Amburana cearensis (Cumaru) and Its Active Principles as Source of Anti-Leishmania Drugs: Immunomodulatory Activity of Coumarin (1,2-Benzopyrone)
Abstract
1. Introduction
2. Materials and Methods
2.1. Botanical Material
2.2. Chemicals
2.3. Preparation and Chemical Characterization of Dried Extract from Amburana Cearensis (DEAC)
2.4. Amburana Cearensis Phenolic Fraction (ACPF) Obtainment and Characterization
2.5. Parasites
2.6. Leishmanicidal Effect Evaluation
2.6.1. Effect Evaluation Against the Promastigote Forms
2.6.2. Cytotoxicity Assessment
2.6.3. Effect Evaluation Against the Amastigote Forms
Measurement of Nitric Oxide (NO) and Cytokines
2.7. Statistical Analysis
3. Results
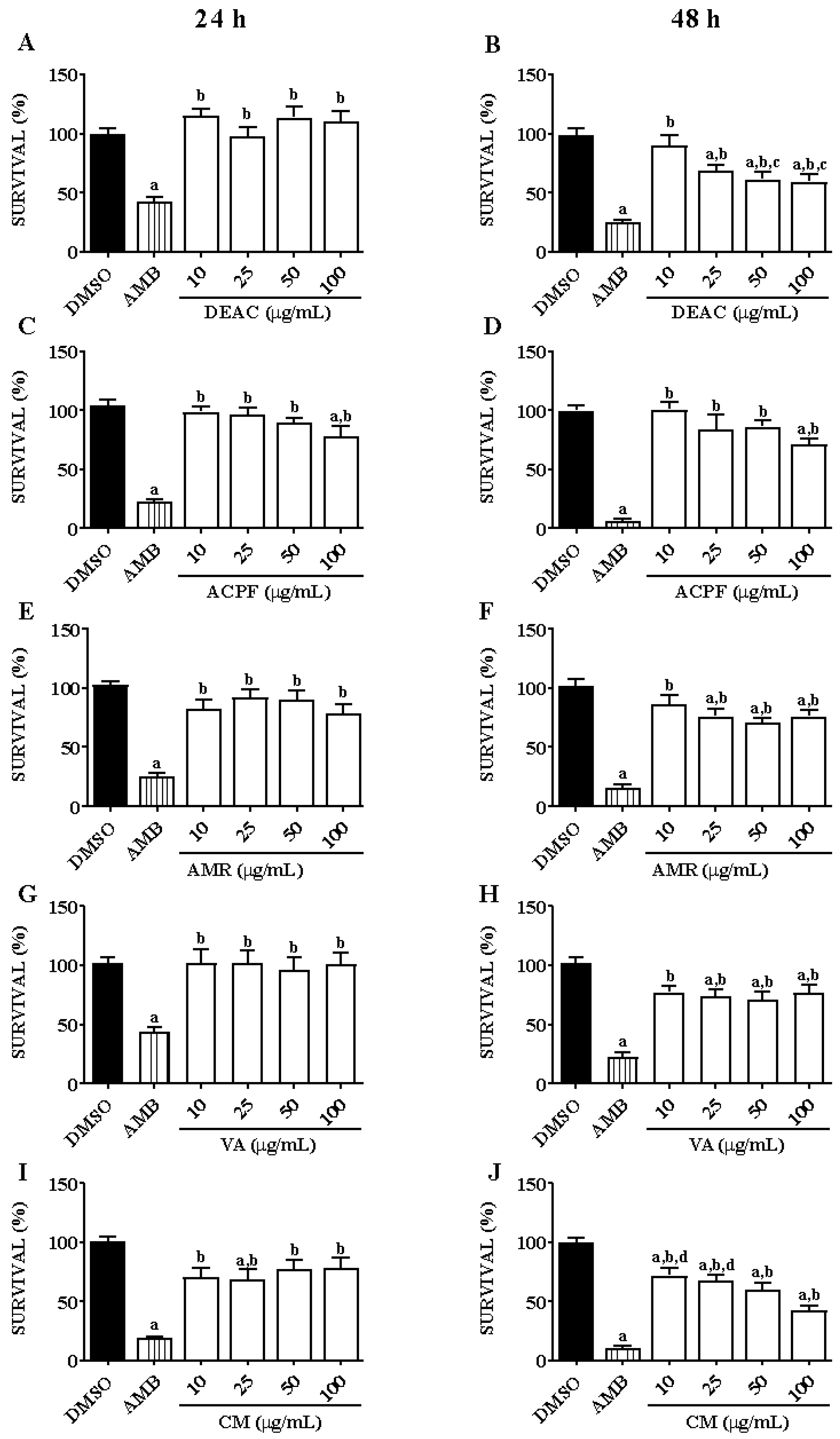
3.1. Anti-Leishmanial Activity of Dried Extract, Phenolic Fraction, and Molecules of A. cearensis in L. braziliensis Promastigotes
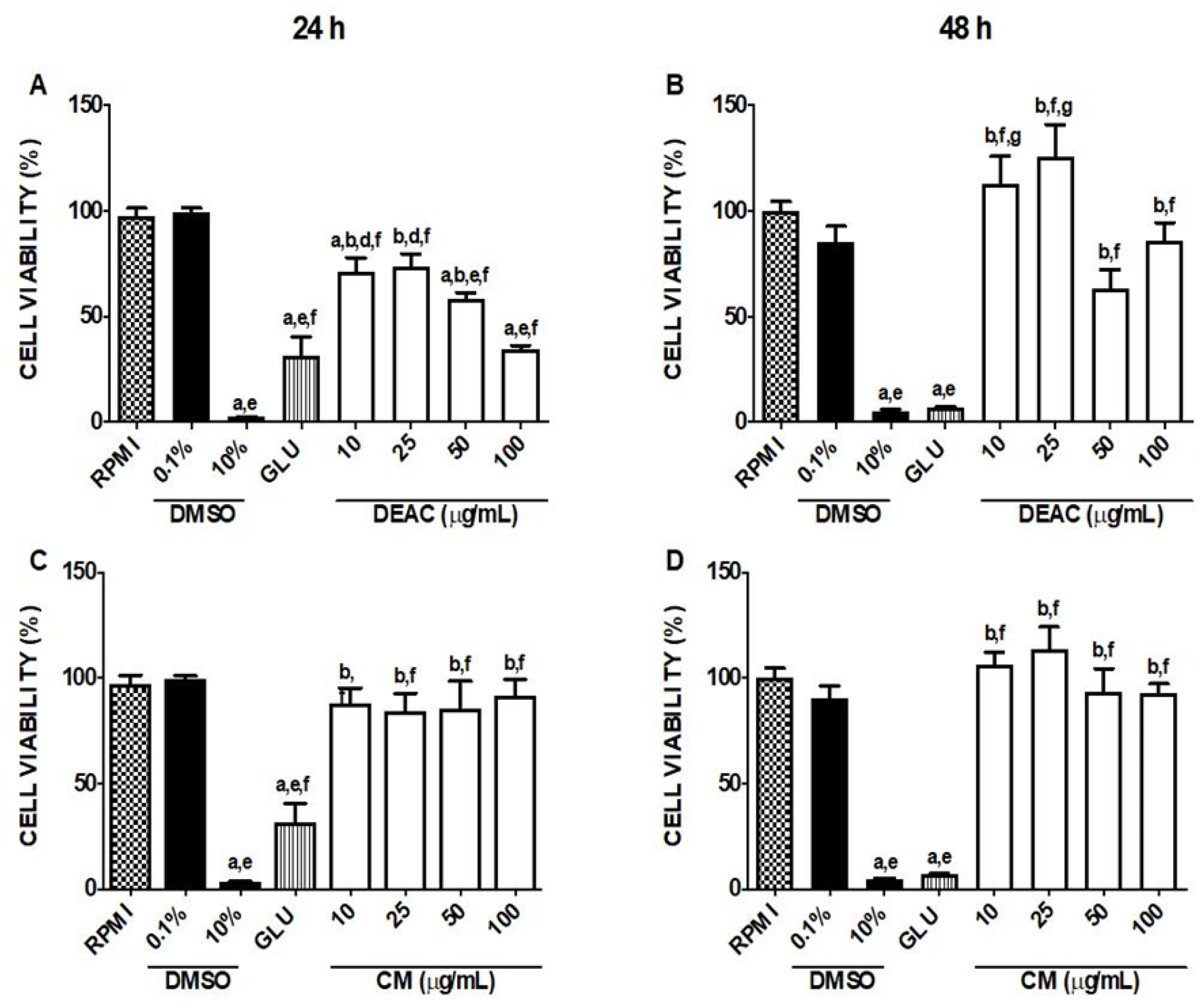
3.2. Cytotoxicity Assessment of DEAC and CM in Macrophages
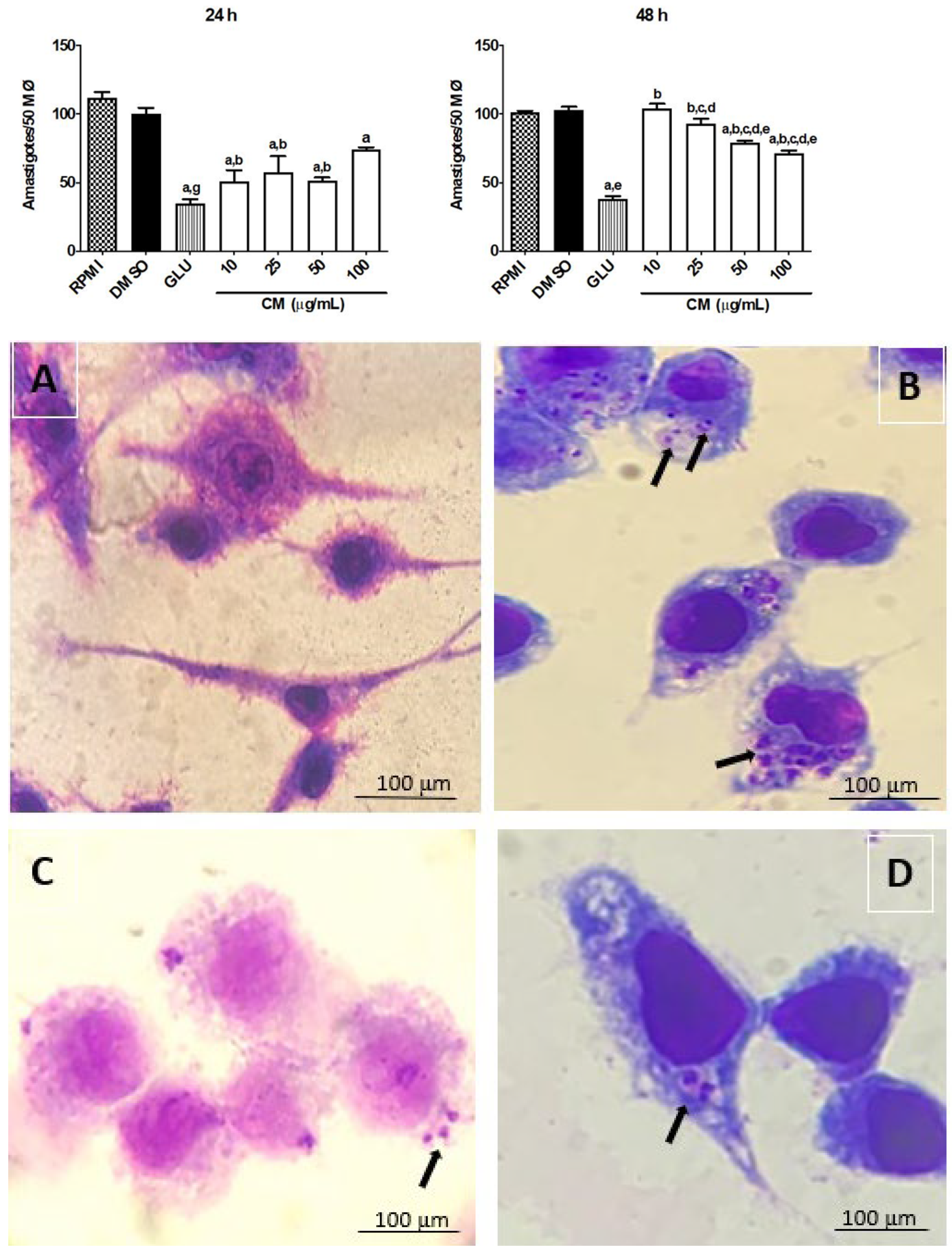
3.3. Effect of CM on Leishmania braziliensis Amastigotes
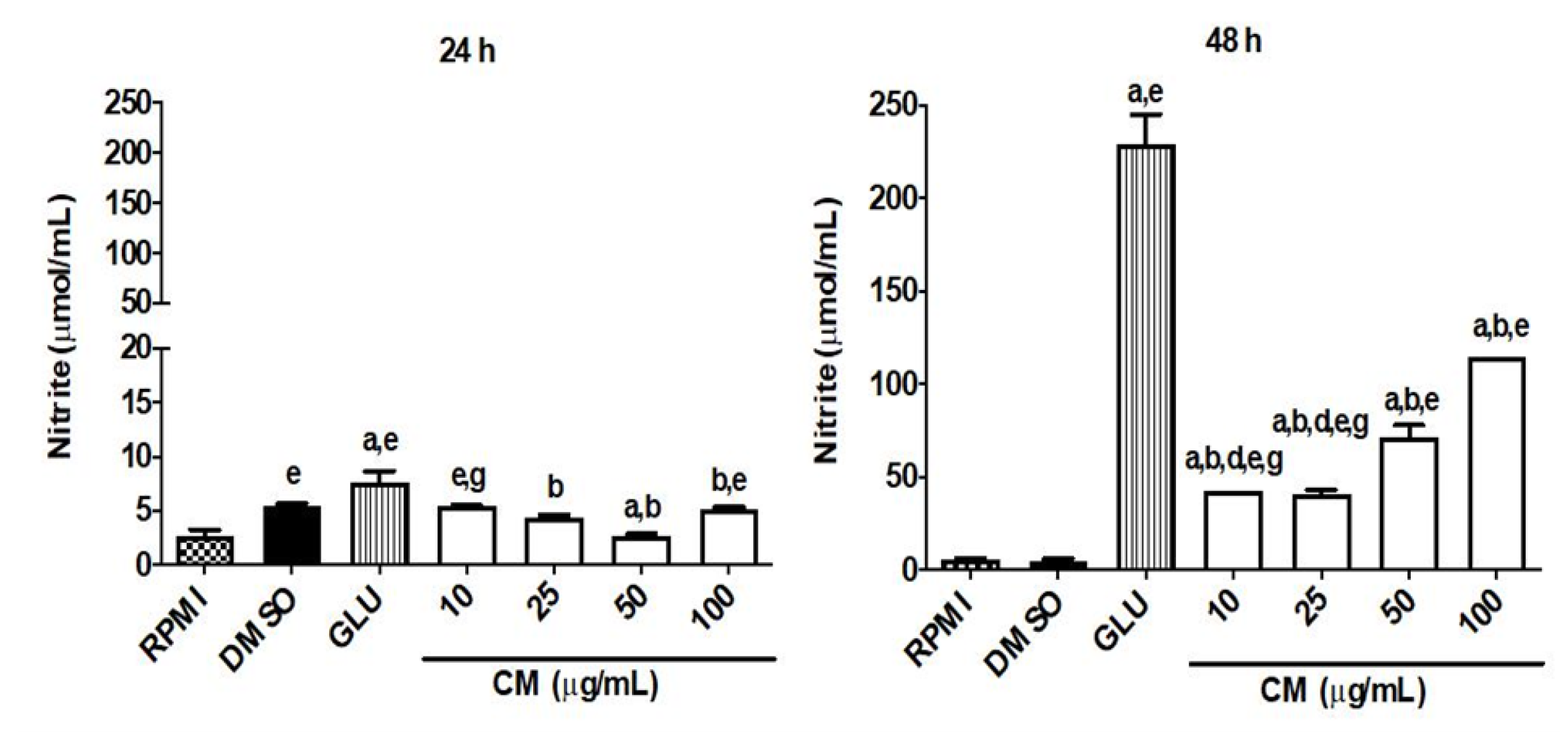
CM Induces an Increase in Nitric Oxide Production and Modulates the Cytokine Profile in Macrophages Infected by L. braziliensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCL | Localized cutaneous leishmaniasis |
| Th1 | T helper type 1 lymphocytes |
| TNF- α | Tumor necrosis factor α |
| IFN- γ | Interferon (IFN) γ |
| Th1 | T helper type 2 |
| IL | Interleukin |
| CM | Coumarin |
| AMR | Amburoside A |
| VA | Vanillic acid |
| JNK | C-Jun N-terminal kinase |
| ERK1/2 | Extracellular signal-regulated kinase |
| MAPKs | Mitogen-activated protein kinases |
| HPLC-PDA | High-performance liquid chromatography–photodiode array |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| DMSO | Dimethyl sulfoxide |
| DEAC | Dried extract from Amburana cearensis |
| ACPF | Amburana cearensis phenolic fraction |
| ACME | A. cearensis methanolic extract |
| FBS | Fetal bovine serum |
| GLU | Glucantime |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
References
- Alcântara, L.M.; Ferreira, T.C.S.; Gadelha, F.R.; Miguel, D.C. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. IJP Drugs Drug Resist. 2018, 8, 430–439. [Google Scholar] [CrossRef]
- Bahrami, F.; Harandi, A.M.; Rafati, S. Biomarkers of Cutaneous Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 222. [Google Scholar] [CrossRef]
- World Health Organization. Neglected Tropical Diseases; Leishmaniasis Control Team: Geneva, Switzerland, 2020; Available online: https://www.who.int/neglected_diseases/en/ (accessed on 20 February 2021).
- BRASIL-Ministério da Saúde. Ministério da Saúde Secretaria de Vigilância em Saúde Departamento de Vigilância das Doenças Transmissíveis Coordenação Geral de Doenças Transmissíveis. 2019. Available online: https://www.gov.br (accessed on 23 March 2021).
- Lewnard, J.A.; Jirmanus, L.; Júnior, N.N.; Machado, P.R.; Glesby, M.J.; Carvalho, E.M.; Schriefer, A.; Weinberger, D.M. Forecasting Temporal Dynamics of Cutaneous Leishmaniasis in Northeast Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3283. [Google Scholar] [CrossRef]
- Santos, D.M.; Petersen, A.L.O.A.; Celes, F.S.; Veras, P.S.T.; Oliveira, C.I. Chemotherapeutic Potential of 17-AAG against Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis. PLoS Negl. Trop. Dis. 2014, 8, e3275. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Alexander, J.; Bryson, K. T helper (h)1/Th2 and Leishmania: Paradox rather than paradigm. Immun. Lett. 2005, 99, 17–23. [Google Scholar] [CrossRef]
- Oliaee, R.T.; Sharifi, I.; Afgar, A.; Jafarzadeh, A.; Kareshk, A.T.; Bamorovat, M.; Sharifi, H.; Babaei, Z.; Keyhani, A.; Keyhani, A.; et al. Differential expression of TLRs 2, 4, 9, iNOS and TNF-α and arginase activity in peripheral blood monocytes from glucantime unresponsive and responsive patients with anthroponotic cutaneous leishmaniasis caused by Leishmania tropica. Microb. Pathog. 2019, 126, 368–378. [Google Scholar] [CrossRef]
- Govender, M.; Hurdayal, R.; Martinez-Salazar, B.; Gqada, K.; Pillay, S.; Gcanga, L.; Passelli, K.; Nieuwenhuizen, N.E.; Tacchini-Cottier, F.; Guler, R.; et al. Deletionof Interleukin-4 Receptor Alpha-Responsive Keratinocytes in BALB/c Mice Does Not Alter Susceptibility to Cutaneous Leishmaniasis. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Bekhit, A.A.; El-Agroudy, E.; Helmy, A.; Ibrahim, T.M.; Shavandi, A.; Bekhit, A.E.A. Leishmania treatment and prevention: Natural and synthesized drugs. Eur. J. Med. Chem. 2018, 160, 229–2445. [Google Scholar] [CrossRef]
- Maia, G.N. Caatinga: Árvores e Arbustos e suas Utilidades; D&Z Editora: São Paulo, Brazil, 2004. [Google Scholar]
- Pereira, E.P.L.; Souza, C.S.; Amparo, J.; Ferreira, R.S.; Nuñez-Figueredo, Y.; Fernandez, L.G.; Ribeiro, P.R.; Braga-de-Souza, S.; Silva, V.D.A.; Costa, S.L. Amburana cearensis seed extract protects brain mitochondria from oxidative stress and cerebellar cells from excitotoxicity induced by glutamate. J. Ethnopharmacol. 2017, 14, 157–166. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais no Brasil Nativas e Exóticas; Instituto Plantarum: Nova Odessa, SP, Brazil, 2002. [Google Scholar]
- Lima, S.C.G.; Teixeira, M.J.; Lopes-Júnior, J.E.G.; Morais, S.M.; Torres, A.F.; Braga, M.A.; Rodrigues, R.O.; Santiago, G.M.P.; Martins, A.C.; Nagao-Dias, A.T. In Vitro and In Vivo Leishmanicidal Activity of Astronium fraxinifolium (Schott) and Plectranthus amboinicus (Lour.) Spreng against Leishmania (Viannia) braziliensis. BioMed Res. Int. 2014, 2014, 848293. [Google Scholar] [CrossRef]
- Araujo, A.B.; Serra-Azul, F.V.C.; Silva, F.R.M.; Almeida, T.S.; Oliveira, J.V.N.; Pimenta, A.T.A.; Bezerra, A.M.E.; Machado, N.J.; Leal, L.K.A.M. Antineuroinflammatory Effect of Amburana cearensis and Its Molecules Coumarin and Amburoside A by Inhibiting the MAPK Signaling Pathway in LPS-Activated BV-2 Microglial Cells. Oxidative Med. Cell. Longev. 2022, 2022, 6304087. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.K.A.M.; Ferreira, A.A.G.; Bezerra, G.A.; Matos, F.J.A.; Viana, G.S.B. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: A comparative study. J. Ethnopharmacol. 2000, 70, 151–159. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Nechio, M.; Silveira, E.R.; Canuto, K.M.; Fontenele, R.A.; Viana, G.S.B. Anti-inflammatory and Smooth Muscle relaxant activities of the hydroalcoholic extract and chemical constituents from Amburana cearensis A. C. Smith. Phytother. Res. 2003, 17, 335–340. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Matos, M.E.; Matos, F.J.A.; Ribeiro, R.A.; Ferreira, F.V.; Viana, G.S.B. Antinociceptive and antiedematogenic effects of the hydroalcoholic extract and coumarin from Torresea cearensis Fr. All. Phytomedicine 1997, 4, 221–227. [Google Scholar] [CrossRef]
- Michaeli, D.; Molavi, A.; Mirelman, D.; Hanoch, A.; Weinstein, L. Mode of action of coumermycin A: Comparisons with novobiocin, Antimicrob. Agents Chemother. 1970, 10, 95–99. [Google Scholar]
- Higgins, N.P.; Peebles, C.L.; Sugino, A.; Cozzarelli, N.R. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc. Natl. Acad. Sci. USA 1978, 75, 1773–1777. [Google Scholar] [CrossRef]
- Mandlik, V.; Singh, S. Molecular docking and molecular dynamics simulation study of inositol phosphorylceramide synthase—Inhibitor complex in leishmaniasis: Insight into the structure based drug design. F1000Research 2016, 5, 1610. [Google Scholar] [CrossRef]
- Bravo, J.A.B.; Sauvain, M.; Gimenez, A.T.; Victoria-Munoz, V.O.; Callapa, J.; Men-Olivier, L.L.; Massiot, G.; Lavaud, C. Bioactive phenolic glycosides from Amburana cearensis. Phytochemistry 1999, 50, 71–74. [Google Scholar] [CrossRef]
- Canuto, K.M.; Silveira, E.R. Constituintes químicos da casca do caule de Amburana cearensis A.C. Smith. Química Nova 2006, 29, 1241–1243. [Google Scholar] [CrossRef]
- Araruna, S.M.; Silva, A.H.; Canuto, K.M.; Silveira, E.R.; Leal, L.K.A.M. Influence of process conditions on the physicochemical characteristics of cumaru (Amburana cearensis) powder produced by spray drying. Braz. J. Pharmacog. 2013, 23, 134–135. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysisofnitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Spillere, A.R.; Neves, G.M.D.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V.L. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef]
- Napolitano, H.B.; Silva, M.; Ellena, J.; Rodrigues, B.D.G.; Almeida, A.L.C.; Vieira, P.C.; Oliva, G.; Thiemann, O.H. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz. J. Med. Biol. Res. 2004, 37, 1847–1852. [Google Scholar] [CrossRef]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene coumarins from Ferulaszowitsiana and in vitro antileishmanicidal activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar] [CrossRef]
- Vila-Nova, N.S.; De Morais, S.M.; Falcão, M.J.; Alcantara, T.T.; Ferreira, P.A.; Cavalcanti, E.S.; Vieira, I.G.; Campello, C.C.; Wilson, M. Different susceptibilities of Leishmania spp. promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol. 2013, 133, 334–338. [Google Scholar] [CrossRef]
- De Muylder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A Screen against Leishmania Intracellular Amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Kar, N.; Chakraborty, S.; Kumar, A.; Ghosh, S.; Bera, T. Development and evaluation of a cedrol-loaded nanostructured lipid carrier system for in vitro and in vivo susceptibilities of wild and drug resistant Leishmania donovani amastigotes. Science 2017, 104, 196–211. [Google Scholar]
- Parizi, M.H.; Pardakhty, A.; Sharifi, I.; Farajzadeh, S.; Daie-Parizi, M.H.; Sharifi, H.; Keyhani, A.R.; Mostafavi, M.; Bamorovat, M.; Ghaffari, D. Antileishmanial activity and immune modulatory effects of benzoxonium chloride and its entrapped forms in niosome on Leishmania tropica. J. Parasit. Dis. 2019, 43, 406–415. [Google Scholar]
- Guan, X.L.; Maser, P. Comparative sphingolipidomics of disease-causing trypanosomatids reveal unique lifecycle- and taxonomy-specific lipid chemistries. Sci. Rep. 2017, 7, 13617. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host. Pathog. Interface Nat. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Sarkar, D.; De Sarkar, S.; Gille, L.; Chatterjee, M. Ascaridole exerts the leishmanicidal activity by inhibiting parasite glycolysis. Phytomedicine 2022, 103, 154221. [Google Scholar] [CrossRef]
- Costa, A.B.G.; Vieira, T.S.S.; Silva, R.P.; Mesquita, A.L.F.; Fernandes, J.R.M.; Saraiva, E.M. 3′nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect. Immun. 2014, 82, 1732–1740. [Google Scholar] [CrossRef]
- Duarte, M.C.; Tavares, G.S.; Valadares, D.G.; Lage, D.P.; Ribeiro, T.G.; Lage, L.M.; Rodrigues, M.R.; Faraco, A.A.; Soto, M.; Da Silva, E.S.; et al. Antileishmanial activity and mechanism of action from a purified fraction of Zingiber officinalis Roscoe against Leishmania amazonensis. Exp. Parasitol. 2016, 166, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.E.; Sobrinho-Junior, E.P.; Brito, L.M.; Nicolau, L.A.; Carvalho, T.P.; Moura, A.K.; Rodrigues, K.A.; Carneiro, S.M.; Arcanjo, D.D.; Citó, A.M.; et al. Anti-Leishmania activity of essential oil of Myracrodruon urundeuva (Engl.) Fr. All.: Composition, cytotoxity and possible mechanisms of action. Exp. Parasitol. 2017, 175, 59–67. [Google Scholar] [CrossRef]
- Brenzan, M.A.; Ferreira, I.C.P.; Lonardoni, M.V.C.; Honda, P.A.; Rodrigues-Filho, E.; Nakamura, C.V.; Dias-Filho, B.P.; Ueda-Nakamura, T.; Cortez, D.A.G. Activity of Extracts and Coumarins from the Leaves of Calophyllum brasiliense. on Leishmania braziliensis. J. Pharm. Biol. 2008, 46, 380–386. [Google Scholar] [CrossRef]
- Kermani, E.K.; Sajjadi, S.E.; Hejazi, S.H.; Arjmand, R.; Saberi, S.; Eskandarian, A.A. Anti-Leishmania Activity of Osthole. Pharmacogn. Res. 2016, 85, 1–4. [Google Scholar] [CrossRef]
- Mandlik, V.; Patil, S.; Bopanna, R.; Basu, S.; Singh, S. Biological Activity of Coumarin Derivatives as Anti-Leishmanial Agents. PLoS ONE 2016, 11, e0164585. [Google Scholar] [CrossRef]
- Saha, P.; Mukhopadhyay, D.; Chatterjee, M. Immunomodulation by chemotherapeutic agents against Leishmaniasis. Int. Immunopharmacol. 2011, 11, 1668–1679. [Google Scholar] [CrossRef]
- Gupta, G.; Majumdar, S.; Adhikari, A.; Bhattacharya, P.; Mukherjee, A.K.; Majumdar, S.B.; Majumdar, S. Treatment with IP-10 induces host-protective immune response by regulating the T regulatory cell functioning in Leishmania donovani-infected mice. Med. Microbiol. Immunol. 2011, 200, 241–253. [Google Scholar] [CrossRef]
- Grimaldi-Junior, G.; Porrozzi, R.; Friedrich, K.; Teva, A.; Marchevsky, R.S.; Vieira, F.; Miekeley, N.; Paumgartten, F.J.R. Comparative Efficacies of Two Antimony Regimens To Treat Leishmania braziliensis-Induced Cutaneous Leishmaniasis in Rhesus Macaques (Macaca mulatta). Antimicrob. Agents Chemother. 2010, 54, 502–505. [Google Scholar] [CrossRef]
- Kalantari, H.; Hemmati, A.; Bavarsad, N.; Rezaie, A.; Ahmadi, S. Effect of topical Nanoliposomes of Paromomycin on Rats Liver and Kidney. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e17565. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Camada, I.; Leopoldo, P.T.G.; Pereira, J.M.B.; Riley, L.W.; Wilson, M.E.; Ho, J.L.; De Jesus, A.R.; Carvalho, E.M.; Almeida, R.P. Resistance of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis tonitric oxide correlates with disease severity in Tegumentary Leishmaniasis. BMC Infect. Dis. 2007, 7, 7. [Google Scholar] [CrossRef]
- Cronemberger-Andrade, A.; Aragão-França, L.; Araujo, C.F.; Rocha, V.J.; Borges-Silva, M.C.; Figueiras, C.P.; Oliveira, P.R.; Freitas, L.A.R.; Veras, P.S.T.; Pontes-de-Carvalho, L. Extracellular Vesicles from Leishmania-Infected Macrophages Confer an Anti-infection Cytokine-Production Profile to Naïve Macrophages. PLoS Negl. Trop. Dis. 2014, 8, e3161. [Google Scholar] [CrossRef]
- Vasquez, R.E.; Xin, L.; Soong, L. Effects of CXCL10 on Dendritic Cell and CD4+ T-Cell Functions during Leishmania amazonensis. Infect. Immun. 2008, 76, 161. [Google Scholar] [CrossRef]
- Naderer, T.; Mcconville, M.J. The Leishmania macrophage interaction: A metabolic perspective. Cell. Microbiol. 2008, 10, 301–308. [Google Scholar] [CrossRef]
- Costa, D.; Carregaro, V.; Lima-Júnior, D.S.; Silva, N.M.; Milanezi, C.M.; Cardoso, C.R.; Giudice, A.; Jesus, A.R.; Carvalho, E.M.; Almeida, R.P.; et al. BALB/c Mice Infected with Antimony Treatment Refractory Isolate of Leishmania braziliensis Present Severe Lesions due to IL-4 Production. PLoS Negl. Trop. Dis. 2011, 5, e965. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infec. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef]
- Salhi, A.; Rodrigues, V.; Santoro, F.; Dessein, H.; Romano, A.; Castellano, L.R.; Sertorio, M.; Rafati, S.; Chevillard, C.; Prata, A.; et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J. Immunol. 2008, 180, 6138–6148. [Google Scholar] [CrossRef]
- Díaz, N.L.; Zerpa, O.; Tapia, F.J. Chemokines and chemokine receptors expression in the lesions of patients with American cutaneous leishmaniasis. Mem. Instit. Oswaldo Cruz. 2013, 108, 446–452. [Google Scholar] [CrossRef]
- Van Assche, T.; Deschacht, M.; Da Luz, R.A.I.; Maes, L.; Cos, P. Leishmania-macrophage interactions: Insights in to the redox biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Castro Rodrigues, N.L.; Silveira, E.S.; Marciano Fonseca, F.R.; Abreu, T.M.; Silveira, E.R.; de Araújo, A.B.; Teixeira, M.J.; Almeida Moreira Leal, L.K. Amburana cearensis (Cumaru) and Its Active Principles as Source of Anti-Leishmania Drugs: Immunomodulatory Activity of Coumarin (1,2-Benzopyrone). Biomedicines 2025, 13, 979. https://doi.org/10.3390/biomedicines13040979
de Castro Rodrigues NL, Silveira ES, Marciano Fonseca FR, Abreu TM, Silveira ER, de Araújo AB, Teixeira MJ, Almeida Moreira Leal LK. Amburana cearensis (Cumaru) and Its Active Principles as Source of Anti-Leishmania Drugs: Immunomodulatory Activity of Coumarin (1,2-Benzopyrone). Biomedicines. 2025; 13(4):979. https://doi.org/10.3390/biomedicines13040979
Chicago/Turabian Stylede Castro Rodrigues, Naya Lúcia, Elizama Shirley Silveira, Francisco Rafael Marciano Fonseca, Ticiana Monteiro Abreu, Edilberto Rocha Silveira, Ana Bruna de Araújo, Maria Jania Teixeira, and Luzia Kalyne Almeida Moreira Leal. 2025. "Amburana cearensis (Cumaru) and Its Active Principles as Source of Anti-Leishmania Drugs: Immunomodulatory Activity of Coumarin (1,2-Benzopyrone)" Biomedicines 13, no. 4: 979. https://doi.org/10.3390/biomedicines13040979
APA Stylede Castro Rodrigues, N. L., Silveira, E. S., Marciano Fonseca, F. R., Abreu, T. M., Silveira, E. R., de Araújo, A. B., Teixeira, M. J., & Almeida Moreira Leal, L. K. (2025). Amburana cearensis (Cumaru) and Its Active Principles as Source of Anti-Leishmania Drugs: Immunomodulatory Activity of Coumarin (1,2-Benzopyrone). Biomedicines, 13(4), 979. https://doi.org/10.3390/biomedicines13040979





