 19) | Control Group (n = 19) | |
|---|---|---|
| Age (years), mean (SD) | 66.4 (14.2) | 65.8 (16.7) |
| Sex, n (%) | ||
| Men | 10 (52.6) | 8 (42.1) |
| Women | 9 (47.4) | 11(57.9) |
| Body mass index (kg/m2) (SD) | 24.1 (2.2) | 27.5 (4.1) |
| Grade, n (%) | ||
| I | 0 | 0 |
| II | 3 (15.8) | 1 (5.3) |
| III | 12 (63.2) | 9 (47.35) |
| IV | 4 (21.0) | 9 (47.35) |
| SD—standard deviation |
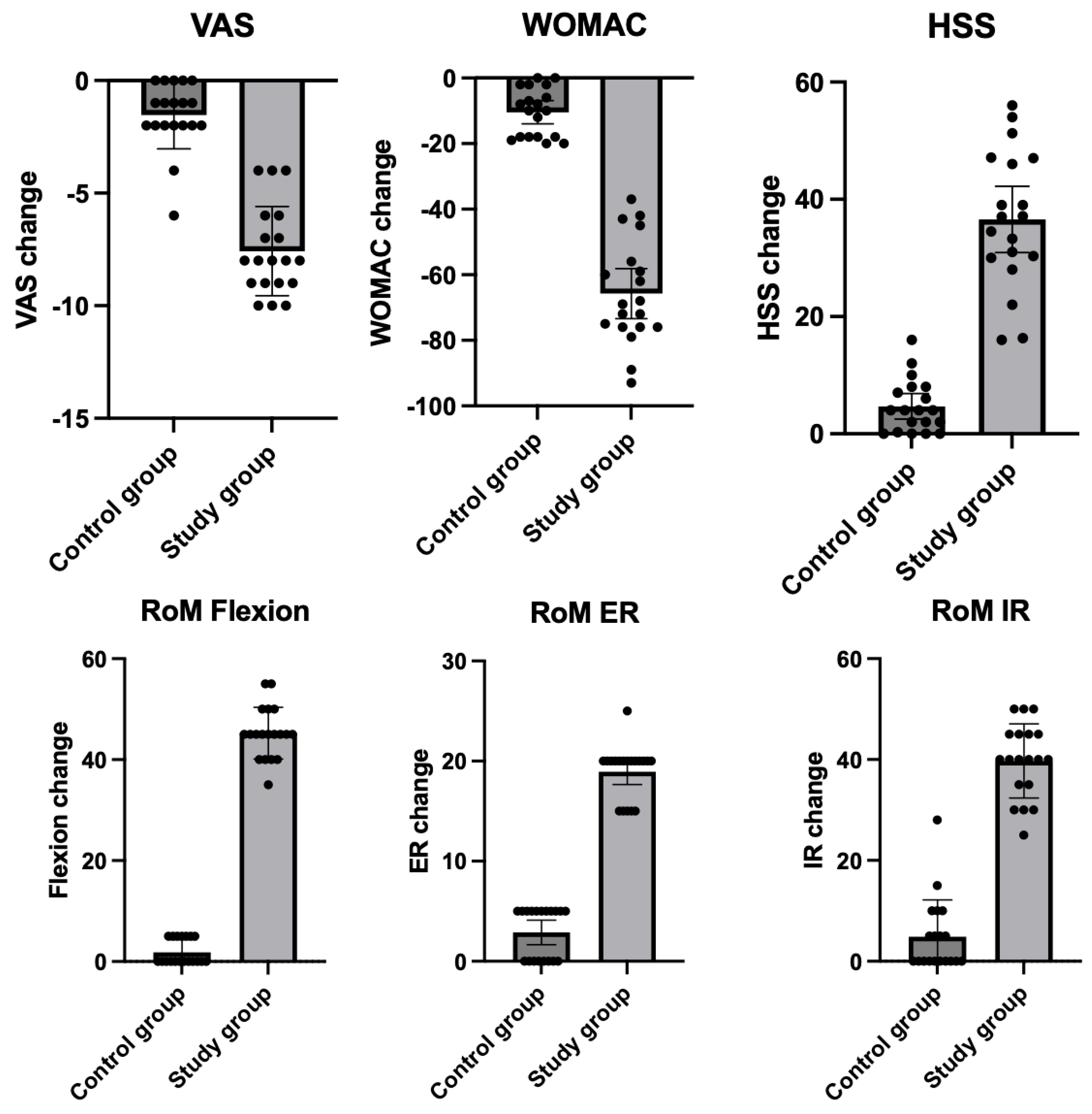
| Study Group (N = 19) | Control Group (N = 19) | p | ||||
|---|---|---|---|---|---|---|
| Variable | Mean | 95% CI | Mean | 95% CI | ||
| VAS | At baseline | 7.8 | 6.9–8.6 | 8.8 | 8.3–9.3 | 0.09 |
| After treatment | 0.2 | 0.0–0.4 | 7.3 | 6.3–8.2 | <0.001 | |
| Change | −7.6 | −8.5–−6.6 | −1.5 | −2.2–−0.8 | <0.001 | |
| p for change | <0.001 | 0.004 | ||||
| WOMAC | At baseline | 76.2 | 71.1–81.3 | 88.7 | 85.1–92.3 | 0.0002 |
| After treatment | 10.5 | 5.1–15.9 | 78.3 | 73.2–83.4 | <0.001 | |
| Change | −65.7 | −73.3–−58.1 | −10.4 | −19.9–−6.9 | <0.001 | |
| p for change | <0.001 | <0.001 | ||||
| HSS | At baseline | 56.4 | 51.3–61.5 | 48.7 | 42.4–55.0 | 0.052 |
| After treatment | 93.0 | 90.5–95.5 | 53.4 | 47.5–59.3 | <0.0001 | |
| Change | 36.6 | 30.9–42.2 | 4.7 | 2.5–6.8 | <0.0001 | |
| p for change | <0.001 | 0.004 | ||||
| RoM flexion | At baseline | 94.0 | 91.6–96.3 | 90.8 | 87.8–93.8 | 0.09 |
| After treatment | 139.2 | 136.6–141.8 | 92.63 | 90.2–95.1 | <0.001 | |
| Change | 45.3 | 42.8–47.7 | 1.84 | 0.6–3.0 | <0.001 | |
| p for change | <0.001 | 0.24 | ||||
| RoM ER | At baseline | 9.7 | 8.4–10.6 | 8.7 | 7.6–9.7 | 0.29 |
| After treatment | 28.4 | 27.0–29.8 | 11.6 | 10.4–12.7 | <0.001 | |
| Change | 18.7 | 17.7–20.2 | 2.9 | 1.7–4.1 | <0.001 | |
| p for change | <0.001 | 0.012 | ||||
| RoM IR | At baseline | 21.6 | 18.7–24.5 | 21.7 | 19.0–24.4 | 0.95 |
| After treatment | 61.3 | 57.3–65.3 | 26.6 | 24.5–28.7 | <0.001 | |
| Change | 39.7 | 36.2–43.3 | 4.9 | 1.4–8.4 | <0.001 | |
| p for change | <0.001 | 0.048 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilczyk, C.; Wasilczyk, B. Nanosurgery and Bioengineered Regenerative Protocols for the Treatment of Hip Osteoarthritis: A Double-Blind Randomized Controlled Trial as an Alternative to Surgical Hip Replacement. Biomedicines 2025, 13, 987. https://doi.org/10.3390/biomedicines13040987
Wasilczyk C, Wasilczyk B. Nanosurgery and Bioengineered Regenerative Protocols for the Treatment of Hip Osteoarthritis: A Double-Blind Randomized Controlled Trial as an Alternative to Surgical Hip Replacement. Biomedicines. 2025; 13(4):987. https://doi.org/10.3390/biomedicines13040987
Chicago/Turabian StyleWasilczyk, Cezary, and Bartosz Wasilczyk. 2025. "Nanosurgery and Bioengineered Regenerative Protocols for the Treatment of Hip Osteoarthritis: A Double-Blind Randomized Controlled Trial as an Alternative to Surgical Hip Replacement" Biomedicines 13, no. 4: 987. https://doi.org/10.3390/biomedicines13040987
APA StyleWasilczyk, C., & Wasilczyk, B. (2025). Nanosurgery and Bioengineered Regenerative Protocols for the Treatment of Hip Osteoarthritis: A Double-Blind Randomized Controlled Trial as an Alternative to Surgical Hip Replacement. Biomedicines, 13(4), 987. https://doi.org/10.3390/biomedicines13040987






