The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet Treatment
2.2. Metabolic Tests
2.3. Blood Biochemistry
2.4. Oil Red O Staining of Lipids in Liver Tissue
2.5. Analysis of the Fatty Acid Composition
2.6. qPCR Analysis
2.7. LC-MS/MS Analysis
2.8. Western Blot
2.9. Statistical Analyses
3. Results
3.1. Body Weight and Metabolic Parameters
3.2. Liver Lipid Analysis
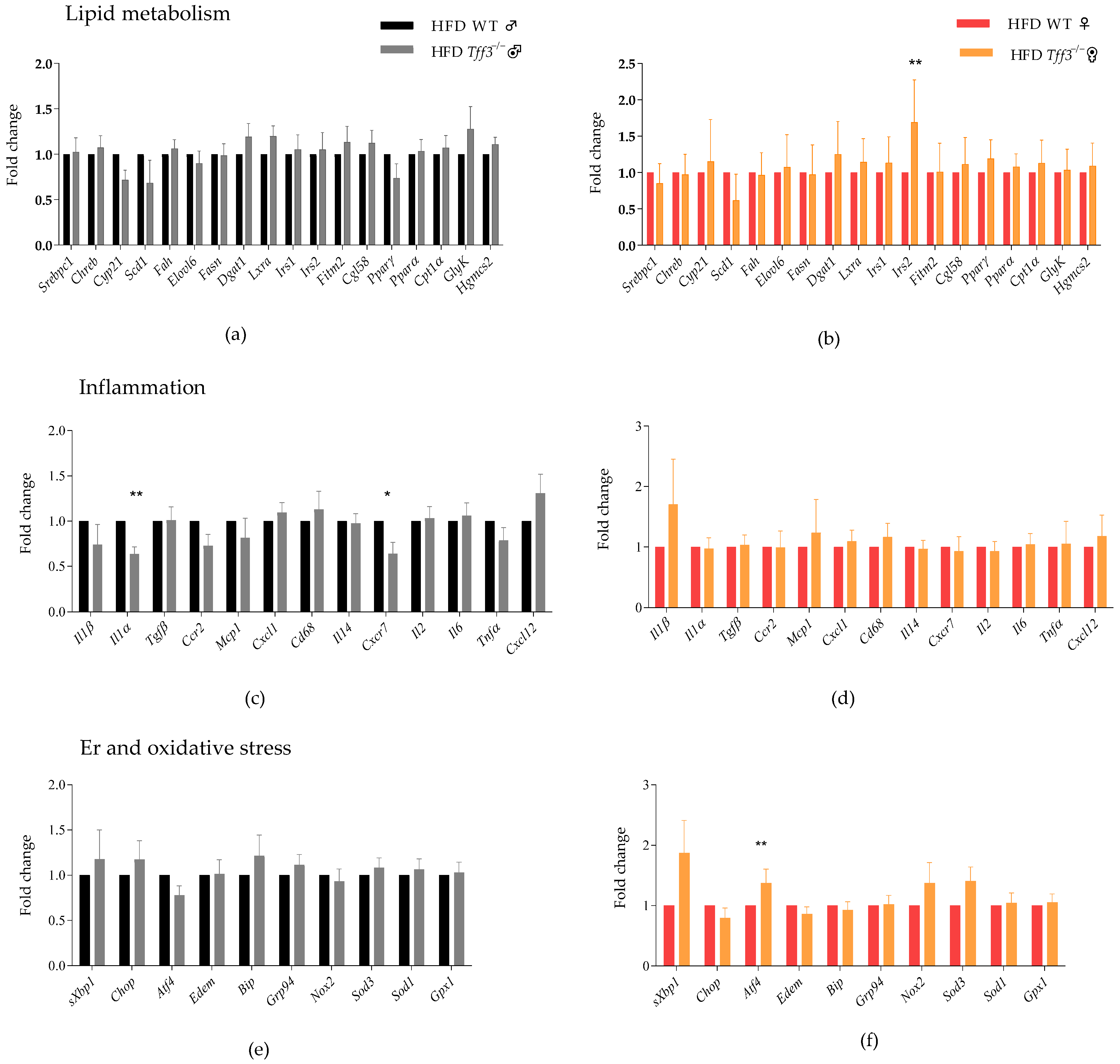
3.3. Expression of Genes Related to Relevant Pathophysiological Pathways
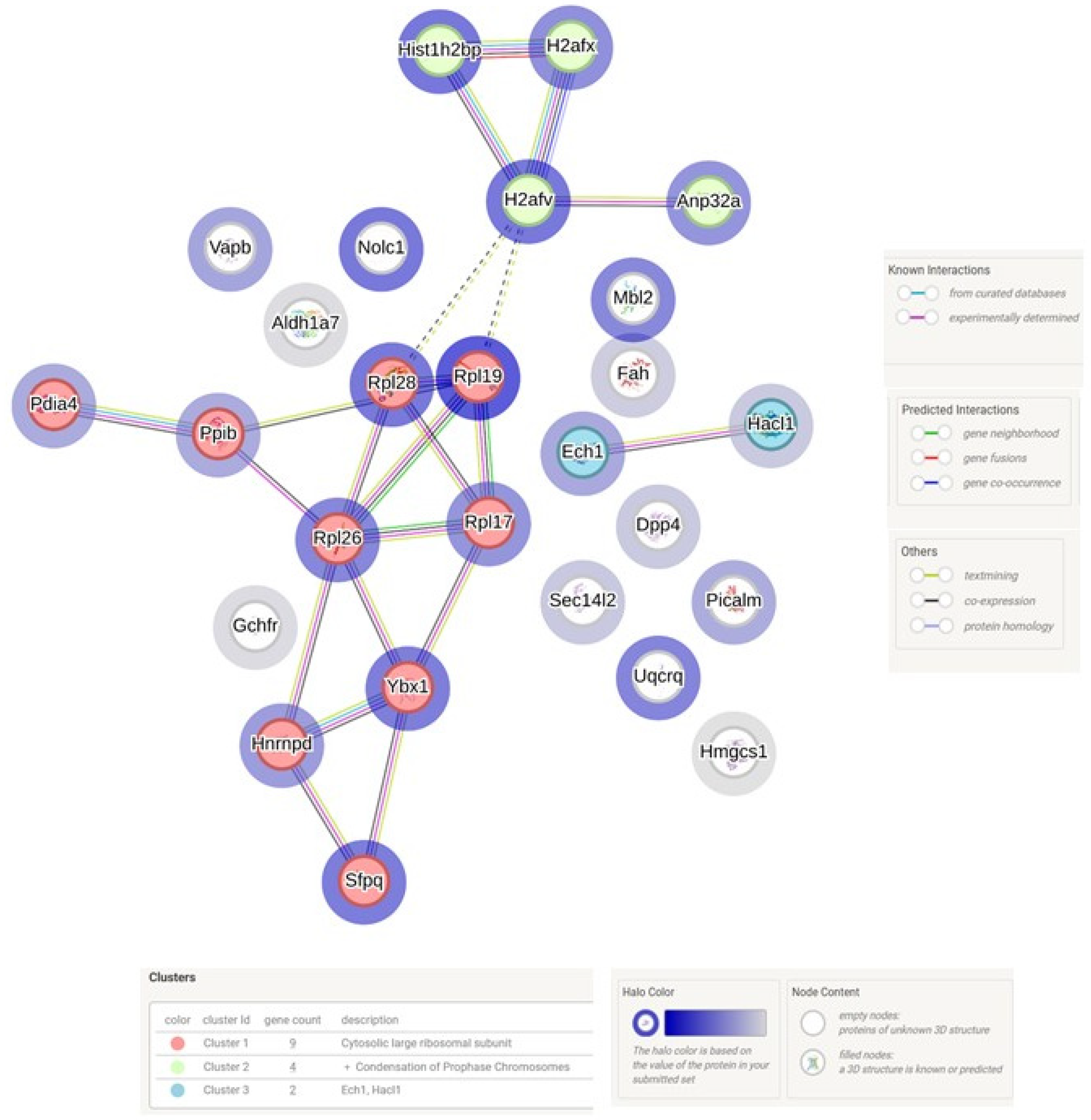
3.4. LC MS/MS Analysis of Liver in WT and Tff3−/− Mice Exposed to Short-Term HFD Treatment
Western Blot Analysis of Ybx1 and Anp32a Protein Expression
4. Discussion
4.1. Body Weight and Metabolic Parameters
4.2. Liver Lipid Analysis
4.3. Expression of Genes Related to Relevant Pathophysiological Pathways
4.4. LC MS/MS Analysis of Liver in WT and Tff3−/− Mice Exposed to Short Term High Fat Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The Complex Link between NAFLD and Type 2 Diabetes Mellitus—Mechanisms and Treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Olver, W.I.; Donnelly, C.J.; May, M.E.; Naggert, J.K.; Shaffer, D.J.; Roopenian, D.C. Searching QTL by Gene Expression: Analysis of Diabesity. BMC Genet. 2005, 6, 12. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, L.; Cui, Y.; Zhang, H.; Chen, Q.; Cui, A.; Fang, F.; Chang, Y. Tff3, as a Novel Peptide, Regulates Hepatic Glucose Metabolism. PLoS ONE 2013, 8, e75240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, H.; Yang, R.; Luan, X.; Zhang, L.; Jin, Q.; Jin, Y.; Xue, J. Mouse Trefoil Factor 3 Ameliorated High-Fat-Diet-Induced Hepatic Steatosis via Increasing Peroxisome Proliferator-Activated Receptor-α-Mediated Fatty Acid Oxidation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E436–E445. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Lin, Q.; Bei, W.; Guo, J. Pathological and Therapeutic Roles of Bioactive Peptide Trefoil Factor 3 in Diverse Diseases: Recent Progress and Perspective. Cell Death Dis. 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Guillén, N.; Navarro, M.A.; Arnal, C.; Noone, E.; Arbonés-Mainar, J.M.; Acín, S.; Surra, J.C.; Muniesa, P.; Roche, H.M.; Osada, J. Microarray Analysis of Hepatic Gene Expression Identifies New Genes Involved in Steatotic Liver. Physiol. Genom. 2009, 37, 187–198. [Google Scholar] [CrossRef]
- Ge, H.; Gardner, J.; Wu, X.; Rulifson, I.; Wang, J.; Xiong, Y.; Ye, J.; Belouski, E.; Cao, P.; Tang, J.; et al. Trefoil Factor 3 (TFF3) is Regulated by Food Intake, Improves Glucose Tolerance and Induces Mucinous Metaplasia. PLoS ONE 2015, 10, e0126924. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Bujak, M.; Bujak, I.T.; Sobočanec, S.; Mihalj, M.; Novak, S.; Cosić, A.; Levak, M.T.; Kopačin, V.; Mihaljević, B.; Balog, T.; et al. Trefoil Factor 3 Deficiency Affects Liver Lipid Metabolism. Cell. Physiol. Biochem. 2018, 47, 827–841. [Google Scholar] [CrossRef]
- Barrera Roa, J.; Sanchez Tortolero, G.; Gonzalez, E. Trefoil Factor 3 (TFF3) Expression is Regulated by Insulin and Glucose. J. Health Sci. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Mohammadi-Shemirani, P.; Sjaarda, J.; Gerstein, H.C.; Treleaven, D.J.; Walsh, M.; Mann, J.F.; McQueen, M.J.; Hess, S.; Paré, G. A Mendelian Randomization-Based Approach to Identify Early and Sensitive Diagnostic Biomarkers of Disease. Clin. Chem. 2019, 65, 427–436. [Google Scholar] [CrossRef]
- Brott, D.A.; Furlong, S.T.; Adler, S.H.; Hainer, J.W.; Arani, R.B.; Pinches, M.; Rossing, P.; Chaturvedi, N. Characterization of Renal Biomarkers for Use in Clinical Trials: Effect of Preanalytical Processing and Qualification Using Samples from Subjects with Diabetes. Drug Des. Devel. Ther. 2015, 9, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Šešelja, K.; Bazina, I.; Welss, J.; Schicht, M.; Paulsen, F.; Bijelić, N.; Roðak, E.; Horvatić, A.; Gelemanović, A.; Mihalj, M.; et al. Effect of Tff3 Deficiency and ER Stress in the Liver. Int. J. Mol. Sci. 2019, 20, 4389. [Google Scholar] [CrossRef]
- Šešelja, K.; Bazina, I.; Vrecl, M.; Welss, J.; Schicht, M.; Mihalj, M.; Kopačin, V.; Paulsen, F.; Pirman, T.; Baus Lončar, M. Tff3 Deficiency Protects against Hepatic Fat Accumulation after Prolonged High-Fat Diet. Life 2022, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-Fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. NIH to Balance Sex in Cell and Animal Studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Intraperitoneal Glucose Tolerance Test (IPGTT) Protocol—IMPReSS. Available online: https://web.mousephenotype.org/impress/ProcedureInfo?action=list&procID=536 (accessed on 6 April 2025).
- Fawaz, G. Haj Intraperitoneal Insulin Tolerance Test. Available online: https://www.mmpc.org/shared/document.aspx?id=84&docType=Protocol (accessed on 6 April 2025).
- Schmöcker, C.; Weylandt, K.H.; Kahlke, L.; Wang, J.; Lobeck, H.; Tiegs, G.; Berg, T.; Kang, J.X. Omega-3 Fatty Acids Alleviate Chemically Induced Acute Hepatitis by Suppression of Cytokines. Hepatology 2007, 45, 864–869. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, S.; Deng, Y.; Gordillo, R.; Ghaben, A.L.; Shao, M.; Zhang, F.; Xu, P.; Li, Y.; Cao, H.; et al. Hepatic Gale Regulates Whole-Body Glucose Homeostasis by Modulating Tff3 Expression. Diabetes 2017, 66, 2789–2799. [Google Scholar] [CrossRef]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A High-Fat Diet Impairs Neurogenesis: Involvement of Lipid Peroxidation and Brain-Derived Neurotrophic Factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Shah, A.A.; Leidinger, P.; Keller, A.; Wendschlag, A.; Backes, C.; Baus-Loncar, M.; Meese, E.; Blin, N. The Intestinal Factor Tff3 and a MiRNA Network Regulate Murine Caloric Metabolism. RNA Biol. 2011, 8, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.M.; Greenaway, S.; White, J.K.; Fuchs, H.; Gailus-Durner, V.; Wells, S.; Sorg, T.; Wong, K.; Bedu, E.; Cartwright, E.J.; et al. A Comparative Phenotypic and Genomic Analysis of C57BL/6J and C57BL/6N Mouse Strains. Genome Biol. 2013, 14, R82. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular Mechanisms of Metabolic Associated Fatty Liver Disease (MAFLD): Functional Analysis of Lipid Metabolism Pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Role of Bioactive Fatty Acids in Nonalcoholic Fatty Liver Disease. Nutr. J. 2016, 15, 72. [Google Scholar] [CrossRef]
- Fridén, M.; Rosqvist, F.; Ahlström, H.; Niessen, H.G.; Schultheis, C.; Hockings, P.; Hulthe, J.; Gummesson, A.; Wanders, A.; Rorsman, F.; et al. Hepatic Unsaturated Fatty Acids Are Linked to Lower Degree of Fibrosis in Non-Alcoholic Fatty Liver Disease. Front. Med. 2022, 8, 814951. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared with Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Blaak, E.E. Mitochondrial Dysfunction Is a Key Pathway That Links Saturated Fat Intake to the Development and Progression of NAFLD. Mol. Nutr. Food Res. 2021, 65, e1900942. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated Fatty Acids Promote Endoplasmic Reticulum Stress and Liver Injury in Rats with Hepatic Steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular Mechanisms and the Role of Saturated Fatty Acids in the Progression of Non-Alcoholic Fatty Liver Disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef]
- Lee, C.-H. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2769. [Google Scholar] [CrossRef] [PubMed]
- Shantanam, S. Mueller Omega-3 Fatty Acids and Nonalcoholic Fatty Liver Disease in Adults and Children: Where Do We Stand? HHS Public Access. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Masterton, G.S.; Plevris, J.N.; Hayes, P.C. Review Article: Omega-3 Fatty Acids—A Promising Novel Therapy for Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2010, 31, 679–692. [Google Scholar] [CrossRef]
- Jeyapal, S.; Kona, S.R.; Mullapudi, S.V.; Putcha, U.K.; Gurumurthy, P.; Ibrahim, A. Substitution of Linoleic Acid with α-Linolenic Acid or Long Chain n-3 Polyunsaturated Fatty Acid Prevents Western Diet Induced Nonalcoholic Steatohepatitis. Sci. Rep. 2018, 8, 10953. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepää, A.L.; Orešič, M.; Yki-Järvinen, H. Hepatic Stearoyl-CoA Desaturase (SCD)-1 Activity and Diacylglycerol but Not Ceramide Concentrations Are Increased in the Nonalcoholic Human Fatty Liver. Diabetes 2009, 58, 203–208. [Google Scholar] [CrossRef]
- Chiappini, F.; Coilly, A.; Kadar, H.; Gual, P.; Tran, A.; Desterke, C.; Samuel, D.; Duclos-Vallée, J.C.; Touboul, D.; Bertrand-Michel, J.; et al. Metabolism Dysregulation Induces a Specific Lipid Signature of Nonalcoholic Steatohepatitis in Patients. Sci. Rep. 2017, 7, 46658. [Google Scholar] [CrossRef]
- Allard, J.P.; Aghdassi, E.; Mohammed, S.; Raman, M.; Avand, G.; Arendt, B.M.; Jalali, P.; Kandasamy, T.; Prayitno, N.; Sherman, M.; et al. Nutritional Assessment and Hepatic Fatty Acid Composition in Non-Alcoholic Fatty Liver Disease (NAFLD): A Cross-Sectional Study. J. Hepatol. 2008, 48, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. ALowω-6 Toω-3 PUFA Ratio (n–6 n–3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. The Importance of the Long-Chain Polyunsaturated Fatty Acid n-6/n-3 Ratio in Development of Non-Alcoholic Fatty Liver Associated with Obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Sertoglu, E.; Kayadibi, H.; Uyanik, M. A Biochemical View: Increase in Polyunsaturated Fatty Acid ω-6/ω-3 Ratio in Relation to Hepatic Steatosis in Patients with Non-Alcoholic Fatty Liver Disease. J. Diabetes Complicat. 2015, 29, 157. [Google Scholar] [CrossRef]
- Hertel, S.C.; Chwieralski, C.E.; Hinz, M.; Rio, M.C.; Tomasetto, C.; Hoffmann, W. Profiling Trefoil Factor Family (TFF) Expression in the Mouse: Identification of an Antisense TFF1-Related Transcript in the Kidney and Liver. Peptides 2004, 25, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Thim, L. A New Family of Growth Factor-like Peptides “Trefoil” Disulphide Loop Structures as a Common Feature in Breast Cancer Associated Peptide (PS2), Pancreatic Spasmolytic Polypeptide (PSP), and Frog Skin Peptides (Spasmolysins). FEBS Lett. 1989, 250, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Sawada, S.; Ueno, Y.; Murakami, K.; Yamada, T.; Gao, J.; Kodama, S.; Izumi, T.; Takahashi, K.; Tsukita, S.; et al. Selective Insulin Resistance with Differential Expressions of IRS-1 and IRS-2 in Human NAFLD Livers. Int. J. Obes. 2018, 42, 1544–1555. [Google Scholar] [CrossRef]
- Kubota, N.; Tobe, K.; Terauchi, Y.; Eto, K.; Yamauchi, T.; Suzuki, R.; Tsubamoto, Y.; Komeda, K.; Nakano, R.; Miki, H.; et al. Disruption of Insulin Receptor Substrate 2 Causes Type 2 Diabetes Because of Liver Insulin Resistance and Lack of Compensatory β-Cell Hyperplasia. Diabetes 2000, 49, 1880–1889. [Google Scholar] [CrossRef]
- Kubota, N.; Kubota, T.; Kajiwara, E.; Iwamura, T.; Kumagai, H.; Watanabe, T.; Inoue, M.; Takamoto, I.; Sasako, T.; Kumagai, K.; et al. Differential Hepatic Distribution of Insulin Receptor Substrates Causes Selective Insulin Resistance in Diabetes and Obesity. Nat. Commun. 2016, 7, 12977. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Geißler, C.; Tackenberg, H.; El Gammal, A.T.; Wolter, S.; Spranger, J.; Mann, O.; Lehnert, H.; Kirchner, H. Multi-Layered Epigenetic Regulation of IRS2 Expression in the Liver of Obese Individuals with Type 2 Diabetes. Diabetologia 2020, 63, 2182–2193. [Google Scholar] [CrossRef]
- Teng, X.; Xu, L.F.; Zhou, P.; Sun, H.W.; Sun, M. Effects of Trefoil Peptide 3 on Expression of TNF-α, TLR4, and NF-ΚB in Trinitrobenzene Sulphonic Acid Induced Colitis Mice. Inflammation 2009, 32, 120–129. [Google Scholar] [CrossRef]
- Fu, T.; Znalesniak, E.B.; Kalinski, T.; Möhle, L.; Biswas, A.; Salm, F.; Dunay, I.R.; Hoffmann, W. TFF Peptides Play a Role in the Immune Response Following Oral Infection of Mice with Toxoplasma Gondii. Eur. J. Microbiol. Immunol. 2015, 5, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Effenberger, M.; Adolph, T.E. A Role for IL-1 Inhibitors in the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD)? Expert Opin. Investig. Drugs 2020, 29, 103–106. [Google Scholar] [CrossRef]
- Almog, T.; Kandel Kfir, M.; Levkovich, H.; Shlomai, G.; Barshack, I.; Stienstra, R.; Lustig, Y.; Leikin Frenkel, A.; Harari, A.; Bujanover, Y.; et al. Interleukin-1α Deficiency Reduces Adiposity, Glucose Intolerance and Hepatic de-Novo Lipogenesis in Diet-Induced Obese Mice. BMJ Open Diabetes Res. Care 2019, 7, e000650. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.; Li, Y.; Qian, X.; Luan, J.; Lv, X. Emerging Importance of Chemokine Receptor CXCR4 and Its Ligand in Liver Disease. Front. Cell Dev. Biol. 2021, 9, 716842. [Google Scholar] [CrossRef] [PubMed]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 Mediate TFF3-Induced Cell Migration Independently from the ERK1/2 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, C.F.; Yu, C.H.; Chen, W.X.; Li, Y.M. Role of Endoplasmic Reticulum Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Kozina, N.; Mihaljević, Z.; Lončar, M.B.; Mihalj, M.; Mišir, M.; Radmilović, M.D.; Justić, H.; Gajović, S.; Šešelja, K.; Bazina, I.; et al. Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57bl/6n Knockout and Wt (C57bl/6n) Control Mice. Int. J. Mol. Sci. 2019, 20, 5188. [Google Scholar] [CrossRef]
- Torres, L.; Karam, S.M.; Wendling, C.; Chenard, M.; Kershenobich, D.; Tomasetto, C.; Rio, M. TreFoil Factor 1 (TFF1/PS2) Deficiency Activates the Unfolded Protein Response. Mol. Med. 2002, 8, 273–282. [Google Scholar] [CrossRef]
- Ren, C.; Dokter-Fokkens, J.; Figueroa Lozano, S.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, 1700572. [Google Scholar] [CrossRef]
- Asada, R.; Saito, A.; Kawasaki, N.; Kanemoto, S.; Iwamoto, H.; Oki, M.; Miyagi, H.; Izumi, S.; Imaizumi, K. The Endoplasmic Reticulum Stress Transducer OASIS Is Involved in the Terminal Differentiation of Goblet Cells in the Large Intestine. J. Biol. Chem. 2012, 287, 8144–8153. [Google Scholar] [CrossRef] [PubMed]
- Wortel, I.M.N.; van der Meer, L.T.; Kilberg, M.S.; van Leeuwen, F.N. Sivanand Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells. Trends Endocrinol. Metab. 2017, 28, 794–806. [Google Scholar] [CrossRef]
- Li, H.; Meng, Q.; Xiao, F.; Chen, S.; Du, Y.; Yu, J.; Wang, C.; Guo, F. ATF4 Deficiency Protects Mice from High-Carbohydrate-Diet-Induced Liver Steatosis. Biochem. J. 2011, 438, 283–289. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, T.; Yu, S.; Lee, S.; Calabuig-Navarro, V.; Yamauchi, J.; Ringquist, S.; Dong, H.H. ATF4 Protein Deficiency Protects against High Fructose-Induced Hypertriglyceridemia in Mice. J. Biol. Chem. 2013, 288, 25350–25361. [Google Scholar] [CrossRef]
- Seo, J.; Fortuno, E.S.; Jae, M.S.; Stenesen, D.; Tang, W.; Parks, E.J.; Adams, C.M.; Townes, T.; Graff, J.M. Atf4 Regulates Obesity, Glucose Homeostasis, and Energy Expenditure. Diabetes 2009, 58, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Thomas, G.; Volarevic, S. Players and Therapeutic Avenues. Nat. Publ. Gr. 2017, 18, 51–63. [Google Scholar]
- Oie, S.; Matsuzaki, K.; Yokoyama, W.; Tokunaga, S.; Waku, T.; Han, S.I.; Iwasaki, N.; Mikogai, A.; Yasuzawa-Tanaka, K.; Kishimoto, H.; et al. Hepatic RRNA Transcription Regulates High-Fat-Diet-Induced Obesity. Cell Rep. 2014, 7, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Lyabin, D.N.; Eliseeva, I.A.; Ovchinnikov, L.P. YB-1 Protein: Functions and Regulation. Wiley Interdiscip. Rev. RNA 2014, 5, 95–110. [Google Scholar] [CrossRef]
- Wang, L.; Gulappa, T.; Menon, K.M.J. Identification and Characterization of Proteins That Selectively Interact with the LHR MRNA Binding Protein (LRBP) in Rat Ovaries. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.G.; Rentz, T.; Dorighello, G.G.; Marques, A.C.; Lorza-Gil, E.; Wanschel, A.C.B.A.; De Moraes, A.; Vercesi, A.E.; Oliveira, H.C.F. Lack of Mitochondrial NADP(H)-Transhydrogenase Expression in Macrophages Exacerbates Atherosclerosis in Hypercholesterolemic Mice. Biochem. J. 2019, 476, 3769–3789. [Google Scholar] [CrossRef]
- Pihlajamäki, J.; Lerin, C.; Itkonen, P.; Boes, T.; Floss, T.; Schroeder, J.; Dearie, F.; Crunkhorn, S.; Burak, F.; Jimenez-Chillaron, J.C.; et al. Expression of the Splicing Factor Gene SFRS10 Is Reduced in Human Obesity and Contributes to Enhanced Lipogenesis. Cell Metab. 2011, 14, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.T.; Yu, Y.; Hamiche, A.; Wang, L. Cracking the ANP32 Whips: Important Functions, Unequal Requirement, and Hints at Disease Implications. BioEssays 2014, 36, 1062–1071. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone Acetylation and the Role of Histone Deacetylases in Normal Cyclic Endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, Y.; Shin, M.H.; Cho, S.I.; Zouboulis, C.C.; Kim, M.K.; Lee, D.H.; Chung, J.H. Histone Deacetylase 1 Reduces Lipogenesis by Suppressing Srebp1 Transcription in Human Sebocyte Cell Line Sz95. Int. J. Mol. Sci. 2021, 22, 4477. [Google Scholar] [CrossRef]
- Yang, X.; Lu, B.; Sun, X.; Han, C.; Fu, C.; Xu, K.; Wang, M.; Li, D.; Chen, Z.; Opal, P.; et al. ANP32A Regulates Histone H3 Acetylation and Promotes Leukemogenesis. Leukemia 2018, 32, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, N.; Najafi, B.; Schueder, F.; Quagliarini, F.; Steger, M.; Seitz, S.; Kasper, R.; Salinas, F.; Cox, J.; Uhlenhaut, N.H.; et al. Organellar Proteomics and Phospho-Proteomics Reveal Subcellular Reorganization in Diet-Induced Hepatic Steatosis. Dev. Cell. 2018, 47, 205–221.e7. [Google Scholar] [CrossRef] [PubMed]



| Main Fatty Acid Group (g of Fatty Acids/100 g of Total Fatty Acids) | HFD WT ♂ | HFD Tff3−/− ♂ | HFD WT ♀ | HFD Tff3−/− ♀ |
|---|---|---|---|---|
| C 14:0 | 0.43 | 0.41 | 0.42 | 0.44 |
| C 16:0 | 25.38 | 24.58 | 24.76 | 25.20 |
| C 16:1 | 4.06 | 3.85 | 3.07 | 2.88 |
| C 18:0 | 8.41 | 9.45 | 8.76 | 10.21 |
| C 18:1 | 32.76 | 30.45 | 37.40 | 31.80 |
| C 18:2, n-6 | 10.19 | 11.27 | 9.27 § | 10.47 |
| C 18:3, n-6 | 0.33 | 0.37 | 0.32 § | 0.49 |
| C 18:3, n-3 | 0.14 | 0.17 | 0.12 | 0.16 |
| C 20:0 | 0.34 ‡,* | 0.27 † | 0.03 | 0.04 |
| C 20:1, n-9 | 0.56 | 0.46 † | 0.42 § | 0.25 |
| C 20:3, n-6 | 0.91 * | 0.97 † | 0.60 | 0.62 |
| C 20:4, n-6 | 10.00 | 10.73 | 8.69 | 9.81 |
| C 20:5, n-3 | 0.12 * | 0.14 | 0.07 § | 0.13 |
| C 22:4, n-6 | 0.33 | 0.32 | 0.32 | 0.37 |
| C 22:5, n-6 | 0.49 | 0.49 † | 0.57 | 0.62 |
| C 22:5, n-3 | 0.31 * | 0.33 | 0.16 § | 0.28 |
| C 22:6, n-3 | 4.39 | 4.78 | 4.25 | 5.37 |
| ∑ SFA 1 | 35.00 | 35.19 | 34.35 | 36.34 |
| ∑ MUFA 2 | 37.47 | 34.81 | 40.90 | 34.96 |
| ∑ PUFA 3 | 27.19 | 29.68 | 24.44 | 28.33 |
| n-6 PUFA | 22.14 | 24.08 | 19.73 | 22.13 |
| n-3 PUFA | 4.98 | 5.46 | 4.63 § | 5.99 |
| n-6/n-3 PUFA 4 | 4.59:1 | 4.42:1 † | 4.29:1 § | 3.71:1 |
| Total fat content (g/100 g liver) | 6.50 ± 2.36 | 5.22 ± 0.24 | 7.41 ± 1.11 | 5.96 ± 1.58 |
| Protein Name (Short in STRING) | Fold Change | FDR | p Value |
|---|---|---|---|
| ribosomal protein L19 (Rpl19) | 1.491 | 0.046 | 0.002 |
| histone H2B type 1-P isoform 1 (Hist1h2bp) | 1.341 | 0.046 | 0.002 |
| nucleolar and coiled-body phosphoprotein 1 (Nolc1) | 1.334 | 0.046 | 0.002 |
| histone H2afv (H2afv) | 1.331 | 0.046 | 0.002 |
| 60S ribosomal protein L28 isoform X1 (Rpl28) | 1.327 | 0.046 | 0.002 |
| splicing factor proline/glutamine-rich (polypyrimidine tract binding protein-associated) (Sfpq) | 1.314 | 0.046 | 0.002 |
| nuclease-sensitive element-binding protein 1 isoform X1 (Ybx1) | 1.305 | 0.046 | 0.002 |
| signal recognition particle subunit (Srp27) | 1.264 | 0.046 | 0.002 |
| ubiquinol-cytochrome c reductase, complex III subunit VII (Uqcrq) | 1.264 | 0.046 | 0.002 |
| ribosomal proteinL26 (Rpl26) | 1.261 | 0.046 | 0.002 |
| mannose-binding protein C precursor (Mbl2) | 1.247 | 0.046 | 0.002 |
| histone H2A.X (H2afx) | 1.215 | 0.046 | 0.002 |
| ribosomal protein (Rpl17) | 1.186 | 0.046 | 0.002 |
| acidic nuclear phosphoprotein pp32 (Anp32a) | 1.182 | 0.046 | 0.002 |
| heterogeneous nuclear ribonucleoprotein AU-rich element RNA-binding protein 1 (Hnrnpd) | 1.151 | 0.046 | 0.002 |
| ribosomal protein S13 (Rsp13) | 1.117 | 0.046 | 0.002 |
| vesicle-associated membrane protein, associated protein B and C (Vapb) | 1.107 | 0.046 | 0.002 |
| enoyl coenzyme A hydratase 1, peroxisomal (Ech1) | 1.082 | 0.046 | 0.002 |
| protein disulfide-isomerase A4 isoform 1 precursor (Pdia4) | 1.073 | 0.046 | 0.002 |
| peptidylprolyl isomerase B (Ppib) | 1.073 | 0.046 | 0.002 |
| phosphatidylinositol binding clathrin assembly protein (Picalm) | 1.055 | 0.046 | 0.002 |
| sec14-like protein 2 (Sec14l2) | 0.920 | 0.046 | 0.002 |
| dipeptidyl peptidase 4 isoform 1 (Dpp4) | 0.914 | 0.046 | 0.002 |
| 2-hydroxyacyl-CoA lyase 1 (Hacl1) | 0.911 | 0.046 | 0.002 |
| fumarylacetoacetase hydrolase (Fah) | 0.895 | 0.046 | 0.002 |
| gtp cyclohydrolase I feedback regulator (Gchfr) | 0.820 | 0.046 | 0.002 |
| aldehyde dehydrogenase, cytosolic 1 (Aldh1a7) | 0.808 | 0.046 | 0.002 |
| 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (Hmgcs1) | 0.792 | 0.046 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazina, I.; Šešelja, K.; Pirman, T.; Horvatić, A.; Erman, A.; Mihalj, M.; Baus Lončar, M. The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet. Biomedicines 2025, 13, 1024. https://doi.org/10.3390/biomedicines13051024
Bazina I, Šešelja K, Pirman T, Horvatić A, Erman A, Mihalj M, Baus Lončar M. The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet. Biomedicines. 2025; 13(5):1024. https://doi.org/10.3390/biomedicines13051024
Chicago/Turabian StyleBazina, Iva, Kate Šešelja, Tatjana Pirman, Anita Horvatić, Andreja Erman, Martina Mihalj, and Mirela Baus Lončar. 2025. "The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet" Biomedicines 13, no. 5: 1024. https://doi.org/10.3390/biomedicines13051024
APA StyleBazina, I., Šešelja, K., Pirman, T., Horvatić, A., Erman, A., Mihalj, M., & Baus Lončar, M. (2025). The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet. Biomedicines, 13(5), 1024. https://doi.org/10.3390/biomedicines13051024








