Abstract
Background: Coronavirus Disease 2019 has been associated with dysfunction in multiple endocrine organs, including the thyroid gland. While evidence suggests SARS-CoV-2 may influence thyroid function and promote oncogenesis through inflammation and cytokine storms, its role in thyroid cancer remains unclear. This study investigates whether COVID-19 is associated with an increased risk of thyroid cancer development. Methods: We conducted a retrospective cohort study using the TriNetX global federated health research database, encompassing data from 151 healthcare organizations. Adult patients with confirmed COVID-19 between 1 December 2019 and 31 December 2023, were included and compared to a matched cohort without COVID-19. Patients with prior thyroid cancer history or who had received COVID-19 vaccination were excluded in both groups. Propensity score matching (1:1) was performed for age, gender, and overweight/obesity status. The primary outcome was that new-onset thyroid cancer was diagnosed at least one year after COVID-19 diagnosis. Hazard ratios were calculated using Cox proportional hazards models, and subgroup analyses were performed based on age, gender, thyroid function status and treatment modalities. Results: After matching, a significantly higher thyroid cancer incidence was observed between the post-COVID and non-COVID groups. Subgroup analysis revealed a significantly higher risk of thyroid cancer development following COVID-19 diagnosis in patients who developed hyperthyroidism (HR 2.14, 95% CI: 1.04–4.46) or hypothyroid-ism (HR 1.83, 95% CI: 1.12–2.97) compared with the non-COVID population. Male patients also exhibited a higher risk of thyroid cancer after COVID-19 (HR 1.22, 95% CI 1.02–1.46). For patients with hyperthyroidism or hypothyroidism, those who had prior COVID-19 exhibited a relatively higher risk of developing thyroid cancer than those without a history of COVID-19 (HR 4.387, 95% CI: 2.08–9.24 for hyperthyroidism; HR 2.58, 95% CI: 1.58–4.22 for hypothyroidism). Conclusions: Patients with COVID-19 exhibited an increase in thyroid cancer risk, with specific subgroups—male adults and those with post-infectious thyroid dysfunction—also exhibiting increased risk. These findings suggest a potential relationship between SARS-CoV-2 and thyroid oncogenesis, warranting further prospective research.
1. Introduction
Coronavirus Disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, has had a profound impact on global health and economies [1]. As of April 2025, over 770 million confirmed infections and 7 million deaths have been reported worldwide [2]. COVID-19 presents with a wide spectrum of clinical manifestations, ranging from asymptomatic cases to severe respiratory failure and multi-organ dysfunction [3]. In addition to the direct effect of SARS-CoV-2 viral invasion, indirect effects—such as abnormal inflammatory responses and cytokine storms—can also impair various endocrine organs, including the pancreas, adrenal glands, pituitary glands, and thyroid gland [4].
Thyroid cancer is the most prevalent endocrine malignancy [5], and its incidence has been rising rapidly worldwide in recent years [6]. It is classified into differentiated thyroid cancer (including papillary and follicular thyroid carcinoma), medullary thyroid cancer, and anaplastic thyroid cancer [7]. Among these, papillary thyroid carcinoma is the most common type, accounting for approximately 90% of cases [8,9]. Established risk factors for thyroid cancer include childhood exposure to ionizing radiation and obesity. Other potential risk factors include endocrine-disrupting chemicals and thyroid dysfunction [6]. The incidence of thyroid cancer is higher in women than in men [9]. In the United States, thyroid cancer ranks as the 13th most commonly diagnosed cancer overall and the 6th most common among women [10]. Prognosis is generally favorable; the 5-year survival rate exceeds 90% for localized or regionally metastatic disease but declines to approximately 54.9% in cases with distant metastases [10].
The thyroid gland produces thyroid hormones, including the prohormone tetraiodothyronine (T4) and the active hormone triiodothyronine (T3). T3 regulates basal metabolic rate by influencing the central nervous system, respiratory system, cardiovascular system, and skeletal muscle [11]. SARS-CoV-2 infection can disrupt thyroid function, potentially leading to conditions such as thyrotoxicosis, hypothyroidism, and the nonthyroidal illness syndrome [12].
SARS-CoV-2 RNA has been detected in thyroid tissue [13], suggesting direct viral invasion. SARS-CoV-2 enters target cells through angiotensin-converting enzyme II (ACE2) receptors, which are highly expressed in endocrine tissues, including the thyroid and pituitary glands [14,15]. The binding of SARS-CoV-2 to ACE2 receptors downregulates the angiotensin I receptor, leading to dysregulation of the renin–angiotensin–aldosterone system. This, in turn, results in inflammation, fibrosis, vasoconstriction, oxidation, and increased capillary permeability [16,17,18,19], all of which may contribute to cancer development. Additionally, SARS-CoV-2 infection induces profound inflammation and cytokine storms [20], which could promote cancer stem cell growth in target organs and are associated with the development of thyroid cancer [21,22]. The inflammation and oxidative stress induced by SARS-CoV-2 may also play a role in the development and progression of thyroid cancer [23]. Infection with SARS-CoV-2 may also induce the expression of nonstructural protein (nsp) 15, which affects the function of the tumor suppressor retinoblastoma protein [24,25], and nsp 3, which degrades the tumor suppressor gene P53 [26,27]. This pathway is considered one of the most likely mechanisms by which SARS-CoV-2 could promote cancer development [28].
However, the literature on the relationship between COVID-19 and thyroid cancer development remains limited, and no consensus has been established. To address this gap, we conducted a retrospective cohort study to investigate whether COVID-19 contributes to the development of thyroid cancer.
2. Materials and Methods
2.1. Setting
In this retrospective cohort study, we utilized data from the Global Collaborative Network within the TriNetX database, which includes information from 151 healthcare organizations (HCOs). TriNetX is a global federated health research network that provides access to electronic medical records, encompassing diagnoses, procedures, medications, laboratory results, and genomic data from large HCOs.
The TriNetX platform complies with the Health Insurance Portability & Accountability Act and the General Data Protection Regulation. The Western Institutional Review Board granted TriNetX a waiver of informed consent, as the platform only aggregates counts and statistical summaries of deidentified information.
2.2. Cohort
We included adult patients (aged ≥18 years) with a positive SARS-CoV-2 PCR test (Table 1) or a COVID-19 diagnosis (ICD-10 codes: U07.1, U07.2, or J12.82) recorded in the TriNetX database during the study period. Patients with a prior history of thyroid cancer (ICD-10 codes: C73 or Z85.850) or those who had received any COVID-19 vaccine (Table 2) were excluded. For the subgroup analysis on abnormal thyroid function, we also excluded patients with a history of hyperthyroidism (ICD-10 codes: E05, E05.8, E05.3, E05.31, and E06) or hypothyroidism (ICD10 codes: E03.3 and E03.8). The index date was defined as the date of the COVID-19 diagnosis.

Table 1.
Detailed criteria used to define a positive SARS-CoV-2 PCR test.

Table 2.
Detailed criteria for defining SARS-CoV-2 vaccination status.
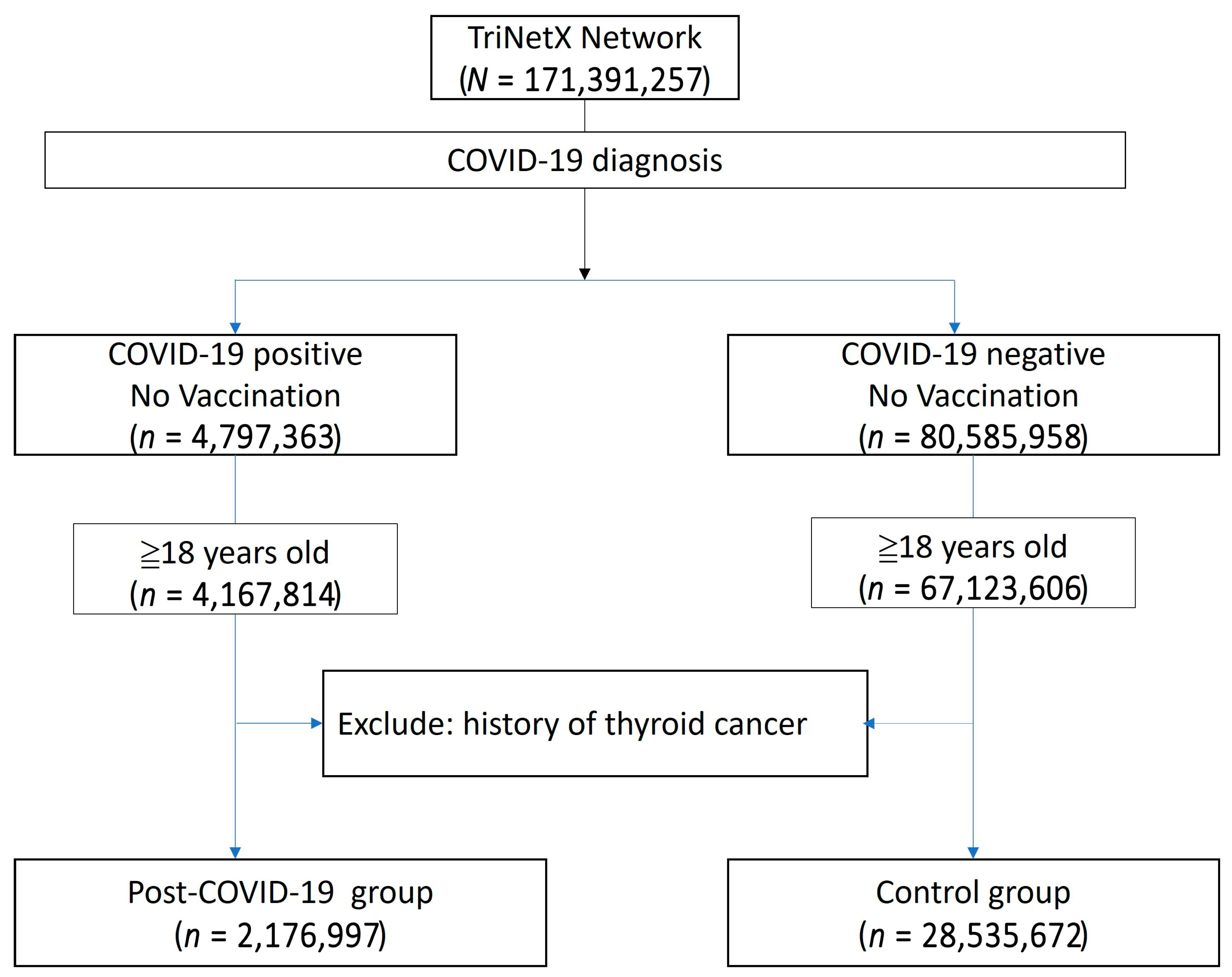
The study population was compared to a control group comprising individuals without a COVID-19 diagnosis, no history of COVID-19 vaccination, and no prior history of thyroid cancer. The primary outcome was a new diagnosis of thyroid cancer occurring at least one year after the COVID-19 diagnosis. A flowchart illustrating the cohort construction, including participants enrolled between 1 December 2019 and 31 December 2023, is presented in Figure 1. Detailed query criteria are provided in Supplementary File S1.
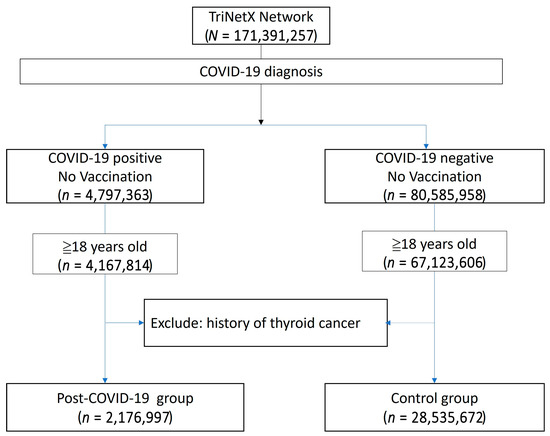
Figure 1.
Flowchart illustrating the construction of the study cohort from the TriNetX database.
A subgroup analysis was performed to compare the risk of thyroid cancer development between patients with and without COVID-19 across different genders and age groups (18–40 years, 41–60 years, and over 60 years). We also assessed the risk among patients who received various antiviral therapies, as well as those who developed hyperthyroidism or hypothyroidism following COVID-19. Additional comparisons were made between COVID-19 patients aged 60 years and older and those aged 18 to 60 years, between those aged 40 years and older and those aged 18 to 40 years; between those who received antiviral therapy and those who did not, and between individuals with post-infectious thyroid dysfunction (hyperthyroidism or hypothyroidism) and those without any thyroid function alterations following infection. Finally, we compared COVID-19 patients who developed thyroid dysfunction with patients who had thyroid dysfunction in the absence of COVID-19. Detailed query criteria are provided in Supplementary Files S2 and S3.
2.3. Statistical Analysis
We performed 1:1 propensity score matching based on age at index, gender and overweight/obesity status, with the non-COVID cohort serving as the control group. In subsequent analyses, we additionally included personal history of irradiation and comorbidities (hypertension, ischemic heart disease, and diabetes mellitus) as matching covariates. Propensity score matching is an integrated function within the TriNetX platform. We selected the covariates to be included in the matching process, and the system conducted propensity score matching to compare outcomes between the cohorts.
The outcomes were compared using built-in functions within the TriNetX platform. We calculated the hazard ratio (HR) for thyroid cancer development in both groups. The proportional hazards assumption was tested using the generalized Schoenfeld approach, available in TriNetX. Survival probabilities were estimated using the Kaplan–Meier method. Statistical significance was defined as a p-value < 0.05, and a 95% confidence interval (CI) was used as additional evidence of statistical significance.
3. Results
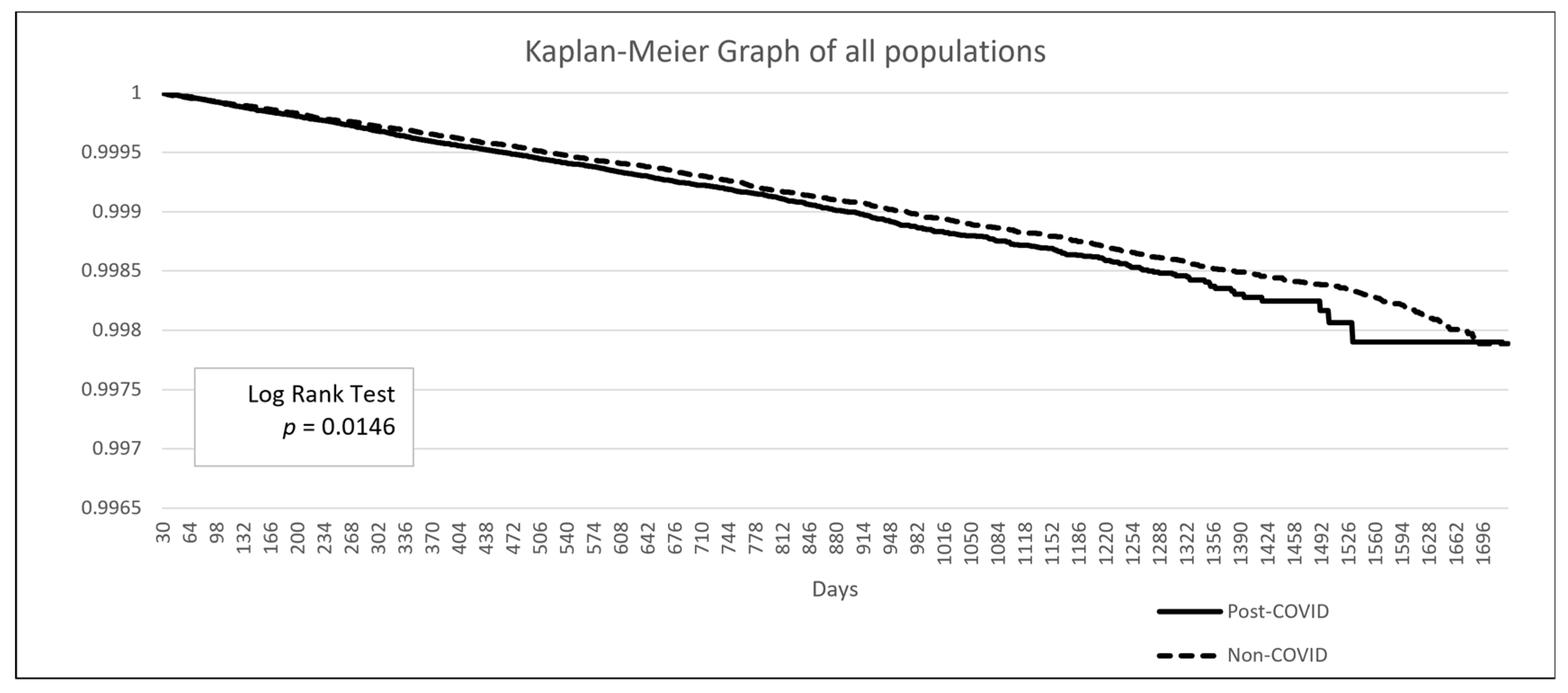
The baseline demographic data is presented in Table 3. The post-COVID group included 2,176,997 patients, while the non-COVID group comprised 28,535,672 patients. Due to the limitations of the TriNetX built-in function, propensity score matching in such large populations can only be performed on age, genders, and overweight/obesity status. After matching, the mean age was 48.8 years, and 57.9% of the population was female. A higher proportion of patients in the post-COVID group had comorbidities such as hypertensive diseases, ischemic heart disease, or diabetes mellitus. Additionally, a greater proportion of patients in the post-COVID group had a personal history of irradiation, although this represented less than 1% of the population (0.9% vs. 0.5%). The Kaplan–Meier analysis revealed a significant difference in the incidence of thyroid cancer following a COVID-19 diagnosis (HR: 1.104, 95% CI: 1.02–1.20, p = 0.0146) (Figure 2).

Table 3.
Characteristics of the post-COVID group (Cohort 1) and non-COVID group (Cohort 2) before and after propensity score matching.
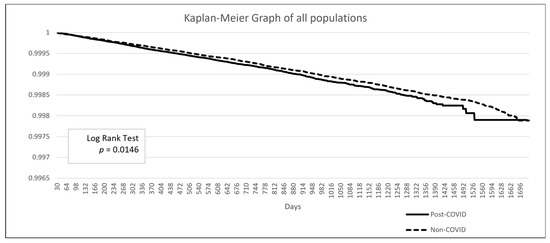
Figure 2.
Kaplan–Meier survival curve comparing the risk of thyroid cancer between the post-COVID and non-COVID groups.
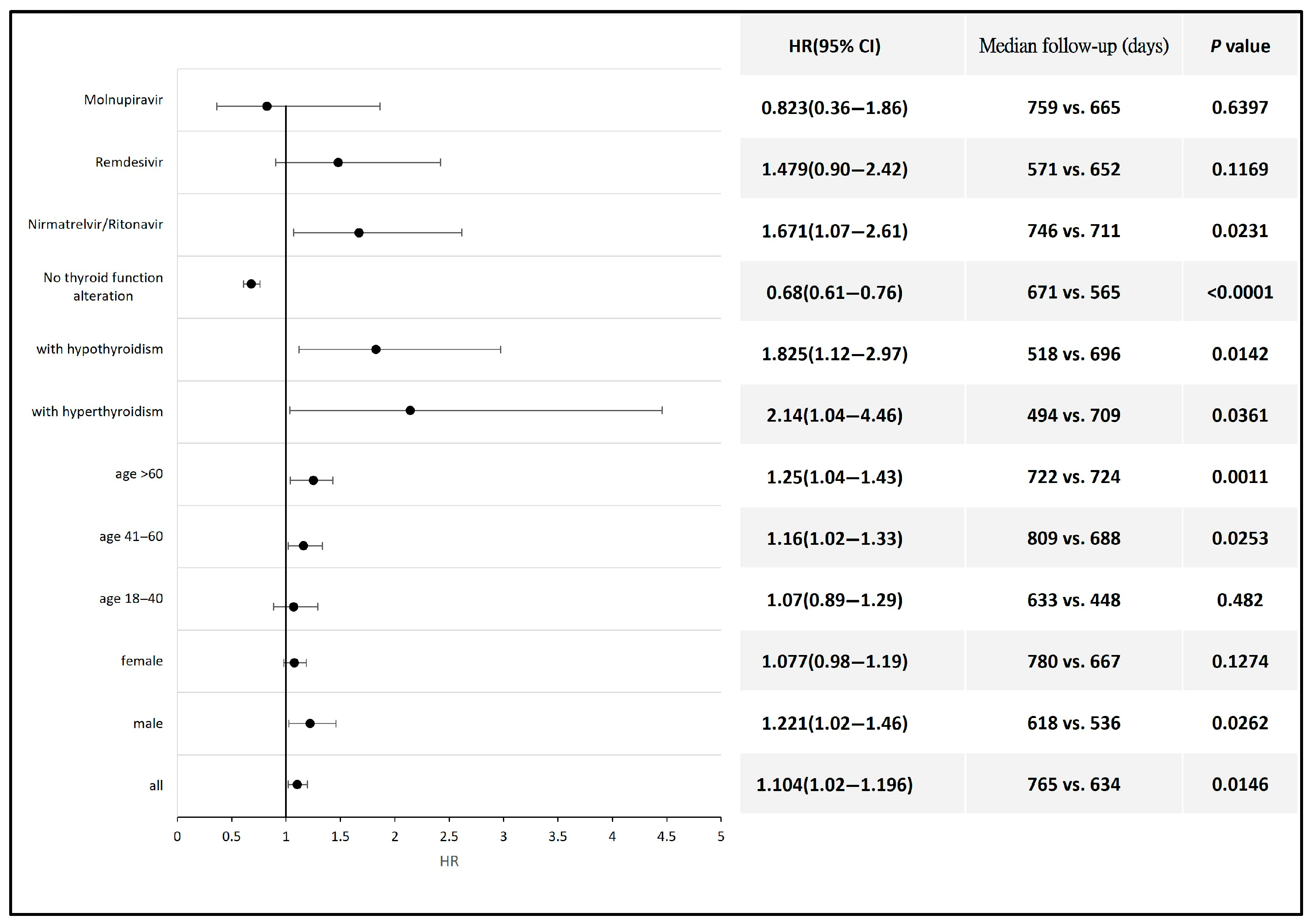
The subgroup analysis revealed a significantly higher risk of thyroid cancer development following COVID-19 diagnosis in patients aged 60 years and older (HR 1.25, 95% CI: 1.04–1.43) and those aged between 41 and 60 years (HR 1.16, 95% CI: 1.02–1.33) compared with the non-COVID population within the same age range. Male patients also exhibited a higher risk of thyroid cancer after COVID-19 (HR 1.22, 95% CI 1.02–1.46). Additionally, the risk of thyroid cancer was also elevated in patients who developed hyperthyroidism (HR 2.14, 95% CI: 1.04–4.46) or hypothyroidism (HR 1.83, 95% CI: 1.12–2.97) compared with the non-COVID population. Antiviral therapy with Nirmatrelvir/Ritonavir was also associated with an increased risk of thyroid cancer (HR 1.67, 95% CI 1.07–2.61) compared with the non-COVID population. Antiviral agents such as Remdesivir and Molnupiravir had no significant impact on the incidence of thyroid cancer (Figure 3). Detailed data and a Kaplan–Meier graph are provided in Supplementary File S4.
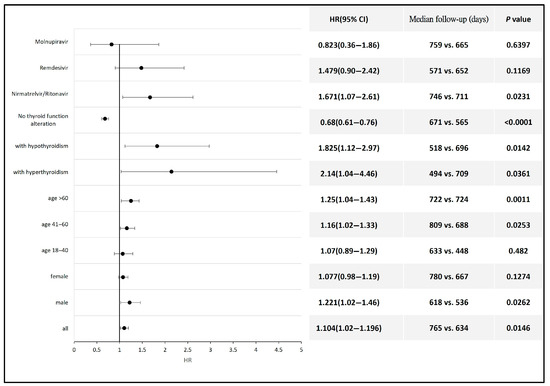
Figure 3.
Forest plot showing the risk of thyroid cancer across different subgroups of COVID-19 patients compared to non-COVID-19 individuals or to those of the corresponding gender or age group.
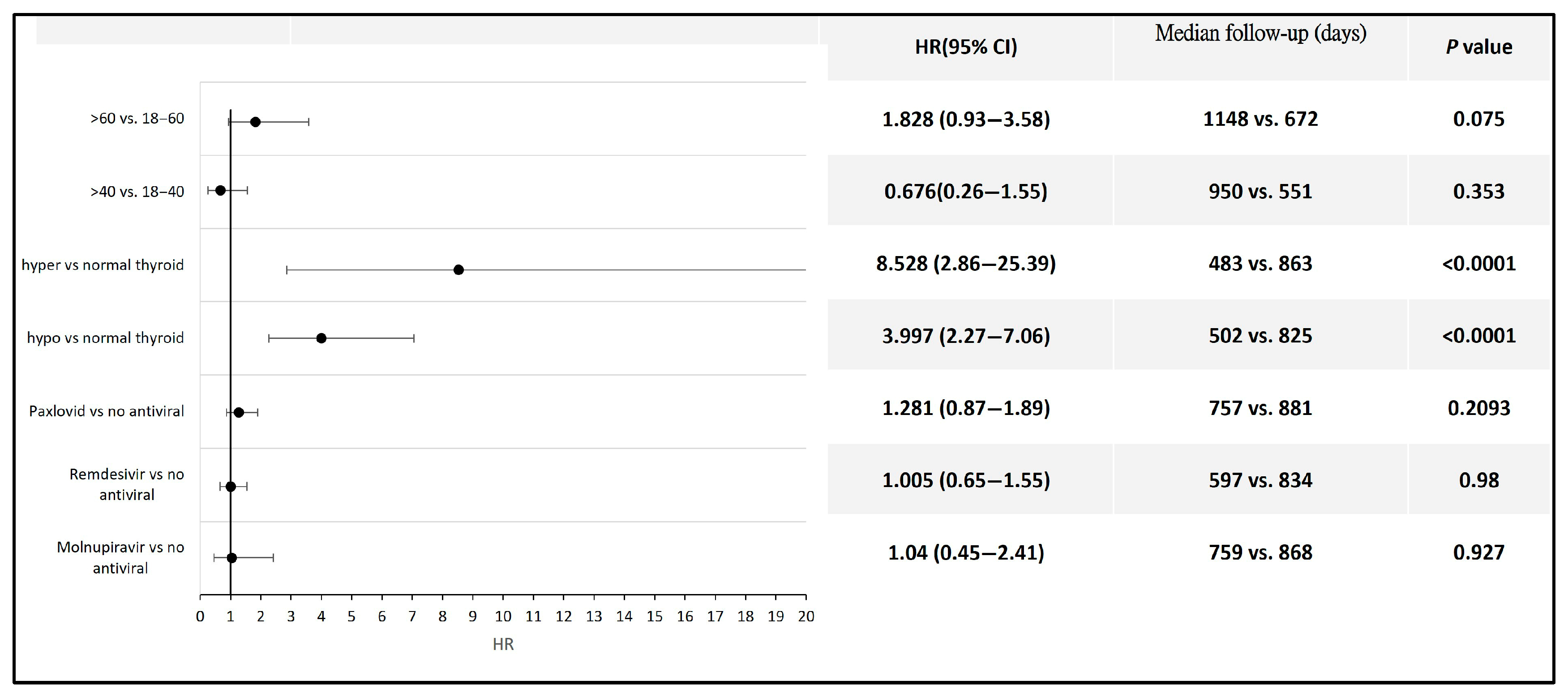
Among the patients with COVID-19, those who developed hyperthyroidism or hypothyroidism had a significantly higher risk of developing thyroid cancer compared to those without thyroid function alterations following infection (HR 8.53, 95% CI: 2.86–25.39 for hyperthyroidism; HR 3.99, 95% CI: 2.27–7.06 for hypothyroidism). No statistically significant difference in thyroid cancer risk was observed between COVID-19 patients aged 60 years and older and those aged 18 to 60 years, between those aged 40 years and older and those aged 18 to 40 years, or between patients who received antiviral therapy and those who did not (Figure 4). Detailed data and Kaplan–Meier curves are provided in Supplementary File S5.
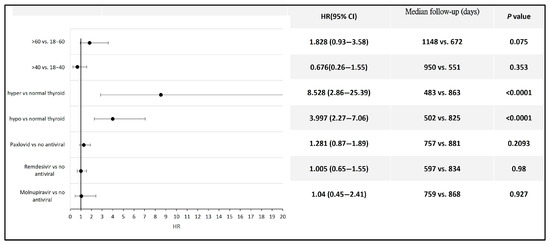
Figure 4.
Forest plot illustrating thyroid cancer risk across different subpopulations following COVID-19.
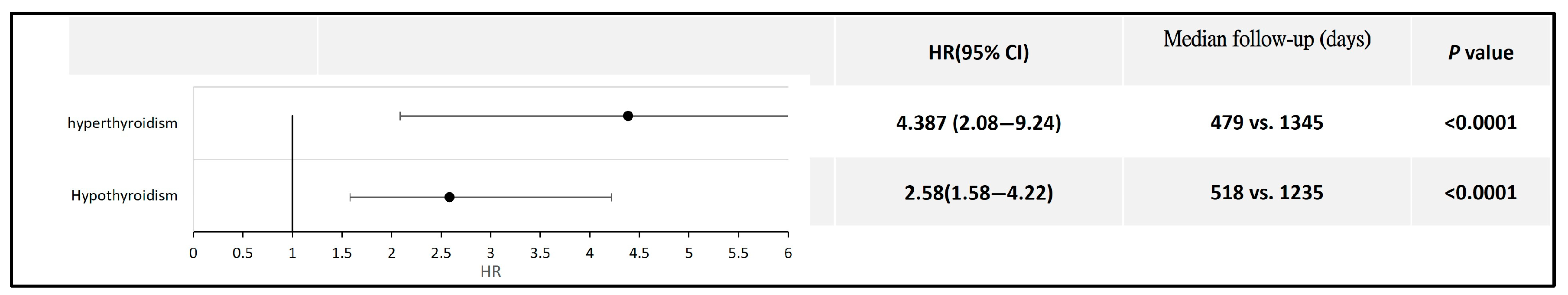
Furthermore, for patients with hyperthyroidism or hypothyroidism, those who had prior COVID-19 exhibited a relatively higher risk of developing thyroid cancer than those without a history of COVID-19 (HR 4.387, 95% CI: 2.08–9.24 for hyperthyroidism; HR 2.58, 95% CI: 1.58–4.22 for hypothyroidism) (Figure 5). Detailed data and Kaplan–Meier curves are provided in Supplementary File S6.
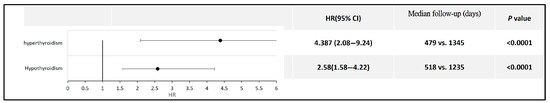
Figure 5.
Forest plot comparing thyroid cancer risk in patients with thyroid dysfunction, with and without a history of COVID-19.
4. Discussion
This retrospective study using the TriNetX database demonstrated a direct association between COVID-19 and the overall development of thyroid cancer. Subgroup analysis revealed an increased risk of thyroid cancer in patients who developed hyperthyroidism or hypothyroidism following COVID-19, as well as in male patients. Individuals aged 60 years and older or those aged between 41 and 60 years, as well as those who received Nirmatrelvir/Ritonavir therapy, also exhibited a higher risk. However, among COVID-19 patients, only those who developed hyperthyroidism or hypothyroidism—compared with those without thyroid dysfunction—showed statistically significant results. Neither age (over 60 vs. 18–60 years or over 41 vs. 18–40 years) nor antiviral therapy (Nirmatrelvir/Ritonavir, Remdesivir, or Molnupiravir vs. no antiviral therapy) had a significant impact on thyroid cancer development.
There is limited evidence regarding the impact of COVID-19 on thyroid cancer development [29]. Xu et al. applied the Mendelian randomization (MR) method to analyze genome-wide association study data on COVID-19 susceptibility and severity in the European population. Their study suggested that hospitalization due to COVID-19 is associated with an increased risk of thyroid cancer development. However, MR results derived from genetic data did not show a significant association between COVID-19 susceptibility (risk of infection) or severity and thyroid cancer [30]. Hassan et al. conducted a retrospective study at an endocrine surgery center in the United Arab Emirates and found not only an increase in thyroid cancer cases undergoing surgery, but also more aggressive pathological features in the post-pandemic era (between January 2021 and December 2022) [31]. Our results demonstrated a direct association between COVID-19 diagnosis and the development of thyroid cancer. However, due to the limitations of the TriNetX database, we were unable to analyze the association between COVID-19 hospitalization and thyroid cancer development.
Given the indolent nature of thyroid cancer, the increased number of diagnoses observed after COVID-19 infection may partially reflect a diagnostic shift rather than a true rise in incidence. Several studies have reported delays in thyroid cancer screening and diagnosis during the pandemic, likely due to reduced healthcare access and concerns about aerosol-generating procedures such as fine-needle aspiration [32,33]. Bell et al. conducted a longitudinal analysis showing a decline in thyroid cancer diagnoses between March 2020 and December 2021, followed by a rebound exceeding pre-pandemic levels [34]. The authors attributed this to delayed diagnoses during the pandemic. Similarly, Virnceanu et al. noted not only a post-pandemic rise in incidence but also an increase in the presentation of giant thyroid cancers [35]. While such delays likely occurred equally across both COVID-19 and non-COVID-19 populations, this would only affect the results if patients with preexisting thyroid cancer had increased susceptibility to COVID-19. To address this issue, we note that to date, there is no evidence indicating increased susceptibility to COVID-19 among patients with thyroid cancer [29]. For example, a single-center study by Prete et al. reported no elevated prevalence of COVID-19 in patients with untreated thyroid cancer compared to the general population [36]. Thus, the likelihood of pre-existing, undiagnosed thyroid cancer should be similar between COVID-19 and non-COVID-19 groups.
Hyperthyroidism was previously thought to be associated with a decreased risk of thyroid cancer [37]. However, a systematic review and meta-analysis published in 2020 found that both hyperthyroidism and hypothyroidism are associated with an increased risk of thyroid cancer [38]. The potential for diagnostic bias may exist in those with pre-existing thyroid dysfunction, particularly due to increased medical attention and surveillance, such as thyroid ultrasonography. In our study, among COVID-19 patients, those who developed hyperthyroidism or hypothyroidism had a higher risk of thyroid cancer compared to those without thyroid dysfunction. To determine whether thyroid dysfunction or COVID-19 was primarily responsible for this increased risk, we specifically compared patients with thyroid dysfunction who had a prior COVID-19 infection to those with thyroid dysfunction who did not. Despite similar underlying thyroid conditions and likely comparable exposure to thyroid imaging, we observed a higher incidence of thyroid cancer in the post-COVID group. These findings suggest that the observed difference may not be solely attributed to increased ultrasound utilization associated with thyroid dysfunction. Instead, both thyroid dysfunction and COVID-19 itself may independently or synergistically contribute to an increased risk of thyroid cancer.
COVID-19 patients, especially those who were hospitalized, may have undergone more medical examinations, such as chest computed tomography scans to assess the severity of infection. These imaging studies could incidentally detect thyroid nodules, potentially leading to a higher likelihood of subsequent cancer diagnosis. This may partly explain our findings.
Many antiviral agents used to treat COVID-19 are known to reduce disease severity and shorten the duration of recovery [39,40,41]. Remdesivir, Nirmatrelvir/Ritonavir, and Molnupiravir have been shown to be effective in treating COVID-19 by inhibiting SARS-CoV-2 replication or viral RNA synthesis [42,43,44,45]. Additionally, Remdesivir has been reported to increase the ratio of T helper 2- to T helper 1-associated cytokines [39], while Nirmatrelvir/Ritonavir has been shown to reduce pro-inflammatory cytokine levels in COVID-19 patients [41,46]. In our study, compared to the non-COVID population, COVID-19 patients who received Nirmatrelvir/Ritonavir therapy exhibited an increased risk of thyroid cancer. However, when compared with COVID-19 patients who did not receive antiviral therapy, there was no significant difference in thyroid cancer risk after treatment with either antiviral agent. Therefore, none of these three antiviral agents appeared to reduce the incidence of thyroid cancer in our study.
Both mRNA and adenovirus-vectored COVID-19 vaccines have been reported to cause thyroiditis [47]. Thyroiditis can lead to alterations in thyroid function, potentially introducing bias into our analysis. Therefore, individuals who received any form of COVID-19 vaccination were excluded from this study.
Our study has several limitations. As noted by Ludwig et al. [48], studies utilizing electronic health records are subject to inherent biases. Retrospective cohort studies based on electronic data may suffer from incomplete access to raw clinical details, including hospitalization status, COVID-19 severity, patients’ vital signs, treatment regimens, thyroid cancer histological subtypes, and other important variables. Furthermore, due to limitations of the TriNetX platform, we were unable to perform full propensity score matching beyond age, gender, and obesity, which may affect the robustness of our comparisons. Differences in follow-up duration across subgroups may also lead to either an overestimation or an underestimation of thyroid cancer incidence. Additional sources of bias may arise from miscoding, inaccurate coding, or missing data related to comorbidities, socioeconomic status, gender and lifestyle factors, and family history of thyroid cancer. Finally, the observed increase in thyroid cancer incidence in certain subgroups may reflect delayed diagnoses or underdiagnosis during the pre-pandemic period, rather than a true increase in risk. The relatively small sample size, multiple comparisons, and absence of multivariable analysis in the subgroup analyses may also introduce bias.
5. Conclusions
This study identified an association between COVID-19 and the subsequent development of thyroid cancer across a large, diverse population. Subgroup analyses further revealed a significantly increased risk in male patients and in those who developed hyperthyroidism or hypothyroidism following COVID-19, suggesting that male sex and post-infectious thyroid dysfunction may amplify susceptibility to thyroid oncogenesis in the context of SARS-CoV-2 infection. However, limitations such as the retrospective design, incomplete clinical information, and constraints in propensity score matching warrant cautious interpretation of the findings. Prospective studies are needed to further clarify the relationship between COVID-19 and thyroid cancer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13081933/s1. The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors. Supplementary File S1: Detailed query criteria of post-COVID and non-COVID group. Query Criteria for Cohort post-COVID; Query Criteria for Cohort non-COVID. Supplementary File S2: Detailed query criteria of subgroups of population in post-COVID and non-COVID groups. Query Criteria for Cohort post-COVID, male; Query Criteria for Cohort non-COVID, male; Query Criteria for Cohort post-COVID, female; Query Criteria for Cohort non-COVID, female; Query Criteria for Cohort post-COVID, >60; Query Criteria for Cohort non-COVID, >60; Query Criteria for Cohort post-COVID, 41–60; Query Criteria for Cohort non-COVID, 41–60; Query Criteria for Cohort post-COVID, 18–40; Query Criteria for Cohort non-COVID, 18–40; Query Criteria for Cohort post-COVID, with hyperthyroidism; Query Criteria for Cohort post-COVID, with hypothyroidism; Query Criteria for Cohort post-COVID, s/p Nirmatrelvir/Ritonavir; Query Criteria for Cohort post-COVID, s/p Molnupiravir; Query Criteria for Cohort post-COVID, s/p Remdesivir. Supplementary File S3. Detailed query criteria of subgroup of population. Query Criteria for Cohort post-COVID, >41; Query Criteria for Cohort post-COVID, 18–40; Query Criteria for Cohort post-COVID, >60; Query Criteria for Cohort post-COVID, 18-60; Query Criteria for Cohort post-COVID, normal thyroid function; Query Criteria for Cohort post-COVID, with hypothyroidism; Query Criteria for Cohort post-COVID, with hyperthyroidism; Query Criteria for Cohort non-COVID, with hypothyroidism; Query Criteria for Cohort non-COVID, with hyperthyroidism. Supplementary File S4. Kaplan-Meier graph of thyroid cancer risk in different subgroups of COVID-19 patients. Supplementary Figure S4.1. Kaplan—Meier survival curve of thyroid cancer risk in male populations. Supplementary Figure S4.2. Kaplan—Meier survival curve of thyroid cancer risk in female populations. Supplementary Figure S4.3. Kaplan—Meier survival curve of thyroid cancer risk in populations aged between 18 and 40 years. Supplementary Figure S4.4. Kaplan—Meier survival curve of thyroid cancer risk in populations aged between 41 and 60 years. Supplementary Figure S4.5. Kaplan—Meier survival curve of thyroid cancer risk in populations aged over 60 years. Supplementary Figure S4.6. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who developed hyperthyroidism vs non-COVID populations. Supplementary Figure S4.7. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who developed hypothyroidism vs non-COVID populations. Supplementary Figure S4.8. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who had no thyroid function alteration vs non-COVID populations. Supplementary Figure S4.9. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Nirmatrelvir/Ritonavir treatment vs non-COVID populations. Supplementary Figure S4.10. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Remdesivir treatment vs non-COVID populations. Supplementary Figure S4.11. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Molnupiravir treatment vs non-COVID populations. Supplementary File S5. Kaplan-Meier graph of thyroid cancer risk in different subpopulations following COVID-19. Supplementary Figure S5.1. Kaplan—Meier survival curve of thyroid cancer risk in different age interval of post-COVID populations. Supplementary Figure S5.2. Kaplan—Meier survival curve of thyroid cancer risk in different age interval of post-COVID populations. Supplementary Figure S5.3. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations with hyperthyroidism vs no thyroid function alteration. Supplementary Figure S5.4. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations with hypothyroidism vs no thyroid function alteration. Supplementary Figure S5.5. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Nirmatrelvir/Ritonavir vs no antiviral treatment. Supplementary Figure S5.6. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Remdesivir vs no antiviral treatment. Supplementary Figure S5.7. Kaplan—Meier survival curve of thyroid cancer risk in post-COVID populations who received Molnupiravir vs no antiviral treatment. Supplementary File S6. Kaplan–Meier curve illustrating the risk of thyroid cancer in subpopulations with thyroid dysfunction, stratified by COVID-19 status. Supplementary Figure S6.1. Kaplan—Meier survival curve of thyroid cancer risk in hyperthyroidism populations with or without COVID-19. Supplementary Figure S6.2. Kaplan—Meier survival curve of thyroid cancer risk in hypothyroidism populations with or without COVID-19.
Author Contributions
H.-Y.W. wrote the draft of the manuscript; H.-Y.W. and C.-H.C. performed data analysis; C.-H.C. and S.-C.T. designed and supervised this study. S.-C.T., Y.-C.L., J.-U.H. and C.-H.C. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The use of TriNetX for this study was approved under the authority of the Institutional Review Board of Taichung Veterans General Hospital (TCVGH-IRB No. CE25331B) on 12 June 2025. The Western Institutional Review Board granted TriNetX a waiver of informed consent, as the platform only aggregates counts and statistical summaries of deidentified information.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Files.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE2 | angiotensin-converting enzyme II |
| CI | confidence interval |
| HCOs | healthcare organizations |
| HR | hazard ratio |
| MR | Mendelian randomization |
| nsp | nonstructural protein |
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 5 July 2023).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; Giustina, A.; Puig-Domingo, M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020, 21, 495–507. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr. Progress in Endocrine Neoplasia. Clin. Cancer Res. 2016, 22, 4981–4988. [Google Scholar] [CrossRef]
- Almansoori, A.; Busch, H.; Bendardaf, R.; Hamoudi, R. Thyroid cancer incidence in the United Arab Emirates: A retrospective study on association with age and gender. F1000Research 2022, 11, 338. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rossetti, C.L.; Cazarin, J.; Hecht, F.; Beltrao, F.E.L.; Ferreira, A.C.F.; Fortunato, R.S.; Ramos, H.E.; de Carvalho, D.P. COVID-19 and thyroid function: What do we know so far? Front. Endocrinol. 2022, 13, 1041676. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2021, 22, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e8. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Kang, J.; Li, G.; Ge, J.; Gu, J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann. Transl. Med. 2020, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Gressens, S.B.; Leftheriotis, G.; Dussaule, J.C.; Flamant, M.; Levy, B.I.; Vidal-Petiot, E. Controversial Roles of the Renin Angiotensin System and Its Modulators During the COVID-19 Pandemic. Front. Physiol. 2021, 12, 624052. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Ferreira, S.; Nahmias, C. G-protein coupled receptors of the renin-angiotensin system: New targets against breast cancer? Front. Pharmacol. 2015, 6, 24. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Jamaati, H.; Tabarsi, P.; Bayram, H.; Varahram, M.; Adcock, I.M. COVID-19: Molecular and Cellular Response. Front. Cell. Infect. Microbiol. 2021, 11, 563085. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Lee, A.J.X.; Purshouse, K. COVID-19 and cancer registries: Learning from the first peak of the SARS-CoV-2 pandemic. Br. J. Cancer 2021, 124, 1777–1784. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Naguib, R. Potential relationships between COVID-19 and the thyroid gland: An update. J. Int. Med. Res. 2022, 50, 3000605221082898. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Liu, P.; Leibowitz, J.L.; Kao, C.C. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J. Virol. 2012, 86, 4294–4304. [Google Scholar] [CrossRef]
- Stingi, A.; Cirillo, L. SARS-CoV-2 infection and cancer: Evidence for and against a role of SARS-CoV-2 in cancer onset. Bioessays 2021, 43, e2000289. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Carbajo-Lozoya, J.; Hein, M.Y.; Muller, M.A.; Deng, W.; Lei, J.; Meyer, B.; Kusov, Y.; von Brunn, B.; Bairad, D.R.; et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA 2016, 113, E5192–E5201. [Google Scholar] [CrossRef]
- Leng, R.P.; Lin, Y.; Ma, W.; Wu, H.; Lemmers, B.; Chung, S.; Parant, J.M.; Lozano, G.; Hakem, R.; Benchimol, S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 2003, 112, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Duntas, L.H.; Jonklaas, J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J. Endocr. Soc. 2021, 5, bvab076. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, X.; Zhou, D. Exploring the Potential Association between COVID-19 and Thyroid Cancer: A Mendelian Randomization Study. ACS Omega 2023, 8, 49158–49164. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Hassan, L.; Bacha, F.; Al Salameh, M.; Gatee, O.; Hassan, W. Papillary Thyroid Cancer Trends in the Wake of the COVID-19 Pandemic: Is There a Shift toward a More Aggressive Entity? Diseases 2024, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Hui, Z.; Shen, Z.; Kan, C.; Hou, N.; Sun, X.; Han, F. Thyroid Cancer and COVID-19: Prospects for Therapeutic Approaches and Drug Development. Front. Endocrinol. 2022, 13, 873027. [Google Scholar] [CrossRef]
- Spartalis, E.; Plakopitis, N.; Theodori, M.A.; Karagiannis, S.P.; Athanasiadis, D.I.; Spartalis, M.; Boutzios, G.; Paschou, S.A.; Nikiteas, N.; Troupis, T. Thyroid cancer surgery during the coronavirus disease 2019 pandemic: Perioperative management and oncological and anatomical considerations. Future Oncol. 2021, 17, 4389–4395. [Google Scholar] [CrossRef]
- Bell, R.; Weinberger, D.M.; Venkatesh, M.; Fernandes-Taylor, S.; Francis, D.O.; Davies, L. Thyroid Cancer Incidence During 2020 to 2021 COVID-19 Variant Waves. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 969–977. [Google Scholar] [CrossRef]
- Vrinceanu, D.; Dumitru, M.; Marinescu, A.; Serboiu, C.; Musat, G.; Radulescu, M.; Popa-Cherecheanu, M.; Ciornei, C.; Manole, F. Management of Giant Thyroid Tumors in Patients with Multiple Comorbidities in a Tertiary Head and Neck Surgery Center. Biomedicines 2024, 12, 2204. [Google Scholar] [CrossRef]
- Prete, A.; Falcone, M.; Bottici, V.; Giani, C.; Tiseo, G.; Agate, L.; Matrone, A.; Cappagli, V.; Valerio, L.; Lorusso, L.; et al. Thyroid cancer and COVID-19: Experience at one single thyroid disease referral center. Endocrine 2021, 72, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Means, J.H. The Thyroid and Its Diseases; Lippincott: Philadelphia, PA, USA, 1937. [Google Scholar]
- Tran, T.V.; Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.C.; Journy, N. Thyroid dysfunction and cancer incidence: A systematic review and meta-analysis. Endocr. Relat. Cancer 2020, 27, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Young, B.E.; Fong, S.W.; Ding, Y.; Goh, Y.S.; Chee, R.S.; Tan, S.Y.; Kalimuddin, S.; Tambyah, P.A.; Leo, Y.S.; et al. Differential Cytokine Responses in Hospitalized COVID-19 Patients Limit Efficacy of Remdesivir. Front. Immunol. 2021, 12, 680188. [Google Scholar] [CrossRef]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef]
- Panza, F.; Fiorino, F.; Pastore, G.; Fiaschi, L.; Tumbarello, M.; Medaglini, D.; Ciabattini, A.; Montagnani, F.; Fabbiani, M. Does Nirmatrelvir/Ritonavir Influence the Immune Response against SARS-CoV-2, Independently from Rebound? Microorganisms 2023, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Hunt, B.J.; Stegemann, M.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.-S.; et al. A living WHO guideline on drugs for covid-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Schinas, G.; Gogos, C. Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir. Viruses 2022, 14, 2540. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Gotte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Sendur, S.N.; Oguz, S.H.; Unluturk, U. COVID-19 vaccination and thyroiditis. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101759. [Google Scholar] [CrossRef]
- Ludwig, R.J.; Anson, M.; Zirpel, H.; Thaci, D.; Olbrich, H.; Bieber, K.; Kridin, K.; Dempfle, A.; Curman, P.; Zhao, S.S.; et al. A comprehensive review of methodologies and application to use the real-world data and analytics platform TriNetX. Front. Pharmacol. 2025, 16, 1516126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).