Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zinc Oxide Nanoparticles (ZnO NPs)
2.2. Assessment of Antibacterial Activity
2.3. Cells and Cell Culture
2.4. Cell Viability Assay
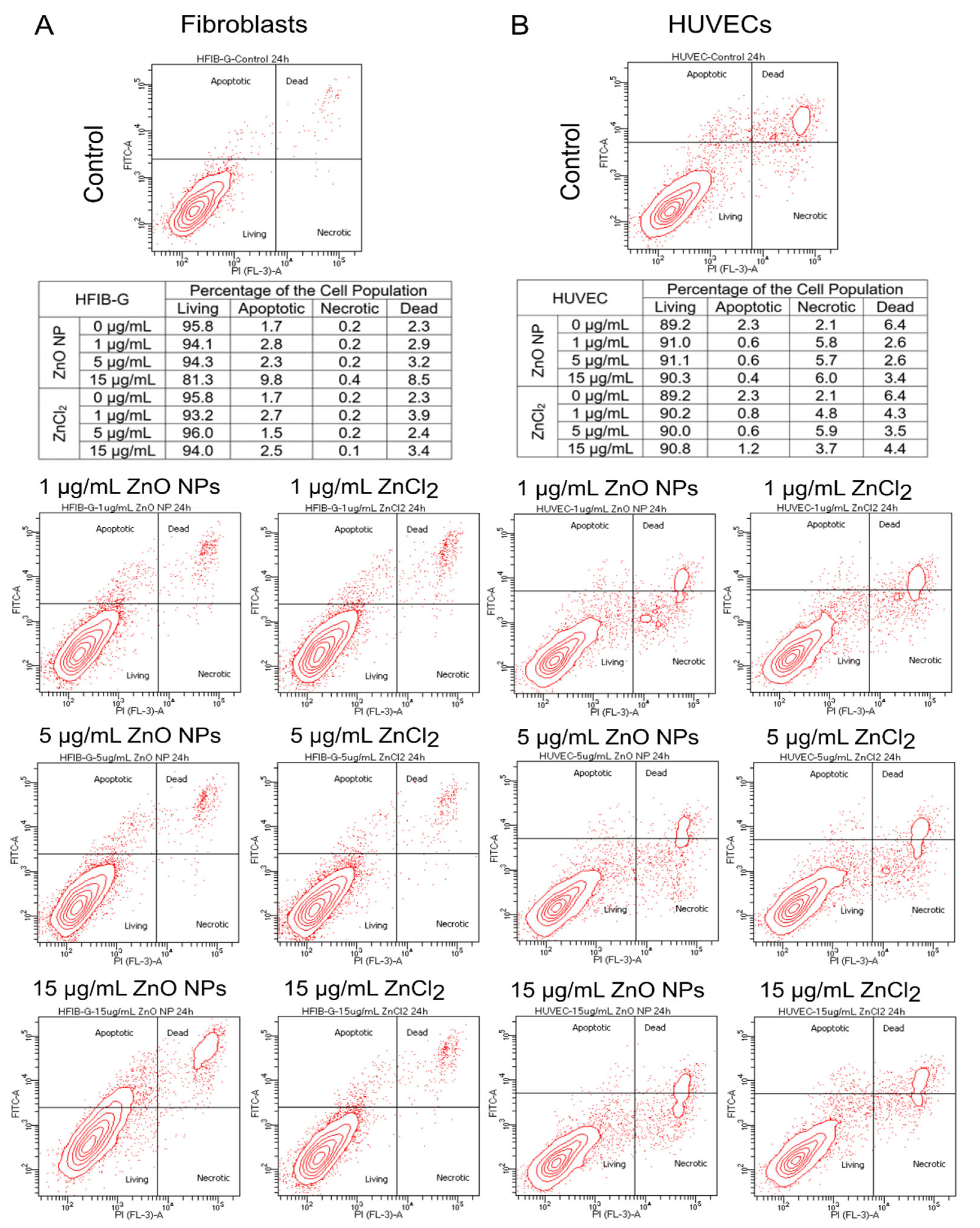
2.5. Analysis of Cell Death by Flow Cytometry
2.6. Chicken Chorioallantoic Membrane Assay (CAM-Assay)
2.7. Statistical Analysis
3. Results
3.1. Antibacterial Properties of ZnO NPs
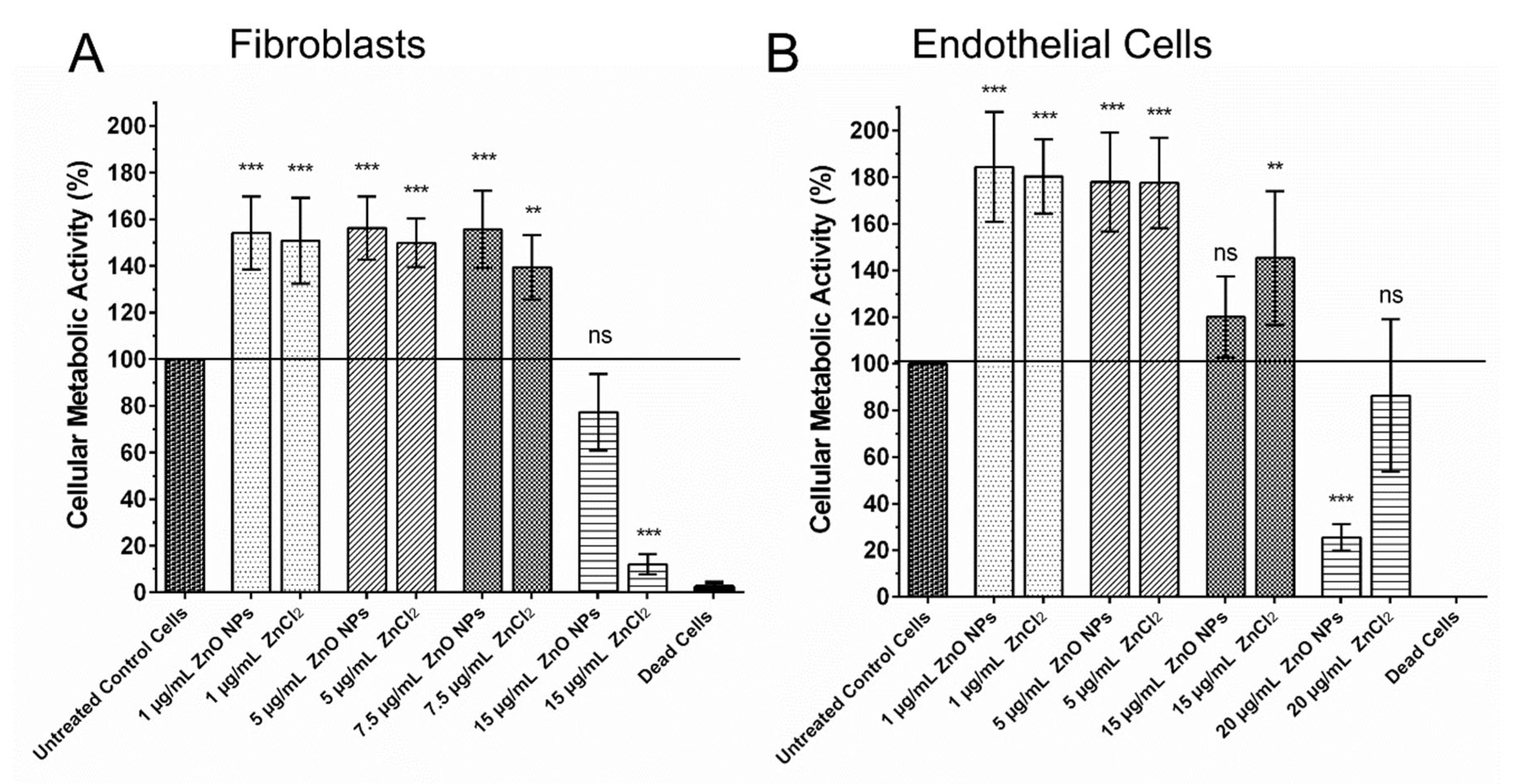
3.2. Cellular Metabolic Activity of Fibroblasts and Endothelial Cells in the Presence of ZnO NPs
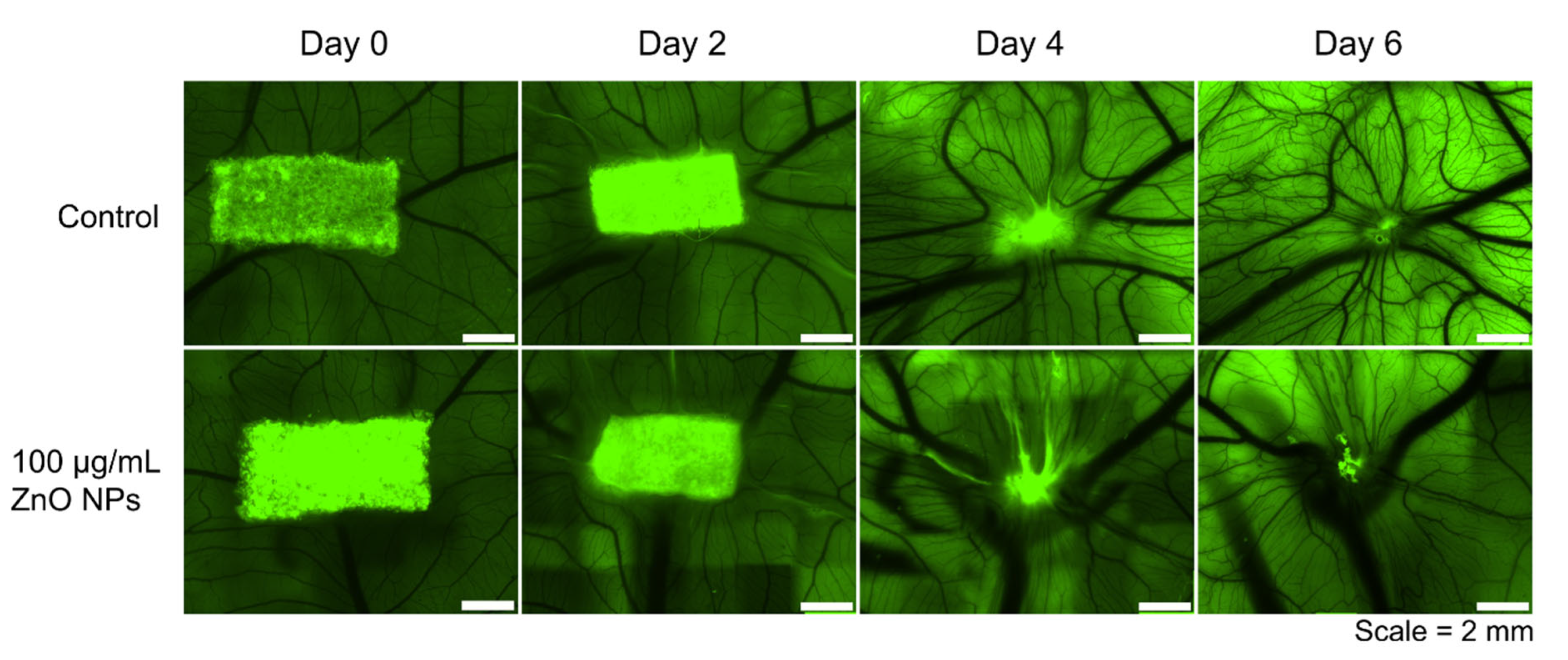
3.3. Biocompatibility of ZnO NPs Assessed in the Chorioallantoic Membrane Assay (CAM Assay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and alendronic acid on titanium surfaces: Adhesion, proliferation and differentiation of bone marrow-derived stem cells. J. Biomed. Mater. Res. A 2020, 108, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Thiem, D.G.; Adam, M.; Ganz, C.; Gerber, T.; Kämmerer, P.W. The implant surface and its role in affecting the dynamic processes of bone remodeling by means of distance osteogenesis: A comparative in vivo study. Int. J. Oral Maxillofac. Implant. 2019, 34, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Heller, M.; Götz, H.; Schiegnitz, E.; Al-Nawas, B.; Kämmerer, P.W. Comparison of growth & function of endothelial progenitor cells cultured on deproteinized bovine bone modified with covalently bound fibronectin and bound vascular endothelial growth factor. Clin. Oral Implant. Res. 2017, 28, 543–550. [Google Scholar] [CrossRef]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Götz, H.; Rommens, P.M.; Hofmann, A. Surface Functionalization of Orthopedic Titanium Implants with Bone Sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Götz, H.; Mattyasovszky, S.G.; Rommens, P.M.; Hofmann, A. Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice. Materials 2018, 11, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 35004. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 207. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.-R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Choi, S.-J.; Choy, J.-H. Biokinetics of zinc oxide nanoparticles: Toxicokinetics, biological fates, and protein interaction. Int. J. Nanomed. 2014, 9 (Suppl. S2), 261–269. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Liang, J.; Liu, H.; Zhang, K.; Li, J. Nanomaterials for Angiogenesis in Skin Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Kumar, P.T.S.; Raja, B.; Lakshmanan, V.-K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10 (Suppl. S1), 53–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, S.; Ranjan Dahiya, U.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; Rehman, I.u. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2019, 51, 226–234. [Google Scholar] [CrossRef]
- Heim, J.; Felder, E.; Tahir, M.N.; Kaltbeitzel, A.; Heinrich, U.R.; Brochhausen, C.; Mailänder, V.; Tremel, W.; Brieger, J. Genotoxic effects of zinc oxide nanoparticles. Nanoscale 2015, 7, 8931–8938. [Google Scholar] [CrossRef] [Green Version]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Hannan, R.T.; Peirce, S.M.; Barker, T.H. Fibroblasts: Diverse Cells Critical to Biomaterials Integration. ACS Biomater. Sci. Eng. 2018, 4, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Rani, R.; Gupta, M. Angiogenesis: Aspects in Wound Healing. Endothelial Signaling in Vascular Dysfunction and Disease; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–90. ISBN 9780128161968. [Google Scholar]
- Brieger, J.; Schroeder, P.; Gosepath, J.; Mann, W. VEGF-subtype specific protection of SCC and HUVECs from radiation induced cell death. Int. J. Mol. Med. 2005, 15, 145–151. [Google Scholar] [CrossRef]
- Buhr, C.R.; Eckrich, J.; Kluenker, M.; Bruns, K.; Wiesmann, N.; Tremel, W.; Brieger, J. Determination of the LD50 with the chick embryo chorioallantoic membrane (CAM) assay as a promising alternative in nanotoxicological evaluation. Nanotoxicology 2021, 15, 690–705. [Google Scholar] [CrossRef]
- Buhr, C.R.; Wiesmann, N.; Tanner, R.C.; Brieger, J.; Eckrich, J. The Chorioallantoic Membrane Assay in Nanotoxicological Research-An Alternative for In Vivo Experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef]
- Merckx, G.; Tay, H.; Lo Monaco, M.; van Zandvoort, M.; Spiegelaere, W.; de Lambrichts, I.; Bronckaers, A. Chorioallantoic Membrane Assay as Model for Angiogenesis in Tissue Engineering: Focus on Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 519–539. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef] [PubMed]
- Heimes, D.; Wiesmann, N.; Eckrich, J.; Brieger, J.; Mattyasovszky, S.; Proff, P.; Weber, M.; Deschner, J.; Al-Nawas, B.; Kämmerer, P.W. In Vivo Modulation of Angiogenesis and Immune Response on a Collagen Matrix via Extracorporeal Shockwaves. Int. J. Mol. Sci. 2020, 21, 7574. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The gelatin sponge-chorioallantoic membrane assay. Nat. Protoc. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Kolenda, A.; Krawczyk-Balska, A.; Prochwicz, E.; Woźniak, B.; Łojkowski, W.; Mierzwińska-Nastalska, E. Zinc Oxide Nanoparticles Cytotoxicity and Release from Newly Formed PMMA-ZnO Nanocomposites Designed for Denture Bases. Nanomaterials 2019, 9, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barui, A.K.; Nethi, S.K.; Patra, C.R. Investigation of the role of nitric oxide driven angiogenesis by zinc oxide nanoflowers. J. Mater. Chem. B 2017, 5, 3391–3403. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B Biol. 2018, 178, 211–218. [Google Scholar] [CrossRef]
- Rahimi Kalateh Shah Mohammad, G.; Homayouni Tabrizi, M.; Ardalan, T.; Yadamani, S.; Safavi, E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019, 44, 845. [Google Scholar] [CrossRef]
- Poier, N.; Hochstöger, J.; Hackenberg, S.; Scherzad, A.; Bregenzer, M.; Schopper, D.; Kleinsasser, N. Effects of Zinc Oxide Nanoparticles in HUVEC: Cyto- and Genotoxicity and Functional Impairment After Long-Term and Repetitive Exposure in vitro. Int. J. Nanomed. 2020, 15, 4441–4452. [Google Scholar] [CrossRef]
- Tada-Oikawa, S.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Wu, W.; Yamada, Y.; Mishima, T.; Ichihara, S. Zn(II) released from zinc oxide nano/micro particles suppresses vasculogenesis in human endothelial colony-forming cells. Toxicol. Rep. 2015, 2, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species in vascular formation and development. Oxid. Med. Cell. Longev. 2013, 2013, 374963. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Lin, L.-S.; Wang, J.-F.; Song, J.; Liu, Y.; Zhu, G.; Dai, Y.; Shen, Z.; Tian, R.; Song, J.; Wang, Z.; et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019, 9, 7200–7209. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Park, Y.-H.; Bae, H.C.; Kim, J.H.; Jeong, S.H.; Lee, H.; Son, S.W. ZnO nanoparticle induces apoptosis by ROS triggered mitochondrial pathway in human keratinocytes. Mol. Cell. Toxicol. 2014, 10, 387–391. [Google Scholar] [CrossRef]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef] [PubMed]
- Elbahri, M.; Abdelaziz, R.; Disci-Zayed, D.; Homaeigohar, S.; Sosna, J.; Adam, D.; Kienle, L.; Dankwort, T.; Abdelaziz, M. Underwater Leidenfrost nanochemistry for creation of size-tailored zinc peroxide cancer nanotherapeutics. Nat. Commun. 2017, 8, 15319. [Google Scholar] [CrossRef] [PubMed]
- Fröber, K.; Bergs, C.; Pich, A.; Conrads, G. Biofunctionalized zinc peroxide nanoparticles inhibit peri-implantitis associated anaerobes and Aggregatibacter actinomycetemcomitans pH-dependent. Anaerobe 2020, 62, 102153. [Google Scholar] [CrossRef] [PubMed]
- Bergs, C.; Brück, L.; Rosencrantz, R.R.; Conrads, G.; Elling, L.; Pich, A. Biofunctionalized zinc peroxide (ZnO2) nanoparticles as active oxygen sources and antibacterial agents. RSC Adv. 2017, 7, 38998–39010. [Google Scholar] [CrossRef] [Green Version]
- Drigotas, M.; Affolter, A.; Mann, W.J.; Brieger, J. Reactive oxygen species activation of MAPK pathway results in VEGF upregulation as an undesired irradiation response. J. Oral Pathol. Med. 2013, 42, 612–619. [Google Scholar] [CrossRef]
- Hanai, Y.; Tokuda, H.; Yasuda, E.; Noda, T.; Ohta, T.; Takai, S.; Kozawa, O. Up-regulation by zinc of FGF-2-induced VEGF release through enhancing p44/p42 MAP kinase activation in osteoblasts. Life Sci. 2006, 80, 230–234. [Google Scholar] [CrossRef]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Hassan, M.S.; Sheikh, F.A.; Lee, H.K.; Seo, K.-S.; Yoon, D.; Hwang, I.H. Zinc oxide-doped poly(urethane) spider web nanofibrous scaffold via one-step electrospinning: A novel matrix for tissue engineering. Appl. Microbiol. Biotechnol. 2013, 97, 1725–1734. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef]
- Arshad, R.; Sohail, M.F.; Sarwar, H.S.; Saeed, H.; Ali, I.; Akhtar, S.; Hussain, S.Z.; Afzal, I.; Jahan, S.; Anees-Ur-Rehman; et al. ZnO-NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: In vitro and in vivo evaluation. PLoS ONE 2019, 14, e0217079. [Google Scholar] [CrossRef] [Green Version]
- Batool, M.; Khurshid, S.; Qureshi, Z.; Daoush, W.M. Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem. Pap. 2021, 75, 893–907. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Lakshmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, L.; Chaudhry, A.A.; Aleem, A.R.; Malik, M.H.; Ijaz, K.; Akhtar, H.; Alvi, F.; Khan, A.F.; Rehman, I.u.; Yar, M. Development of K-doped ZnO nanoparticles encapsulated crosslinked chitosan based new membranes to stimulate angiogenesis in tissue engineered skin grafts. Int. J. Biol. Macromol. 2018, 120, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Han, J.; Liu, Z.; Wei, S.; Su, X.; Zhang, G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated Collagenous Chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: Exhaustive in vivo evaluations. J. Photochem. Photobiol. B Biol. 2019, 197, 111539. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prone, G.; Silva-Bermudez, P.; Bazzar, M.; Focarete, M.L.; Rodil, S.E.; Vidal-Gutiérrez, X.; García-Macedo, J.A.; García-Pérez, V.I.; Velasquillo, C.; Almaguer-Flores, A. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed. Mater. 2020, 15, 35006. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, Y.; Cui, M.; Tey, H.L.; Wang, L.; Xu, C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B 2017, 5, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2019, 117, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Li, C.; Cao, J.; He, E.; Wu, X.; Wang, F.; Wang, L. Facile preparation PCL/ modified nano ZnO organic-inorganic composite and its application in antibacterial materials. J. Polym. Res. 2020, 27, 78. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Hashemi-Najafabadi, S.; Eslaminejad, M.B.; Bagheri, F.; Sayahpour, F.A. The effect of modified electrospun PCL-nHA-nZnO scaffolds on osteogenesis and angiogenesis. J. Biomed. Mater. Res. A 2019, 107, 2040–2052. [Google Scholar] [CrossRef]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [Green Version]
- Raj, I.; Mozetic, M.; Jayachandran, V.P.; Jose, J.; Thomas, S.; Kalarikkal, N. Fracture resistant, antibiofilm adherent, self-assembled PMMA/ZnO nanoformulations for biomedical applications: Physico-chemical and biological perspectives of nano reinforcement. Nanotechnology 2018, 29, 305704. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/Zno Bionanocomposite Films with Positively Charged Zno as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kämmerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials . Biomedicines 2021, 9, 1462. https://doi.org/10.3390/biomedicines9101462
Wiesmann N, Mendler S, Buhr CR, Ritz U, Kämmerer PW, Brieger J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials . Biomedicines. 2021; 9(10):1462. https://doi.org/10.3390/biomedicines9101462
Chicago/Turabian StyleWiesmann, Nadine, Simone Mendler, Christoph R. Buhr, Ulrike Ritz, Peer W. Kämmerer, and Juergen Brieger. 2021. "Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials " Biomedicines 9, no. 10: 1462. https://doi.org/10.3390/biomedicines9101462
APA StyleWiesmann, N., Mendler, S., Buhr, C. R., Ritz, U., Kämmerer, P. W., & Brieger, J. (2021). Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials . Biomedicines, 9(10), 1462. https://doi.org/10.3390/biomedicines9101462





