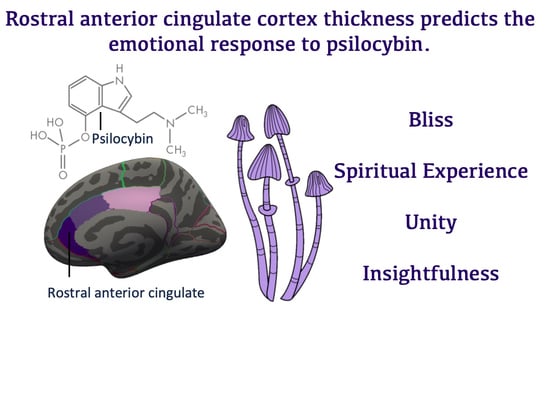
Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design and Psilocybin Administration
2.3. Neuro-Imaging Acquisition
2.4. Image Data Processing
2.5. Statistical Analyses
3. Results
3.1. Dose Comparison
3.2. Cingulate Thickness Predicting Sub-Scales
3.3. Comparing Correlated Correlation Coefficients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Twarog, B.M.; Page, I.H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 1953, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eivindvik, K.; Rasmussen, K.E.; Sund, R.B. Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta Pharm. Nord. 1989, 1, 295–302. [Google Scholar]
- Hasler, F.; Studerus, E.; Lindner, K.; Ludewig, S.; Vollenweider, F.X. Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J. Psychopharmacol. 2009, 23, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mckenna, D.J.; Repke, D.B.; Lo, L.; Peroutka, S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Publ. Gr. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Carter, O.L.; Burr, D.C.; Pettigrew, J.D.; Wallis, G.M.; Hasler, F.; Vollenweider, F.X. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cogn. Neurosci. 2005, 17, 1497–1508. [Google Scholar] [CrossRef] [Green Version]
- Gouzoulis-Mayfrank, E.; Schreckenberger, M.; Sabri, O.; Hermle, L.; Büll, U.; Sass, H. Neurometabolic Effects of Psilocybin, (MDE) and d-Methamphetamine in Healthy Volunteers. Neuropsychopharmacology 1999, 20, 565–581. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.R.; Richards, W. a.; McCann, U.; Jesse, R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl). 2006, 187, 268–283. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Johnson, M.W.; Hurwitz, E.; Griffiths, R.R. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berl). 2018, 235, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Richards, W.A.; Richards, B.D.; McCann, U.; Jesse, R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl). 2011, 218, 649–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, C.R.; Preller, K.H.; Kraehenmann, R.; Michels, L.; Stämpfli, P.; Vollenweider, F.X. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage 2017, 159, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, T.; Preller, K.H.; Kometer, M.; Dziobek, I.; Vollenweider, F.X. Effect of psilocybin on empathy and moral decision-making. Int. J. Neuropsychopharmacol. 2017, 20, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, F.X.; Csomor, P.; Knappe, B.; Geyer, M.; Quednow, B.B. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 2007, 32, 1876–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraehenmann, R.; Schmidt, A.; Friston, K.; Preller, K.H.; Seifritz, E.; Vollenweider, F.X. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. NeuroImage Clin. 2015, 11, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Preller, K.H.; Pokorny, T.; Hock, A.; Kraehenmann, R.; Stãmpfli, P.; Seifritz, E.; Scheidegger, M.; Vollenweider, F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA 2016, 113, 5119–5124. [Google Scholar] [CrossRef] [Green Version]
- Kometer, M.; Schmidt, A.; Bachmann, R.; Studerus, E.; Seifritz, E.; Vollenweider, F.X. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol. Psychiatry 2012, 72, 898–906. [Google Scholar] [CrossRef]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law 2017, 3, 2050324516683325. [Google Scholar] [CrossRef]
- Metzner, R.; Litwin, G.H.; Weil, G.M. The Relation of Expectation and Mood to Psilocybin Reactions: A Questionnaire Study. Psychedelic Rev. 1965, 1, 3–39. [Google Scholar]
- Becker, H.S. History, culture and subjective experience: an exploration of the social bases of drug-induced experiences. J. Health Soc. Behav. 1967, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Studerus, E.; Gamma, A.; Kometer, M.; Vollenweider, F.X. Prediction of psilocybin response in healthy volunteers. PLoS ONE 2012, 7, e30800. [Google Scholar] [CrossRef]
- Panton, Y.; Fischer, R. Hallucinogenic Drug-Induced Behavior Under Sensory Attenuation: Prediction of Response to Psilocybin. Arch. Gen. Psychiatry 1973, 28, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.F.; Cherkasova, M.V.; Larcher, K.; Evans, A.C.; Baker, G.B.; Dagher, A.; Benkelfat, C.; Leyton, M. Individual Differences in Frontal Cortical Thickness Correlate with the d-Amphetamine-Induced Striatal Dopamine Response in Humans. J. Neurosci. 2013, 33, 15285–15294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherkasova, M.V.; Faridi, N.; Casey, K.F.; Larcher, K.; O’Driscoll, G.A.; Hechtman, L.; Joober, R.; Baker, G.B.; Palmer, J.; Evans, A.C.; et al. Differential Associations between Cortical Thickness and Striatal Dopamine in Treatment-Naïve Adults with ADHD vs. Healthy Controls. Front. Hum. Neurosci. 2017, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaworska, N.; Cox, S.M.; Casey, K.F.; Boileau, I.; Cherkasova, M.; Larcher, K.; Dagher, A.; Benkelfat, C.; Leyton, M. Is there a relation between novelty seeking, striatal dopamine release and frontal cortical thickness? PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Chaney, A.; Carballedo, A.; Amico, F.; Fagan, A.; Skokauskas, N.; Meaney, J.; Frodl, T. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J. Psychiatry Neurosci. 2014, 39, 50. [Google Scholar] [CrossRef] [Green Version]
- Redlich, R.; Almeida, J.R.; Grotegerd, D.; Opel, N.; Kugel, H.; Heindel, W.; Arolt, V.; Phillips, M.L.; Dannlowski, U. Brain morphometric biomarkers distinguishing unipolar and bipolar depression: A voxel-based morphometry-pattern classification approach. JAMA Psychiatry 2014, 71, 1222–1230. [Google Scholar] [CrossRef]
- Jung, J.; Kang, J.; Won, E.; Nam, K.; Lee, M.S.; Tae, W.S.; Ham, B.J. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: A voxel-based morphometry study. J. Affect. Disord. 2014, 169, 179–187. [Google Scholar] [CrossRef]
- Young, R.C.; Kalayam, B.; Nambudiri, D.E.; Kakuma, T.; Alexopoulos, G.S. Brain morphology and response to nortriptyline in geriatric depression. Am. J. Geriatr. Psychiatry 1999, 7, 147–150. [Google Scholar] [CrossRef]
- Schilling, C.; Kühn, S.; Paus, T.; Romanowski, A.; Banaschewski, T.; Barbot, A.; Barker, G.J.; Brühl, R.; Büchel, C.; Conrod, P.J.; et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol. Psychiatry 2013, 36, 4038–4049. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.J.; Hollinshead, M.O.; Roffman, J.L.; Smoller, J.W.; Buckner, R.L. Individual Differences in Cognitive Control Circuit Anatomy Link Sensation Seeking, Impulsivity, and Substance Use. J. Neurosci. 2016, 36, 4038–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardini, S.; Cloninger, C.R.; Venneri, A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res. Bull. 2009, 79, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 1998, 31, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Studerus, E.; Gamma, A.; Vollenweider, F.X. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Kraehenmann, R.; Preller, K.H.; Scheidegger, M.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol. Psychiatry 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Mayberg, S. Limbic-Cortical Dysregulation: Depression. In The Neuropsychiatry of Limbic and Subcortical Disorders; American Psychiatric Press: Washington, DC, USA, 1997. [Google Scholar]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A high-resolution in vivo atlas of the human brain’s serotonin system. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Cosman, E., Jr.; Fischl, B.; Wells III, W.; Dale, A. Topology Correction for Cortical Surface Models; Massachusetts Institute of Technology: Cambridge, MA, USA, 1999; pp. 169–170. [Google Scholar]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Han, X.; Jovicich, J.; Jovicich, J.; Salat, D.; Salat, D.; van der Kouwe, A.; van der Kouwe, A.; Quinn, B.; Quinn, B.; et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006, 32, 180–194. [Google Scholar] [CrossRef]
- Meng, X.L.; Rosenthal, R.; Rubin, D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992, 111, 172. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebedev, A.V.; Lövdén, M.; Rosenthal, G.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum. Brain Mapp. 2015, 36, 3137–3153. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Fadok, R.A.; Purcell, M.; Liu, S.; Stonnington, C.; Spetzler, R.F.; Baxter, L.C. Localizing sadness activation within the subgenual cingulate in individuals: A novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging Behav. 2011, 5, 229–239. [Google Scholar] [CrossRef]
- Liotti, M.; Mayberg, H.S.; Brannan, S.K.; McGinnis, S.; Jerabek, P.; Fox, P.T. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol. Psychiatry 2000, 48, 30–42. [Google Scholar] [CrossRef]
- Braden, B.B.; Pipe, T.B.; Smith, R.; Glaspy, T.K.; Deatherage, B.R.; Baxter, L.C. Brain and behavior changes associated with an abbreviated 4-week mindfulness-based stress reduction course in back pain patients. Brain Behav. 2016, 6, e00443. [Google Scholar] [CrossRef]
- LeDoux, J. The amygdala. Curr. Biol. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [Green Version]
- Blair, K.S.; Smith, B.W.; Mitchell, D.G.V.; Morton, J.; Vythilingam, M.; Pessoa, L.; Fridberg, D.; Zametkin, A.; Sturman, D.; Nelson, E.E.; et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage 2007, 35, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Etkin, A.; Egner, T.; Peraza, D.M.; Kandel, E.R.; Hirsch, J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 2006, 51, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Demaree, H.A.; Everhart, D.E.; Youngstrom, E.A.; Harrison, D.W. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance. ” Behav. Cogn. Neurosci. Rev. 2005, 4, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Simon-Thomas, E.R.; Role, K.O.; Knight, R.T. Behavioral and electrophysiological evidence of a right hemisphere bias for the influence of negative emotion on higher cognition. J. Cogn. Neurosci. 2005, 17, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Hermle, L.; Fünfgeld, M.; Oepen, G.; Botsch, H.; Borchardt, D.; Gouzoulis, E.; Fehrenbach, R.A.; Spitzer, M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol. Psychiatry 1992, 32, 976–991. [Google Scholar] [CrossRef]
- Riba, J.; Romero, S.; Grasa, E.; Mena, E.; Carrió, I.; Barbanoj, M.J. Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology (Berl). 2006, 186, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, F.X.; Leenders, K.L.; Scharfetter, C.; Maguire, P.; Stadelmann, O.; Angst, J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 1997, 16, 357–372. [Google Scholar] [CrossRef]
- Ley, R.G.; Bryden, M.P. Hemispheric differences in processing emotions and faces. Brain Lang. 1979, 7, 127–138. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Nettleton, N.C. The nature of hemispheric specialization in man. Behav. Brain Sci. 1981, 4, 51–63. [Google Scholar] [CrossRef]
- Bottini, G.; Corcoran, R.; Sterzi, R.; Paulesu, E.; Schenone, P.; Scarpa, P.; Frackowiak, R.S.J.; Frith, D. The role of the right hemisphere in the interpretation of figurative aspects of language a positron emission tomography activation study. Brain 1994, 117, 1241–1253. [Google Scholar] [CrossRef]
- Thomas, K.; Malcolm, B.; Lastra, D. Psilocybin-Assisted Therapy: A Review of a Novel Treatment for Psychiatric Disorders. J. Psychoactive Drugs 2017, 49, 446–455. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018, 235, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. The Lancet Psychiatry 2016, 0366, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Roseman, L.; Nutt, D.J.; Carhart-Harris, R.L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 2018, 8, 974. [Google Scholar] [CrossRef]
- Abramson, L.Y.; Metalsky, G.I.; Alloy, L.B. Hopelessness Depression: A Theory-Based Subtype of Depression. Psychol. Rev. 1989, 96, 358. [Google Scholar] [CrossRef]
- Watkins, E.R. Depressive rumination: Investigating mechanisms to improve cognitive behavioural treatments. Cogn. Behav. Ther. 2009, 38, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Sherry, S.B.; Law, A.; Hewitt, P.L.; Flett, G.L.; Besser, A. Social support as a mediator of the relationship between perfectionism and depression: A preliminary test of the social disconnection model. Pers. Individ. Dif. 2008, 45, 339–344. [Google Scholar] [CrossRef]
- Remmers, C.; Michalak, J. Losing your gut feelings. Intuition in depression. Front. Psychol. 2016, 7, 1291. [Google Scholar] [CrossRef] [Green Version]
- Cruwys, T.; Alexander Haslam, S.; Dingle, G.A.; Jetten, J.; Hornsey, M.J.; Desdemona Chong, E.M.; Oei, T.P.S. Feeling connected again: Interventions that increase social identification reduce depression symptoms in community and clinical settings. J. Affect. Disord. 2014, 159, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017, 16, 194–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, C.L.; Kelley, M.E.; Holtzheimer, P.E.; Dunlop, B.W.; Craighead, W.E.; Franco, A.R.; Craddock, R.C.; Mayberg, H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 2013, 70, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Durston, S.; Fossella, J.A.; Casey, B.J.; Hulshoff Pol, H.E.; Galvan, A.; Schnack, H.G.; Steenhuis, M.P.; Minderaa, R.B.; Buitelaar, J.K.; Kahn, R.S.; et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol. Psychiatry 2005, 10, 678–685. [Google Scholar] [CrossRef]
- Shaw, P.; Gornick, M.; Lerch, J.; Addington, A.; Seal, J.; Greenstein, D.; Sharp, W.; Evans, A.; Giedd, J.N.; Castellanos, F.X.; et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2007, 64, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Jaén, A.; López-Martín, S.; Albert, J.; Fernández-Mayoralas, D.M.; Fernández-Perrone, A.L.; de La Peña, M.J.; Calleja-Pérez, B.; Rodríguez, M.R.; López-Arribas, S.; Muñoz-Jareño, N. Cortical thickness differences in the prefrontal cortex in children and adolescents with ADHD in relation to dopamine transporter (DAT1) genotype. Psychiatry Res. -Neuroimaging 2015, 233, 409–417. [Google Scholar] [CrossRef]
- Lesch, K.P. Molecular foundation of anxiety disorders. J. Neural Transm. 2001, 108, 717–746. [Google Scholar] [CrossRef]
- Müller, J.; Dreisbach, G.; Brocke, B.; Lesch, K.P.; Strobel, A.; Goschke, T. Dopamine and cognitive control: The influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Res. 2007, 1131, 155–162. [Google Scholar] [CrossRef]
- Lesch, K.P.; Gutknecht, L. Focus on the 5-HT1A receptor: Emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int. J. Neuropsychopharmacol. 2004, 7, 381–385. [Google Scholar] [CrossRef]
- Lin, J.Y.; Jiang, M.Y.; Kan, Z.M.; Chu, Y. Influence of 5-HTR2A genetic polymorphisms on the efficacy of antidepressants in the treatment of major depressive disorder: a meta-analysis. J. Affect Disord. 2014, 168, 430–438. [Google Scholar] [CrossRef]


| Low (0.16 mg/kg) | High (0.215mg/kg) | p-Value | |||
|---|---|---|---|---|---|
| Construct | Mean | SE | Mean | SE | |
| Unity | 26 | 5 | 33 | 5 | 0.495 |
| Spiritual | 12 | 4 | 16 | 4 | 0.975 |
| Bliss | 38 | 6 | 43 | 6 | 0.75 |
| Insight | 25 | 5 | 36 | 6 | 0.516 |
| Sub-Scale | Rostral Anterior Cingulate Cortex (rACC) | |||
|---|---|---|---|---|
| β | SE | p | FDR p | |
| Unity | ||||
| LH | 0.295 | 17.93 | 0.037 | 0.114 |
| RH | 0.324 | 17.18 | 0.027 | 0.027 |
| Bliss | ||||
| LH | 0.106 | 25.14 | 0.465 | 0.465 |
| RH | 0.386 | 18.95 | 0.008 | 0.011 |
| Spiritual | ||||
| LH | 0.266 | 13.84 | 0.057 | 0.114 |
| RH | 0.465 * | 12.35 | 0.001 | 0.002 |
| Insight | ||||
| LH | 0.179 | 20.26 | 0.202 | 0.269 |
| RH | 0.572 *# | 16.46 | 0.00002 | 0.00008 |
| Sub-Scale | Caudal Cingulate | Posterior Cingulate | ||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Unity | ||||||
| LH | −0.021 | 22.383 | 0.884 | 0.108 | 22.289 | 0.454 |
| RH | 0.275 | 16.976 | 0.065 | 0.062 | 30.219 | 0.676 |
| Bliss | ||||||
| LH | −0.106 | 25.141 | 0.465 | −0.046 | 25.287 | 0.749 |
| RH | 0.006 | 19.852 | 0.969 | 0.01 | 34.181 | 0.947 |
| Spiritual | ||||||
| LH | 0.213 | 16.749 | 0.132 | 0.052 | 17.144 | 0.711 |
| RH | 0.109 | 13.39 | 0.463 | −0.028 | 23.175 | 0.852 |
| Insight | ||||||
| LH | 0.186 | 24.158 | 0.186 | 0.125 | 24.428 | 0.371 |
| RH | 0.241 | 18.781 | 0.099 | −0.033 | 33.242 | 0.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, C.R.; Preller, K.H.; Braden, B.B.; Riecken, C.; Vollenweider, F.X. Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience. Biomedicines 2020, 8, 34. https://doi.org/10.3390/biomedicines8020034
Lewis CR, Preller KH, Braden BB, Riecken C, Vollenweider FX. Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience. Biomedicines. 2020; 8(2):34. https://doi.org/10.3390/biomedicines8020034
Chicago/Turabian StyleLewis, Candace R., Katrin H. Preller, B. Blair Braden, Cory Riecken, and Franz X. Vollenweider. 2020. "Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience" Biomedicines 8, no. 2: 34. https://doi.org/10.3390/biomedicines8020034
APA StyleLewis, C. R., Preller, K. H., Braden, B. B., Riecken, C., & Vollenweider, F. X. (2020). Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience. Biomedicines, 8(2), 34. https://doi.org/10.3390/biomedicines8020034