Hepatokine and Proinflammatory Cytokine Profile in Patients with Carotid Atherosclerosis and Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants
2.1.1. Study Design and Intervention
2.1.2. Statistical Analysis
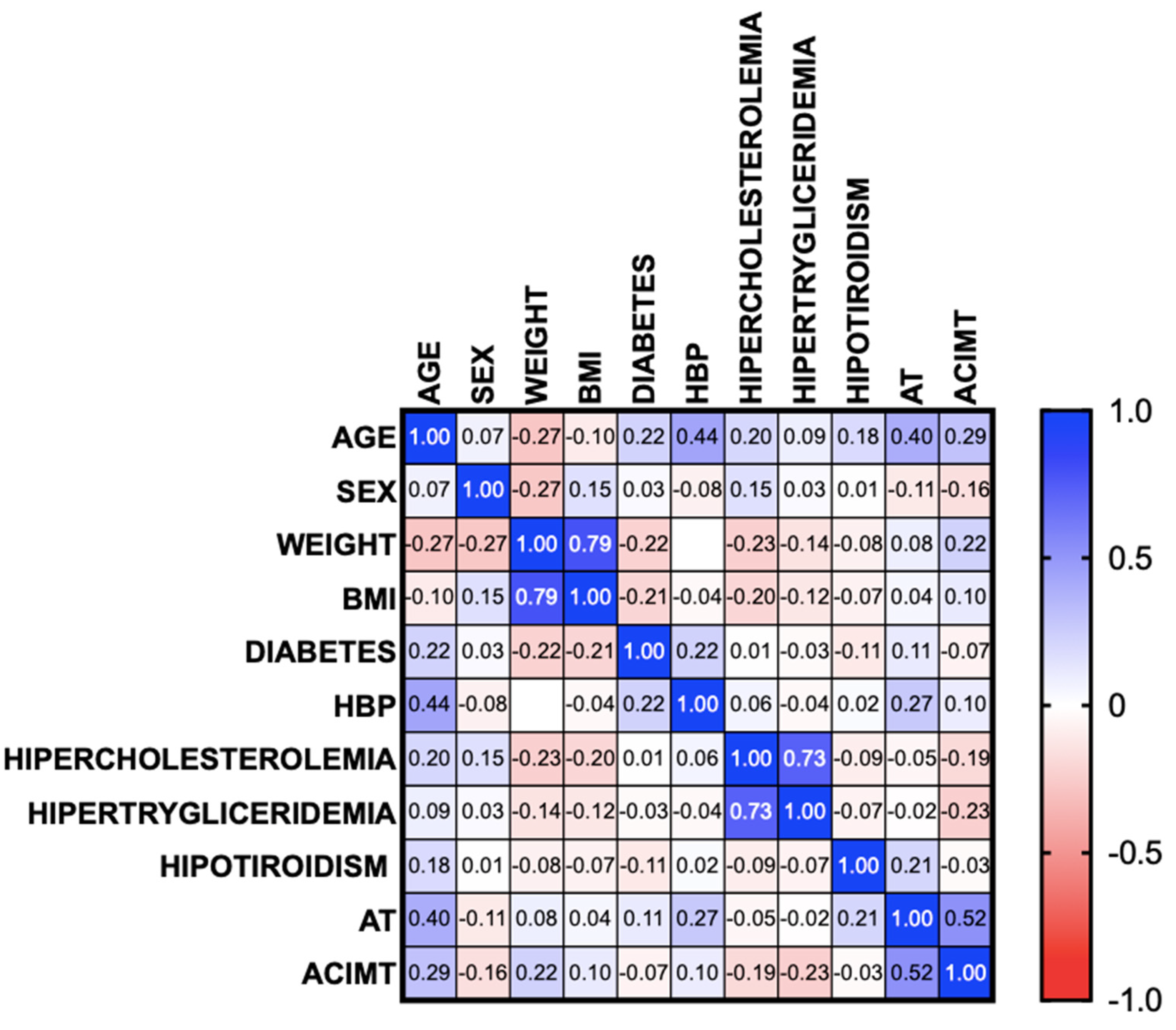
3. Results
3.1. Systemic Inflammation Markers
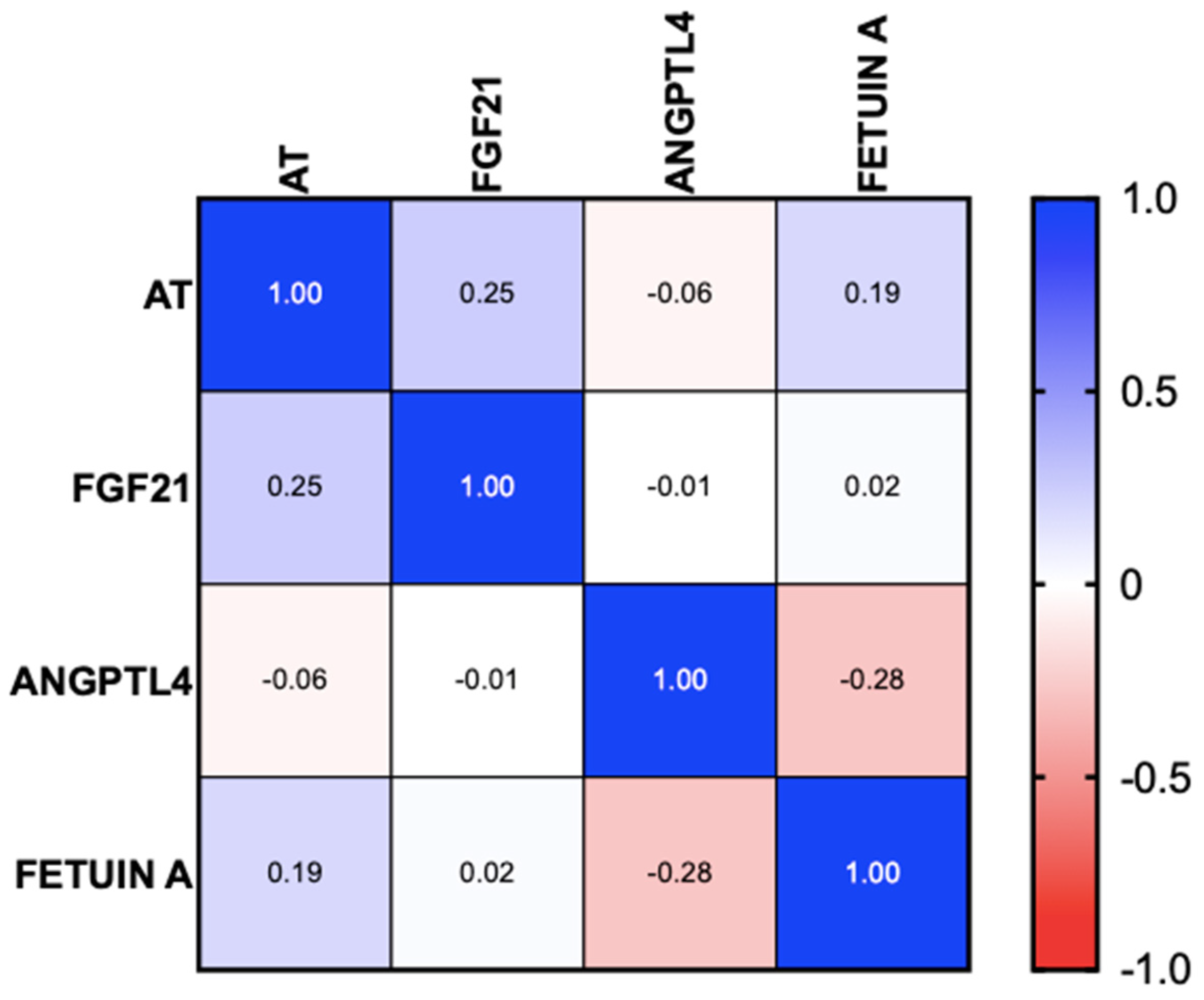
3.2. Hepatokines
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houttu, V.; Csader, S.; Nieuwdorp, M.; Holleboom, A.G.; Schwab, U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 716783. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, S.; Christiansen, S.; Nagel, J.; Wimmer, R.; Artmann, R.; Denk, G.; Bischoff, M.; Bischoff, G.; Rust, C. Lifestyle intervention for morbid obesity: Effects on liver steatosis, inflammation, and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G329–G338. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Hassen, G.; Singh, A.; Belete, G.; Jain, N.; De la Hoz, I.; Camacho-Leon, G.P.; Dargie, N.K.; Carrera, K.G.; Alemu, T.; Jhaveri, S.; et al. Nonalcoholic Fatty Liver Disease: An Emerging Modern-Day Risk Factor for Cardiovascular Disease. Cureus 2022, 14, e25495. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, B.; Aigner, E.; Stickel, F.; Datz, C. NAFLD and Cardiovascular Diseases: Epidemiological, Mechanistic and Therapeutic Considerations. J. Clin. Med. 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Zhang, L.; She, Z.-G.; Li, H.; Zhang, X.-J. Non-alcoholic fatty liver disease: A metabolic burden promoting atherosclerosis. Clin. Sci. 2020, 134, 1775–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Dayal, U.; Soni, U.; Bansal, S.; Aggarwal, K.; Chennupati, C.; Kanagala, S.G.; Gupta, V.; Munjal, R.S.; Jain, R. MAFLD: Exploring the Systemic Effects Beyond Liver. J. Community Hosp. Intern. Med. Perspect. 2025, 15, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Lee, M.-S. Carotid Intima-Media Thickness and Plaque in Cardiovascular Risk Assessment. JACC Cardiovasc. Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-López, M.; Martínez-Alonso, M.; Castro-Boqué, E.; Betriu, À.; Cambray, S.; Farràs, C.; Barbe, F.; Pamplona, R.; Lecube, A.; Mauricio, D.; et al. Subclinical atheromatosis localization and burden in a low-to-moderate cardiovascular risk population: The ILERVAS study. Rev. Esp. Cardiol. 2021, 74, 1042–1053. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Kim, S.U.; Kim, H.C. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2138–2147.e10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, H.; Shou, B.; Cheng, Z.; Jiang, C.; Ye, Y.; Xu, J. Remnant cholesterol and the risk of carotid plaque in hypertension: Results from a community-based screening among old adults in Hangzhou, China. Sci. Rep. 2024, 14, 8407. [Google Scholar] [CrossRef] [PubMed]
- Schonmann, Y.; Yeshua, H.; Bentov, I.; Zelber-Sagi, S. Liver fibrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig. Liver Dis. 2021, 53, 79–85. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Pletikosic, I.; Marasovic Krstulovic, D.; Bakovic, D.; Susilovic Grabovac, Z.; Tandara, L.; Martinovic Kaliterna, D. Association of inflammatory biomarkers and disease activity with subclinical myocardial dysfunction in psoriatic arthritis. Sci. Rep. 2023, 13, 10371. [Google Scholar] [CrossRef] [PubMed]
- Al Ali, L.; van de Vegte, Y.J.; Said, M.A.; Groot, H.E.; Hendriks, T.; Yeung, M.W.; Lipsic, E.; van der Harst, P. Fetuin-A and its genetic association with cardiometabolic disease. Sci. Rep. 2023, 13, 21469. [Google Scholar] [CrossRef]
- Sardana, O.; Goyal, R.; Bedi, O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD. Inflammopharmacology 2021, 29, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Bhattacharya, S.; Biswas, A.; Majumdar, S.S.; Mukhopadhyay, S.; Ray, S.; Bhattacharya, S. NF-κB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem. J. 2010, 429, 451–462. [Google Scholar] [CrossRef]
- Thorin, E.; Labbé, P.; Lambert, M.; Mury, P.; Dagher, O.; Miquel, G.; Thorin-Trescases, N. Angiopoietin-Like Proteins: Cardiovascular Biology and Therapeutic Targeting for the Prevention of Cardiovascular Diseases. Can. J. Cardiol. 2023, 39, 1736–1756. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, H.; Li, X.; Liu, Y.; Mi, Y.; Kong, H.; Xi, D.; Yan, W.; Luo, X.; Ning, Q.; et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics 2020, 10, 9702–9720. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Mangelsdorf, D.J. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.-U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef]
- Liang, J.-Q.; Liu, C.; Zhang, W.-X.; Chen, S.-Y. Interaction between hepatokines and metabolic diseases. Yi Chuan 2022, 44, 853–866. [Google Scholar] [CrossRef] [PubMed]

| Parameter |
MASLD (n = 67) |
MASLD
with AT (n = 11) |
MASLD
Without AT (n = 56) | p |
|---|---|---|---|---|
| Sex | ||||
| Male n(%) | 17 (25) | 4 (36.4) | 13 (23.6) | 0.193 |
| Female n(%) | 49 (72) | 7 (63.6) | 42 (76.4) | |
| Age | 55.9 +11.3 | 63 + 5.4 | 51 + 11.2 | 0.001 |
| Weight | 84.9 + 17.7 | 88.2 + 11.9 | 84.3 +18.7 | 0.379 |
| BMI | 33.2 + 6.4 | 33.8 + 3.7 | 33.1 + 6.8 | 0.610 |
| BMI | ||||
| Normal weight n(%) | 3 (4.4) | - | 3 (5.5) | - |
| Overweight n(%) | 17 (25) | 1 (9.1) | 16 (29.1) | 0.086 |
| Obesity I n(%) | 20 (29) | 4 (36.4) | 16 (29.1) | 0.157 |
| Obesity II n(%) | 19 (27) | 6 (54.5) | 13 (23.6) | 0.086 |
| Obesity III n(%) | 7 (10) | - | 7 (12.7) | - |
| Comorbidities | ||||
| Diabetes n(%) | 17 (25) | 4 (36.4) | 13 (23.6) | 0.386 |
| High blood pressure n(%) | 23 (33.8) | 7 (63.6) | 16 (29.1) | 0.028 |
| Hypercholesterolemia n(%) | 15 (22.1) | 2 (18.2) | 13 (23.6) | 0.692 |
| Hypertriglyceridemia n(%) | 13 (19.1) | 2 (18.2) | 11 (20) | 0.894 |
| Hypothyroidism n(%) | 8 (11.8) | 3 (27.3) | 5 (9.1) | 0.094 |
| Parameter |
MASLD (n = 67) |
MASLD with AT (n = 11) |
MASLD Without AT (n = 56) | p |
|---|---|---|---|---|
| Hb (g/dL) ** | 14.3 ± 1.5 | 13.2 ± 1.8 | 13.5 ± 1.4 | 0.463 |
| Platelets ** | 270 ± 81 | 221 ± 65 | 270 ± 81 | 0.038 |
| TB (g/dL) * | 0.45 (0.2–1.70) | 0.48 (0.27–1.70) | 0.45 (0.2–1.23) | 0.136 |
| AST (g/dL) * | 24 (12–233) | 21 (15–148) | 24 (12–233) | 0.535 |
| ALT (g/dL) * | 20 (5–402) | 28 (12–137) | 29 (5–402) | 0.895 |
| ALP (g/dL) * | 97 (10–244) | 223 (13–236) | 99 (10–244) | 0.561 |
| GGT (g/dL) * | 35 (3–370) | 70 (14–225) | 35 (3–370) | 0.069 |
| TP (g/dL) * | 7.5 (6.3–8.8) | 7.5 (6.3–8.8) | 7.3 (6.7–7.4) | 0.639 |
| ALB (g/dL) | 4.2 (6.3–7.4) | 4.1 (3.7–4.7) | 4.3 (3.7–5.3) | 0.042 |
| Cholesterol (g/dL) ** | 188 ± 51 | 212 ± 45 | 185 ± 51 | 0.114 |
| HDL (g/dL) * | 44 (10–214) | 53 (10–62) | 43 (10–175) | 0.837 |
| LDL (g/dL) ** | 117 ± 46 | 136 ± 49 | 115 ± 43 | 0.215 |
| VLDL (g/dL) * | 30 (13–115) | 31.8 (19–51) | 28 (13–115) | 0.215 |
| Triglycerides (g/dL) * | 150 (45–576) | 158 (99–256) | 147 (45–576) | 0.936 |
| Glucose (g/dL) * | 102 (81–200) | 106 (81–155) | 101 (83–200) | 0.497 |
| Creatinine (g/dL) * | 0.71 (0.4–1.45) | 0.96 (0.66–1.45) | 0.70 (0.4–1.03) | 0.009 |
| CRP (g/dL)* | 3.65 (1–12) | 3.4 (2–7) | 3.5 (1–12) | 0.568 |
| Insulin (g/dL) ** | 20.8 ± 10.9 | 23.7 ± 12 | 20.5 ± 10 | 0.618 |
| HOMA * | 4.2 (2–6–15.38) | 4.1 (3.73–14.18) | 4.3 (2.6–15.3) | 0.590 |
| Parameter |
MASLD (n = 67) |
MASLD
with AT (n = 11) |
MASLD
Without AT (n = 56) | p |
|---|---|---|---|---|
| Systemic inflammation markers | ||||
| TNF-α (pg/mL) * | 0 (0–125) | 0 | (0–125) | - |
| IL-6 (pg/mL) * | 0 (0–50) | 0 | (0–13) | - |
| IL-10 (pg/mL) * | - | - | - | - |
| IL-18 (pg/mL) ** | 819 ± 237 | 824 ± 210 | 824 ± 245 | NS |
| Hepatokines | ||||
| FGF21 (pg/mL) * | 231 (26–2035) | 301 (98–2035) | 230 (26–1419) | 0.067 |
| ANGPL4 (pg/mL) * | 8489 | - | 8489 | - |
| Fetuin-A (ng/mL) * | 18.8 (0–19.2) | 18.9 (18.5–19.0) | 18.8 (0–19.2) | 0.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Contreras, A.D.; Francisco, M.d.R.; Vargas-Basurto, J.L.; González-Gómez, K.D.; Vivanco-Cid, H.; Hernández-Flores, K.G.; Grube-Pagola, P.; Roesch-Dietlen, F.B.; Remes-Troche, J.M. Hepatokine and Proinflammatory Cytokine Profile in Patients with Carotid Atherosclerosis and Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines 2025, 13, 978. https://doi.org/10.3390/biomedicines13040978
Cano-Contreras AD, Francisco MdR, Vargas-Basurto JL, González-Gómez KD, Vivanco-Cid H, Hernández-Flores KG, Grube-Pagola P, Roesch-Dietlen FB, Remes-Troche JM. Hepatokine and Proinflammatory Cytokine Profile in Patients with Carotid Atherosclerosis and Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines. 2025; 13(4):978. https://doi.org/10.3390/biomedicines13040978
Chicago/Turabian StyleCano-Contreras, Ana Delfina, Maria del Rocio Francisco, Jose Luis Vargas-Basurto, Kevin David González-Gómez, Hector Vivanco-Cid, Karina Guadalupe Hernández-Flores, Peter Grube-Pagola, Federico Bernardo Roesch-Dietlen, and Jose Maria Remes-Troche. 2025. "Hepatokine and Proinflammatory Cytokine Profile in Patients with Carotid Atherosclerosis and Metabolic Dysfunction-Associated Steatotic Liver Disease" Biomedicines 13, no. 4: 978. https://doi.org/10.3390/biomedicines13040978
APA StyleCano-Contreras, A. D., Francisco, M. d. R., Vargas-Basurto, J. L., González-Gómez, K. D., Vivanco-Cid, H., Hernández-Flores, K. G., Grube-Pagola, P., Roesch-Dietlen, F. B., & Remes-Troche, J. M. (2025). Hepatokine and Proinflammatory Cytokine Profile in Patients with Carotid Atherosclerosis and Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines, 13(4), 978. https://doi.org/10.3390/biomedicines13040978






