Osteoconductive Properties of a Volume-Stable Collagen Matrix in Rat Calvaria Defects: A Pilot Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Surgical Procedure
2.3. Histological and Histomorphometric Analysis
2.4. Quantitative Backscattered Electron Imaging (qBEI)
2.5. Micro-CT Analysis
3. Results
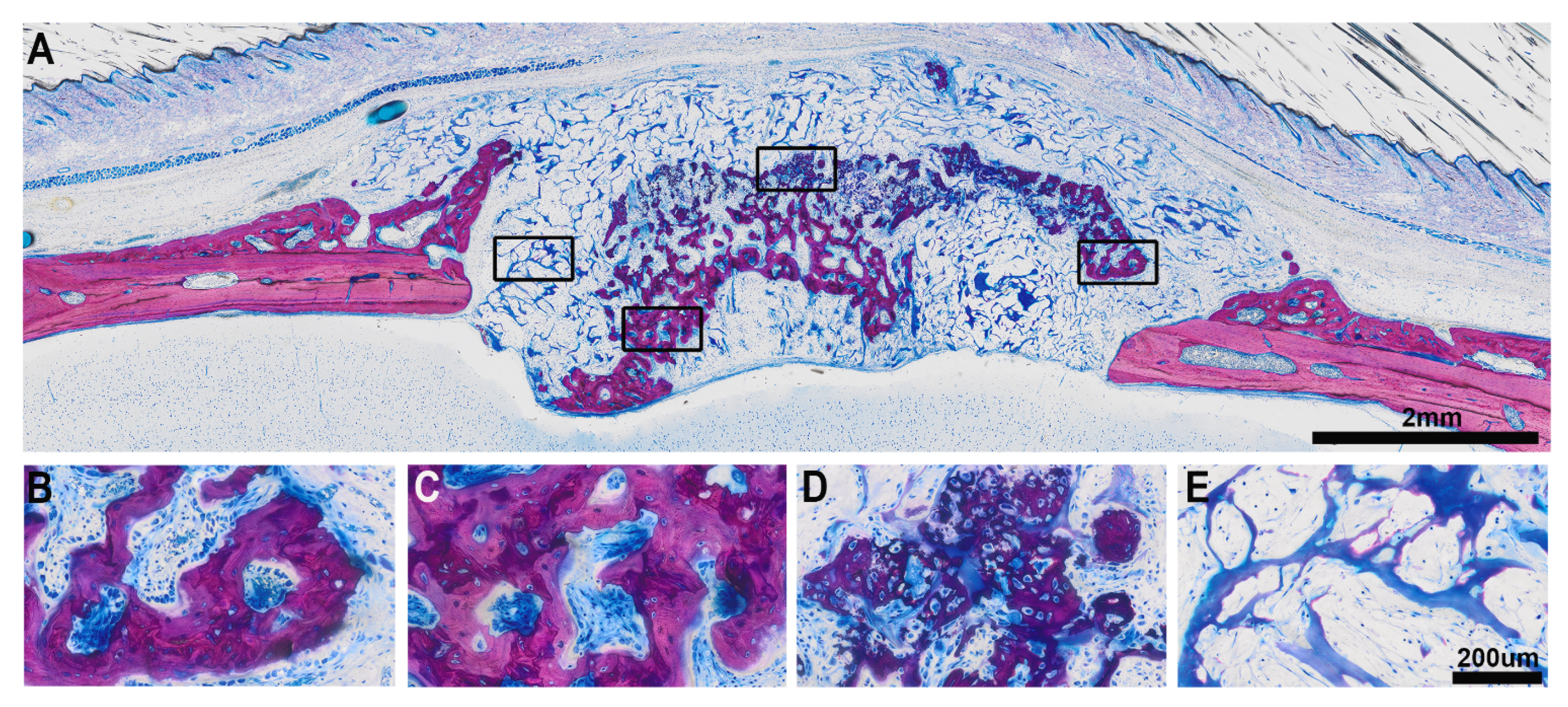
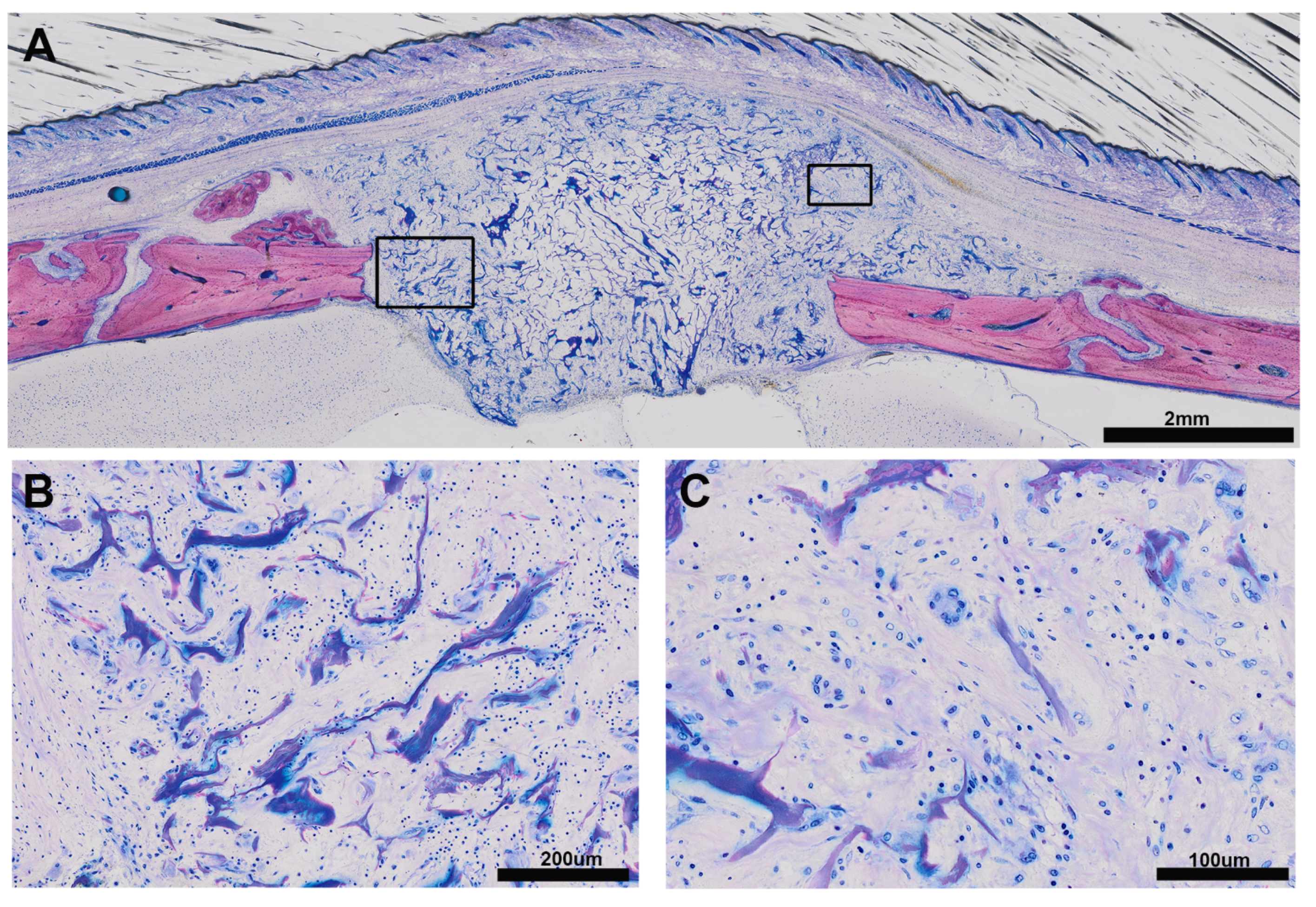
3.1. Histologic Results
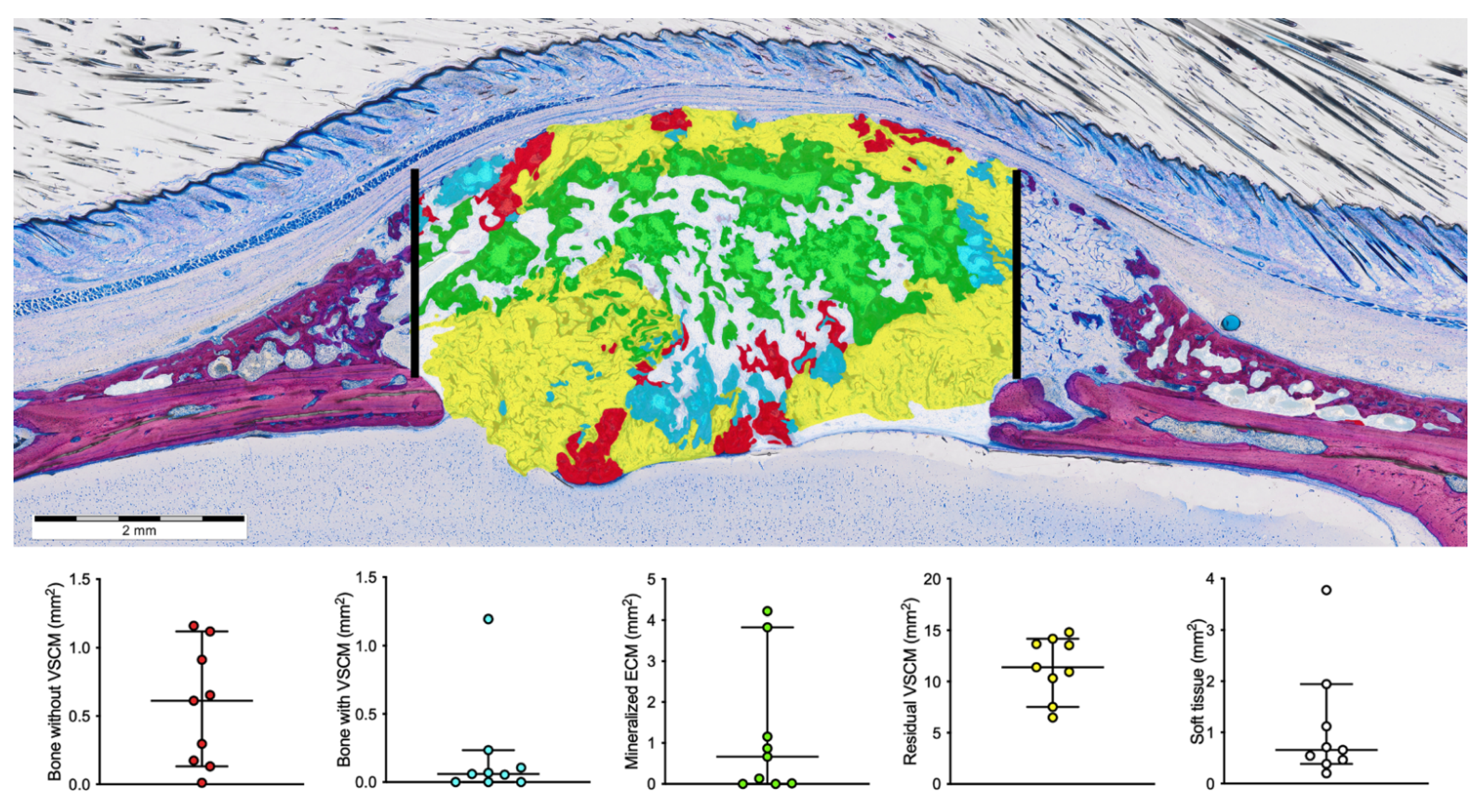
3.2. Histomorphometric Results
3.3. Quantitative Backscattered Electron Imaging (qBEI)
3.4. Microtomographic Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volponi, A.A.; Zaugg, L.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Heal. Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, P.K.; Aghaloo, T. Risk factors in bone augmentation procedures. Periodontol. 2000 2019, 81, 76–90. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef]
- Bertl, K.; Melchard, M.; Pandis, N.; Müller-Kern, M.; Stavropoulos, A. Soft tissue substitutes in non-root coverage procedures: A systematic review and meta-analysis. Clin. Oral Investig. 2017, 21, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Zucchelli, G.; Tavelli, L.; McGuire, M.K.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontol. 2019, 91, 9–16. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 32–49. [Google Scholar] [CrossRef]
- Thoma, D.S.; Lim, H.; Paeng, K.; Kim, M.J.; Jung, R.E.; Hämmerle, C.H.F.; Jung, U. Augmentation of keratinized tissue at tooth and implant sites by using autogenous grafts and collagen-based soft-tissue substitutes. J. Clin. Periodontol. 2019, 47, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Kim, S.; Waller, T.; Cha, J.-K.; Cho, S.-W.; Jung, U.-W.; Thoma, D.S. Soft tissue substitutes to increase gingival thickness: Histologic and volumetric analyses in dogs. J. Clin. Periodontol. 2018, 46, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gasser, T.J.W.; Jung, R.E.; Hämmerle, C.H.F. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, R.; García, V.; Orsini, M.; Martin, C.; Sanz, M. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: A randomized controlled prospective clinical trial. Clin. Oral Implant. Res. 2011, 23, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [Green Version]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef]
- Thoma, D.S.; Hämmerle, C.H.F.; Cochran, D.L.; Jones, A.A.; Görlach, C.; Uebersax, L.; Mathes, S.; Graf-Hausner, U.; Jung, R.E. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible - a histological analysis. J. Clin. Periodontol. 2011, 38, 1063–1070. [Google Scholar] [CrossRef]
- Imber, J.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, F.; Hu, Q.; Krebsbach, P.H. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials 2014, 35, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprinting – Bioprinting from benchside to bedside? Acta Biomater. 2020, 101, 14–25. [Google Scholar] [CrossRef]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef] [Green Version]
- Kuchler, U.; Rybaczek, T.; Dobask, T.; Heimel, P.; Tangl, S.; Klehm, J.; Menzel, M.; Gruber, R. Bone-conditioned medium modulates the osteoconductive properties of collagen membranes in a rat calvaria defect model. Clin. Oral Implant. Res. 2018, 29, 381–388. [Google Scholar] [CrossRef]
- Strauss, F.-J.; Kuchler, U.; Kobatake, R.; Heimel, P.; Tangl, S.; Gruber, R. Acid bone lysates reduce bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.C.E.; Omonte, S.V.; Martins, A.G.V.; De Castro, H.H.O.; Gomes, H.E.; Zenóbio, É.G.; De Oliveira, P.A.D.; Horta, M.C.R.; Souza, P.E.A. Hyaluronic acid on collagen membranes: An experimental study in rats. Arch. Oral Biol. 2017, 73, 214–222. [Google Scholar] [CrossRef]
- De Kok, I.J.; Jere, D.; Padilla, R.J.; Cooper, L.F. Evaluation of a collagen scaffold for cell-based bone repair. Int. J. Oral Maxillofac. Implant. 2014, 29, e122–e129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal Research: Reporting in vivo Experiments—The ARRIVE Guidelines. Br. J. Pharmacol. 2011, 31, 991–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasirzade, J.; Alccayhuaman, K.; Kargarpour, Z.; Kuchler, U.; Strauss, F.; Panahipour, L.; Kampleitner, C.; Heimel, P.; Schwarz, F.; Gruber, R. Acid Dentin Lysate Failed to Modulate Bone Formation in Rat Calvaria Defects. Biology 2021, 10, 196. [Google Scholar] [CrossRef]
- Feher, B.; Alccayhuaman, K.A.A.; Strauss, F.J.; Lee, J.-S.; Tangl, S.; Kuchler, U.; Gruber, R. Osteoconductive properties of upside-down bilayer collagen membranes in rat calvarial defects. Int. J. Implant. Dent. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Roschger, P.; Fratzl, P.; Eschberger, J.; Klaushofer, K. Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone 1998, 23, 319–326. [Google Scholar] [CrossRef]
- Hartmann, M.A.; Blouin, S.; Misof, B.M.; Fratzl-Zelman, N.; Roschger, P.; Berzlanovich, A.; Gruber, G.M.; Brugger, P.C.; Zwerina, J.; Fratzl, P. Quantitative Backscattered Electron Imaging of Bone Using a Thermionic or a Field Emission Electron Source. Calcif. Tissue Int. 2021, 1–13. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Mackie, E.; Ahmed, Y.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, U.E.; Reguzzoni, M.; Pagani, F.; Sibilia, V.; Congiu, T.; Salvi, A.G.; Benetti, A. Study of Endochondral Ossification in Human Fetalcartilage Anlagen of Metacarpals: Comparative Morphology of Mineral Deposition in Cartilage and in the Periosteal Bone Matrix. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2018, 301, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Manjubala, I.; Liu, Y.; Epari, D.; Roschger, P.; Schell, H.; Fratzl, P.; Duda, G. Spatial and temporal variations of mechanical properties and mineral content of the external callus during bone healing. Bone 2009, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.F.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Naenni, N.; Walter, P.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Augmentation of soft tissue volume at pontic sites: A comparison between a cross-linked and a non-cross-linked collagen matrix. Clin. Oral Investig. 2021, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Beaupré, G.S.; Giori, N.J.; A Helms, J. Mechanobiology of Skeletal Regeneration. Clin. Orthop. Relat. Res. 1998, 355S, S41–S55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahipour, L.; Kargarpour, Z.; Luza, B.; Lee, J.-S.; Gruber, R. TGF-β Activity Related to the Use of Collagen Membranes: In Vitro Bioassays. Int. J. Mol. Sci. 2020, 21, 6636. [Google Scholar] [CrossRef]
- Lee, J.-S.; Mitulović, G.; Panahipour, L.; Gruber, R. Proteomic Analysis of Porcine-Derived Collagen Membrane and Matrix. Materials 2020, 13, 5187. [Google Scholar] [CrossRef]
- Gao, L.; Orth, P.; Cucchiarini, M.; Madry, H. Autologous Matrix-Induced Chondrogenesis: A Systematic Review of the Clinical Evidence. Am. J. Sports Med. 2019, 47, 222–231. [Google Scholar] [CrossRef]
- Aldrian, S.; Zak, L.; Wondrasch, B.; Albrecht, C.; Stelzeneder, B.; Binder, H.; Kovar, F.; Trattnig, S.; Marlovits, S. Clinical and Radiological Long-term Outcomes After Matrix-Induced Autologous Chondrocyte Transplantation. Am. J. Sports Med. 2014, 42, 2680–2688. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. New Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Filardo, G.; Andriolo, L.; Angele, P.; Berruto, M.; Brittberg, M.; Condello, V.; Chubinskaya, S.; De Girolamo, L.; Di Martino, A.; Di Matteo, B.; et al. Scaffolds for Knee Chondral and Osteochondral Defects: Indications for Different Clinical Scenarios. A Consensus Statement. Cartilage 2020, 1947603519894729. [Google Scholar] [CrossRef]
- Xing, Z.; Jiang, X.; Si, Q.; Finne-Wistrand, A.; Liu, B.; Xue, Y.; Mustafa, K. Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model. Polymers 2021, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Åberg, T.; Rice, R.; Rice, D.; Thesleff, I.; Waltimo-Sirén, J. Chondrogenic Potential of Mouse Calvarial Mesenchyme. J. Histochem. Cytochem. 2005, 53, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2021, 21, e2000280. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Haidvogl, D.; Donath, K.; Watzek, G. Freeze-dried homogeneous and heterogeneous bone for sinus augmentation in sheep. Clin. Oral Implant. Res. 2002, 13, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Dobsak, T.; Heimel, P.; Tangl, S.; Schwarze, U.Y.; Schett, G.; Gruber, R. Impaired periodontium and temporomandibular joints in tumour necrosis factor-α transgenic mice. J. Clin. Periodontol. 2017, 44, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen-An, P.; Andreassen, K.V.; Henriksen, K.; Li, Y.; Karsdal, M.A.; Bay-Jensen, A.-C. The Inhibitory Effect of Salmon Calcitonin on Tri-Iodothyronine Induction of Early Hypertrophy in Articular Cartilage. PLoS ONE 2012, 7, e40081. [Google Scholar] [CrossRef] [Green Version]



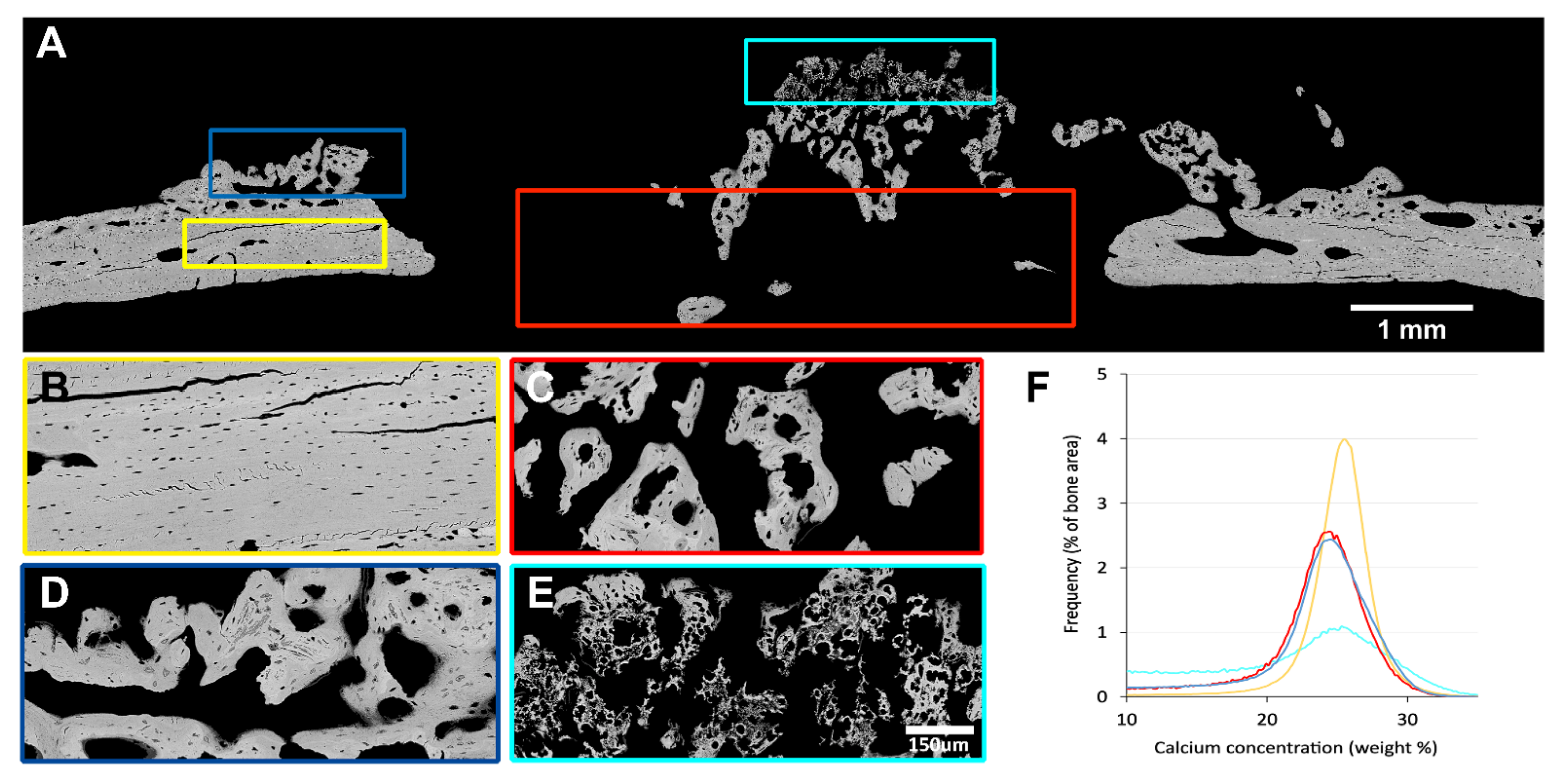

| CaMean (wt % Ca) | CaPeak (wt % Ca) | CaWidth (Δ wt % Ca) | |
|---|---|---|---|
| Cortical bone | 24.9 | 25.5 | 3.8 |
| New bone periost | 22.2 | 24.4 | 5.6 |
| New bone defect | 22.1 | 24.4 | 5.2 |
| Mineralized ECM | 17.8 | 25.1 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alccayhuaman, K.A.A.; Tangl, S.; Blouin, S.; Hartmann, M.A.; Heimel, P.; Kuchler, U.; Lee, J.-S.; Gruber, R. Osteoconductive Properties of a Volume-Stable Collagen Matrix in Rat Calvaria Defects: A Pilot Study. Biomedicines 2021, 9, 732. https://doi.org/10.3390/biomedicines9070732
Alccayhuaman KAA, Tangl S, Blouin S, Hartmann MA, Heimel P, Kuchler U, Lee J-S, Gruber R. Osteoconductive Properties of a Volume-Stable Collagen Matrix in Rat Calvaria Defects: A Pilot Study. Biomedicines. 2021; 9(7):732. https://doi.org/10.3390/biomedicines9070732
Chicago/Turabian StyleAlccayhuaman, Karol Alí Apaza, Stefan Tangl, Stéphane Blouin, Markus A. Hartmann, Patrick Heimel, Ulrike Kuchler, Jung-Seok Lee, and Reinhard Gruber. 2021. "Osteoconductive Properties of a Volume-Stable Collagen Matrix in Rat Calvaria Defects: A Pilot Study" Biomedicines 9, no. 7: 732. https://doi.org/10.3390/biomedicines9070732







