Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review
Abstract
1. Introduction
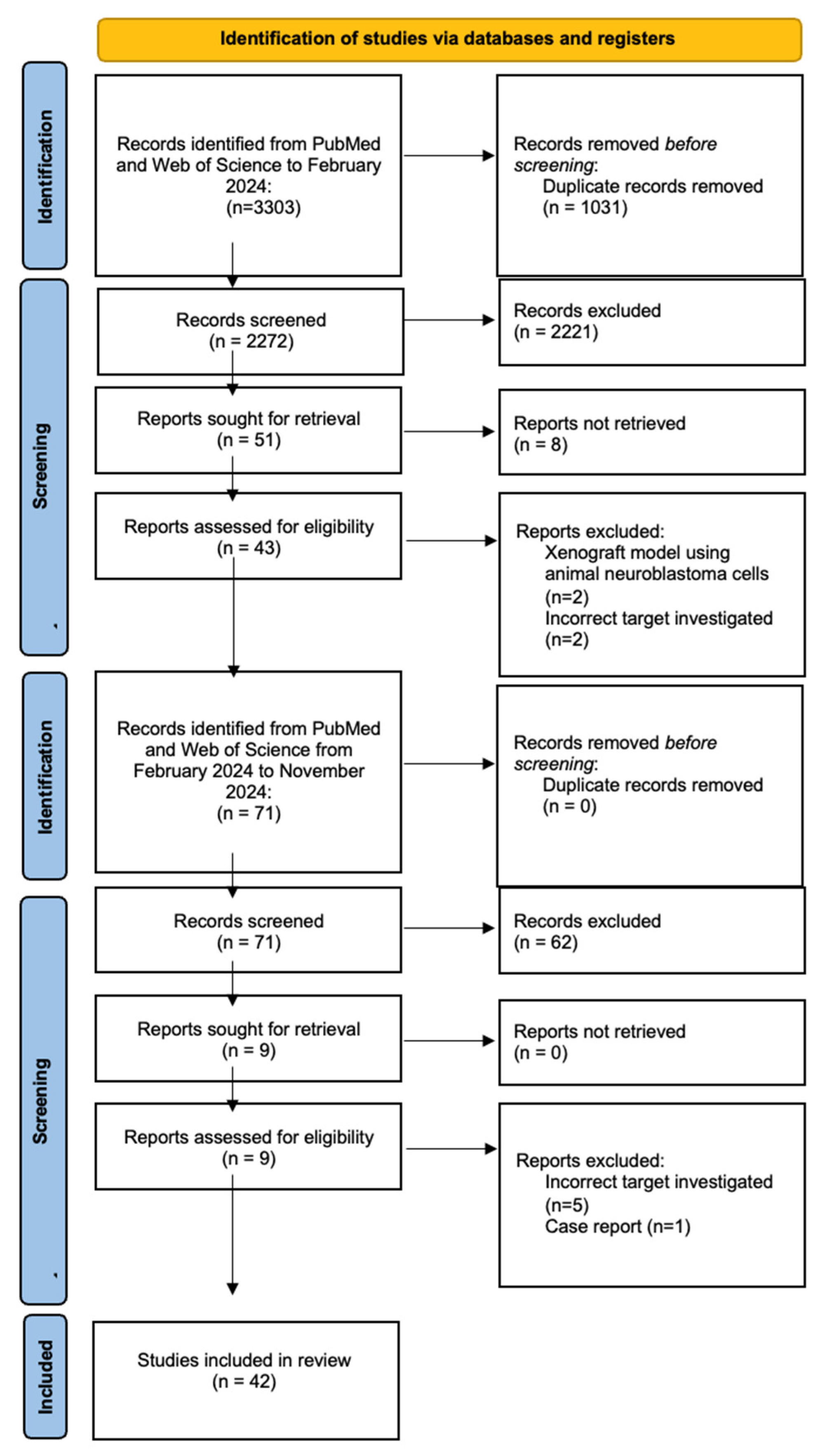
2. Materials and Methods
- Study characteristics: authors, year, country of study, study design, and FMP or target investigated.
- Patient demographics: patient cohort size, age range, sex, number of tumours, tumour location, and tumour stage and grade.
- Pre-clinical study features: sample type (cell lines, tissue samples, xenograft models using human neuroblastoma cells), sample size, study aim, methods, results, and conclusion.
3. Results
3.1. Clinically Investigated FMPs or Targets
3.1.1. Studies Involving Approved Probes
ICG
3.1.2. Studies Involving Probes/Targets in Phase 1–2 Trials
PARP
3.2. Pre-Clinically Investigated FMPs or Targets
3.2.1. Studies Involving Approved Probes or Their Targets
5-Aminolevulinic Acid (5-ALA)
LUM015 and Cathepsins
3.2.2. Studies Involving Probes/Targets in Phase 3 Trials
3.2.3. Studies Involving Probes/Targets in Phase 2 Trials
Epidermal Growth Factor Receptor (EGFR)
Vascular Endothelial Growth Factor Receptor (VEGFR)
Integrins
Matrix Metalloproteinases (MMPs)
Annexin A2
3.2.4. Studies Involving Probes/Targets in Phase 1 Trials
Gastrin-Releasing Peptide Receptor (GRPR)
Carbonic Anhydrase IX (CAIX)
3.3. Clinical Study Quality and Risk of Bias Assessment
4. Discussion
4.1. Future Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FGS | Fluorescence-guided surgery |
| FMP | Fluorescent molecular probes |
| ICG | Indocyanine green |
| 5-ALA | 5-aminolevulinic acid |
| PARP | Poly-ADP ribose polymerase |
| VEGFR | Vascular endothelial growth factor receptor |
| EGFR | Endothelial growth factor receptor |
| EPR | Enhanced permeability and retention |
| CTS | Cathepsin |
| ITG | Integrin |
| GRPR | Gastrin-releasing peptide receptor |
| CAIX | Carbonic anhydrase IX |
| MMP | Matrix metalloproteinase |
References
- Nong, J.; Su, C.; Li, C.; Wang, C.; Li, W.; Li, Y.; Chen, P.; Li, Y.; Li, Z.; She, X.; et al. Global, Regional, and National Epidemiology of Childhood Neuroblastoma (1990–2021): A Statistical Analysis of Incidence, Mortality, and DALYs. eClinicalMedicine 2025, 79, 102964. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and Biological Approach to Risk Stratification and Treatment. Cell Tissue Res. 2018, 372, 195. [Google Scholar] [CrossRef]
- Ara, T.; DeClerck, Y.A. Mechanisms of Invasion and Metastasis in Human Neuroblastoma. Cancer Metastasis Rev. 2006, 25, 645–657. [Google Scholar] [CrossRef]
- Holmes, K.; Pötschger, U.; Pearson, A.D.J.; Sarnacki, S.; Cecchetto, G.; Gomez-Chacon, J.; Squire, R.; Freud, E.; Bysiek, A.; Matthyssens, L.E.; et al. Influence of Surgical Excision on the Survival of Patients With Stage 4 High-Risk Neuroblastoma: A Report From the HR-NBL1/SIOPEN Study. J. Clin. Oncol. 2020, 38, 2902–2915. [Google Scholar] [CrossRef]
- Heneweer, C.; Holland, J.P.; Divilov, V.; Carlin, S.; Lewis, J.S. Magnitude of Enhanced Permeability and Retention Effect in Tumors with Different Phenotypes: 89Zr-Albumin as a Model System. J. Nucl. Med. 2011, 52, 625. [Google Scholar] [CrossRef]
- Preziosi, A.; Paraboschi, I.; Giuliani, S. Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework. Children 2023, 10, 689. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green-Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.H.; Mothi, S.S.; Pio, L.; Mori, M.; Santiago, T.C.; McCarville, M.B.; Kaste, S.C.; Pappo, A.S.; Talbot, L.J.; Murphy, A.J.; et al. Feasibility of Indocyanine Green-Guided Localization of Pulmonary Nodules in Children with Solid Tumors. Pediatr. Blood Cancer 2023, 70, e30437. [Google Scholar] [CrossRef]
- Ak, A.; Kaya, Ö.; Coşan, D.T.; Gülsoy, M. Cytotoxic Effects of Different ICG Concentrations and Laser Parameters on Neuroblastoma. Acta Phys. Pol. A 2015, 128, B-381–B-383. [Google Scholar] [CrossRef]
- Ameis, H.M.; Drenckhan, A.; Freytag, M.; Izbicki, J.R.; Supuran, C.T.; Reinshagen, K.; Holland-Cunz, S.; Gros, S.J. Carbonic Anhydrase IX Correlates with Survival and Is a Potential Therapeutic Target for Neuroblastoma. J. Enzyme Inhib. Med. Chem. 2016, 31, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Fukuzawa, M.; Kusafuka, T.; Komoto, Y.; Oue, T.; Inoue, M.; Okada, A. Immunohistochemical Expression of MMP-2, MMP-9, and TIMP-2 in Neuroblastoma: Association with Tumor Progression and Clinical Outcome. J. Pediatr. Surg. 1998, 33, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Ashok, G.; Miryala, S.K.; Anbarasu, A.; Ramaiah, S. Integrated Systems Biology Approach Using Gene Network Analysis to Identify the Important Pathways and New Potential Drug Targets for Neuroblastoma. Gene Rep. 2021, 23, 101101. [Google Scholar] [CrossRef]
- Bjørnland, K.; Bratland, Å.; Rugnes, E.; Pettersen, S.; Johansen, H.T.; Aasen, A.O.; Fodstad, Ø.; Ree, A.H.; Mælandsmo, G.M. Expression of Matrix Metalloproteinases and the Metastasis-Associated Gene S100A4 in Human Neuroblastoma and Primitive Neuroectodermal Tumor Cells. J. Pediatr. Surg. 2001, 36, 1040–1044. [Google Scholar] [CrossRef]
- Colicchia, V.; Petroni, M.; Guarguaglini, G.; Sardina, F.; Sahún-Roncero, M.; Carbonari, M.; Ricci, B.; Heil, C.; Capalbo, C.; Belardinilli, F.; et al. PARP Inhibitors Enhance Replication Stress and Cause Mitotic Catastrophe in MYCN-Dependent Neuroblastoma. Oncogene 2017, 36, 4682–4691. [Google Scholar] [CrossRef]
- Di Giulio, S.; Colicchia, V.; Pastorino, F.; Pedretti, F.; Fabretti, F.; Nicolis di Robilant, V.; Ramponi, V.; Scafetta, G.; Moretti, M.; Licursi, V.; et al. A Combination of PARP and CHK1 Inhibitors Efficiently Antagonizes MYCN-Driven Tumors. Oncogene 2021, 40, 6143–6152. [Google Scholar] [CrossRef]
- Du, X.; Ding, L.; Huang, S.; Li, F.; Yan, Y.; Tang, R.; Ding, X.; Zhu, Z.; Wang, W. Cathepsin L Promotes Chemresistance to Neuroblastoma by Modulating Serglycin. Front. Pharmacol. 2022, 13, 920022. [Google Scholar] [CrossRef]
- Favrot, M.C.; Combaret, V.; Goillot, E.; Lutz, P.; Frappaz, D.; Thiesse, P.; Thyss, A.; Dolbeau, D.; Bouffet, E.; Tabone, E.; et al. Expression of Integrin Receptors on 45 Clinical Neuroblastoma Specimens. Int. J. Cancer 1991, 49, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Keerthikumar, S.; Fonseka, P.; Edgington, L.E.; Ang, C.S.; Ozcitti, C.; Bogyo, M.; Parker, B.S.; Mathivanan, S. Inhibition of Cathepsin Proteases Attenuates Migration and Sensitizes Aggressive N-Myc Amplified Human Neuroblastoma Cells to Doxorubicin. Oncotarget 2015, 6, 11175. [Google Scholar] [CrossRef]
- Harris, A.C.; Choudhury, S.; Pachl, M. Early Results of Minimally Invasive Fluorescent Guided Pediatric Oncology Surgery with Delivery of Indocyanine Green during Induction of Anesthesia. Photodiagnosis Photodyn. Ther. 2023, 42, 103639. [Google Scholar] [CrossRef]
- Izycka-Swieszewska, E.; Brzeskwiniewicz, M.; Wozniak, A.; Drozynska, E.; Grajkowska, W.; Perek, D.; Balcerska, A.; Klepacka, T.; Limon, J. EGFR, PIK3CA and PTEN Gene Status and Their Protein Product Expression in Neuroblastic Tumours. Folia Neuropathol. 2010, 48, 238–245. [Google Scholar]
- Keller, K.M.; Koetsier, J.; Schild, L.; Amo-Addae, V.; Eising, S.; van den Handel, K.; Ober, K.; Koopmans, B.; Essing, A.; van den Boogaard, M.L.; et al. The Potential of PARP as a Therapeutic Target across Pediatric Solid Malignancies. BMC Cancer 2023, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hu, W.; Kelly, D.R.; Hellmich, M.R.; Evers, B.M.; Chung, D.H. Gastrin-Releasing Peptide Is a Growth Factor for Human Neuroblastomas. Ann. Surg. 2002, 235, 621–630. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Li, X.D.; Almeida, G.S.; Kwok, C.; Gravells, P.; Harrison, D.; Burke, S.; Hallsworth, A.; Jamin, Y.; George, S.; et al. MYCN Expression Induces Replication Stress and Sensitivity to PARP Inhibition in Neuroblastoma. Oncotarget 2020, 11, 2141–2159. [Google Scholar] [CrossRef] [PubMed]
- Langer, I.; Vertongen, P.; Perret, J.; Fontaine, J.; Atassi, G.; Robberecht, P. Expression of Vascular Endothelial Growth Factor (VEGF) and VEGF Receptors in Human Neuroblastomas. Med Pediatr. Oncol. 2000, 34, 386–393. [Google Scholar] [CrossRef]
- Makvandi, M.; Lee, H.; Puentes, L.N.; Reilly, S.W.; Rathi, K.S.; Weng, C.C.; Chan, H.S.; Hou, C.; Raman, P.; Martinez, D.; et al. Targeting PARP-1 with Alpha-Particles Is Potently Cytotoxic to Human Neuroblastoma in Preclinical Models. Mol. Cancer Ther. 2019, 18, 1195–1204. [Google Scholar] [CrossRef]
- Marcus, K.; Johnson, M.; Adam, R.M.; O’Reilly, M.S.; Donovan, M.; Atala, A.; Freeman, M.R.; Soker, S. Tumor Cell-Associated Neuropilin-1 and Vascular Endothelial Growth Factor Expression as Determinants of Tumor Growth in Neuroblastoma. Neuropathology 2005, 25, 178–187. [Google Scholar] [CrossRef]
- McNeil, E.M.; Ritchie, A.M.; Melton, D.W. The Toxicity of Nitrofuran Compounds on Melanoma and Neuroblastoma Cells Is Enhanced by Olaparib and Ameliorated by Melanin Pigment. DNA Repair 2013, 12, 1000–1006. [Google Scholar] [CrossRef]
- Meister, B.; Grünebach, F.; Bautz, F.; Brugger, W.; Fink, F.M.; Kanz, L.; Möhle, R. Expression of Vascular Endothelial Growth Factor (VEGF) and Its Receptors in Human Neuroblastoma. Eur. J. Cancer 1999, 35, 445–449. [Google Scholar] [CrossRef]
- Meyer, A.; Van Golen, C.M.; Kim, B.; Van Golent, K.L.; Feldman, E.L. Integrin Expression Regulates Neuroblastoma Attachment and Migration. Neoplasia 2004, 6, 332–342. [Google Scholar] [CrossRef]
- Navarro, N.; Molist, C.; Sansa-Girona, J.; Zarzosa, P.; Gallo-Oller, G.; Pons, G.; Magdaleno, A.; Guillén, G.; Hladun, R.; Garrido, M.; et al. Integrin Alpha9 Emerges as a Key Therapeutic Target to Reduce Metastasis in Rhabdomyosarcoma and Neuroblastoma. Cell Mol. Life Sci. 2022, 79, 546. [Google Scholar] [CrossRef]
- Norris, R.E.; Adamson, P.C.; Nguyen, V.T.; Fox, E. Preclinical Evaluation of the PARP Inhibitor, Olaparib, in Combination with Cytotoxic Chemotherapy in Pediatric Solid Tumors. Pediatr. Blood Cancer 2014, 61, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kang, J.; Ishola, T.A.; Rychahou, P.G.; Evers, B.M.; Chung, D.H. Gastrin-Releasing Peptide Receptor Silencing Suppresses the Tumorigenesis and Metastatic Potential of Neuroblastoma. Proc. Natl. Acad. Sci. USA 2008, 105, 12891–12896. [Google Scholar] [CrossRef] [PubMed]
- Ramani, P.; Nash, R.; Radevsky, L.; Patel, A.; Luckett, M.; Rogers, C. VEGF-C, VEGF-D and VEGFR-3 Expression in Peripheral Neuroblastic Tumours. Histopathology 2012, 61, 1006–1016. [Google Scholar] [CrossRef]
- Sagulenko, V.; Muth, D.; Sagulenko, E.; Paffhausen, T.; Schwab, M.; Westermann, F. Cathepsin D Protects Human Neuroblastoma Cells from Doxorubicin-Induced Cell Death. Carcinogenesis 2008, 29, 1869–1877. [Google Scholar] [CrossRef]
- Sebesta, J.A.; Young, A.; Bullock, J.; Moore, K.H.; Azarow, K.; Sawin, R.S. Gastrin-Releasing Peptide: A Potential Growth Factor Expressed in Human Neuroblastoma Tumors. Curr. Surg. 2001, 58, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Secomandi, E.; Salwa, A.; Vidoni, C.; Ferraresi, A.; Follo, C.; Isidoro, C. High Expression of the Lysosomal Protease Cathepsin D Confers Better Prognosis in Neuroblastoma Patients by Contrasting EGF-Induced Neuroblastoma Cell Growth. Int. J. Mol. Sci. 2022, 23, 4782. [Google Scholar] [CrossRef]
- Takagi, M.; Yoshida, M.; Nemoto, Y.; Tamaichi, H.; Tsuchida, R.; Seki, M.; Uryu, K.; Nishii, R.; Miyamoto, S.; Saito, M.; et al. Loss of DNA Damage Response in Neuroblastoma and Utility of a PARP Inhibitor. J. Natl. Cancer Inst. 2017, 109, djx062. [Google Scholar] [CrossRef]
- Takagi, M.; Ogawa, C.; Iehara, T.; Aoki-Nogami, Y.; Ishibashi, E.; Imai, M.; Kimura, T.; Nagata, M.; Yasuhara, M.; Masutani, M.; et al. First Phase 1 Clinical Study of Olaparib in Pediatric Patients with Refractory Solid Tumors. Cancer 2022, 128, 2949–2957. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Cai, Y.; Cai, Y.; Yuan, X.; Wang, L.; Wu, Z.; Wu, Y. Annexin A2 Could Enhance Multidrug Resistance by Regulating NF-ΚB Signaling Pathway in Pediatric Neuroblastoma. J. Exp. Clin. Cancer Res. 2017, 36, 111. [Google Scholar] [CrossRef]
- Wang, W.; Song, B.; Zhang, Z. The Roles of MMP-2, MMP-9, TIMP-1, and TIMP-2 in Neuroblastoma. Int. J. Clin. Exp. Med. 2020, 13, 5259–5266. [Google Scholar]
- Watanabe, T.; Nishio, Y.; Yamamoto, Y.; Shimizu, T.; Li, X.K.; Okita, H.; Kuroda, T. Photodynamic Therapy with 5-Aminolevulinic Acid: A New Diagnostic, Therapeutic, and Surgical Aid for Neuroblastoma. J. Pediatr. Surg. 2022, 57, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, F.; Ma, T.; Zeng, Z.; Zhang, N. MiR-338-3p Inhibits Cell Growth, Invasion, and EMT Process in Neuroblastoma through Targeting MMP-2. Open Life Sci. 2021, 16, 198–209. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhang, H.; Jiang, Z.; Lu, H.-T.; Chen, X.; Dong, Q. MiRNA-7 Targets Cathepsin K to Inhibit Invasion and Metastasis of Neuroblastoma Cells. Int. J. Clin. Exp. Pathol. 2017, 10, 5129–5140. [Google Scholar]
- Zhang, S.; Jiang, C.; Su, Y.; Gui, J.; Yue, Z.; Jian, B.; He, S.; Ma, X. Nectin2 Influences Cell Apoptosis by Regulating ANXA2 Expression in Neuroblastoma: Nectin2 Influences SH-SY5Y Cell Migration and Apoptosis. Acta Biochim. Biophys. Sin. 2023, 55, 356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shen, R.; Li, K.; Zheng, N.; Zong, Y.; Ye, D.; Wang, Q.; Wang, Z.; Chen, L.; Ma, Y. Epidermal Growth Factor Receptor Is Overexpressed in Neuroblastoma Tissues and Cells. Acta Biochim. Biophys. Sin. 2016, 48, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, L.; Bleibaum, F.; Voss, M.; Schweizer, M.; Spengler, K.; Winter, D.; Zöphel, F.; Müller, S.; Lichtenthaler, S.; Damme, M.; et al. Cellular Depletion of Major Cathepsin Proteases Reveals Their Concerted Activities for Lysosomal Proteolysis. Cell Mol. Life Sci. 2024, 81, 227. [Google Scholar] [CrossRef]
- Lai, X.; Yoda, H.; Qiao, Y.; Kida, Y.; Takenaga, K.; Shinozaki, Y.; Koshikawa, N.; Takatori, A. Poly (ADP-Ribose) Polymerase Inhibitor Sensitized DNA Damage Caused by an Alkylating Pyrrole-Imidazole Polyamide Targeting MYCN in Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2024, 735, 150794. [Google Scholar] [CrossRef]
- Secomandi, E.; Esposito, A.; Camurani, G.; Vidoni, C.; Salwa, A.; Lualdi, C.; Vallino, L.; Ferraresi, A.; Isidoro, C. Differential Competitive Growth of Transgenic Subclones of Neuroblastoma Cells Expressing Different Levels of Cathepsin D Co-Cultured in 2D and 3D in Response to EGF: Implications in Tumor Heterogeneity and Metastasis. Cancers 2024, 16, 1343. [Google Scholar] [CrossRef]
- Sutton, P.A.; van Dam, M.A.; Cahill, R.A.; Mieog, S.; Polom, K.; Vahrmeijer, A.L.; van der Vorst, J. Fluorescence-Guided Surgery: Comprehensive Review. BJS Open 2023, 7, zrad049. [Google Scholar] [CrossRef]
- Rijs, Z.; Jeremiasse, B.; Shifai, N.; Gelderblom, H.; Sier, C.F.M.; Vahrmeijer, A.L.; van Leeuwen, F.W.B.; van der Steeg, A.F.W.; van de Sande, M.A.J. Introducing Fluorescence-Guided Surgery for Pediatric Ewing, Osteo-, and Rhabdomyosarcomas: A Literature Review. Biomedicines 2021, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.; Lim, D.-K.; Joo, M.K.; Kim, K.; Kim, J.; Cho, H.; Lim, D.-K.; Joo, M.K.; Kim, K. Perspectives for Improving the Tumor Targeting of Nanomedicine via the EPR Effect in Clinical Tumors. Int. J. Mol. Sci. 2023, 24, 10082. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Shah, N.R.; Chukkapalli, S.; King, S.; Grant, C.N.; Brown, E.G.; Avanzini, S.; Lal, D.R.; Sarnacki, S.; Newman, E.A. Modern Surgical Strategies in Pediatric Neuroblastoma: Evolving Approaches and Treatment Principles. Pediatr. Blood Cancer 2024, 72, e31317. [Google Scholar] [CrossRef]
- Vollmer, K.; Gfroerer, S.; Theilen, T.M.; Bochennek, K.; Klingebiel, T.; Rolle, U.; Fiegel, H. Radical Surgery Improves Survival in Patients with Stage 4 Neuroblastoma. World J. Surg. 2018, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
| Probe | Type of FMP | Target/Activator | FDA Approval/Clinical Trial Status | Trial Number | Cancer Studied In |
|---|---|---|---|---|---|
| ICG * | Non-specific dye | - | Approved | - | Gastrointestinal cancers, hepatobiliary cancer, breast cancer, gynaecological cancers, head and neck cancer, lung cancer, paediatric cancers (including neuroblastoma) |
| Methylene blue | Non-specific | - | Approved | - | Breast cancer, neuroendocrine tumours, colorectal cancer |
| Fluoroscein | Non-specific | - | Approved | - | CNS cancer |
| 5-aminolevulinic acid * | Non-specific | - | Approved | - | Head and neck cancer, CNS cancer |
| Pafolacianine (OTL38) | Targeted | Folate receptor alpha | Approved | - | Ovarian cancer, lung cancer |
| Lumicell (LUM015) * | Activatable | Cathepsin | Approved | - | Breast cancer, sarcoma |
| Surgimab-101 | Targeted | CEA | Phase III | NCT03659448 | Colorectal cancer |
| Folate fluorescein isothiocyanate (EC17) | Targeted | Folate receptor | Phase III | 2017-004557-17, NCT01511055 | Ovarian cancer, breast cancer, renal cell carcinoma, lung cancer |
| Panitumumab-IRDye800CW * | Targeted | EGFR | Phase II | NCT04511078, NCT03384238 | Head and neck cancer, pancreatic cancer |
| Bevacizumab IRDye800CW * | Targeted | VEGFR | Phase II | NCT05359874 | Breast cancer, thyroid cancer, rectal cancer, sarcoma, cholangiocarcinoma, oesophageal cancer, pancreatic cancer, head and neck |
| cRGD-ZW800-1 * | Targeted | Integrins | Phase II | NCT05518071, NCT04191460, NCT05752149 | Pancreas adenocarcinoma, head and neck cancer, laryngeal cancer |
| BLZ-100 * | Targeted | Annexin A2, MMP2 | Phase II | NCT02234297, NCT02097875, NCT03579602 | Glioma, skin neoplasms, oral cavity SCC, paediatric CNS tumours |
| Onconano medicine | Targeted | ONM-100 | Phase II | NCT03735680 | Breast cancer, head and neck SCC, colorectal, prostate, ovarian, urothelial, non-small cell lung cancer |
| AVB-620 * | Activatable | MMP2 and 9 | Phase II | NCT03113825 | Breast cancer |
| EMI-137 | Targeted | c-MET | Phase II | NCT03360461, NCT03470259, NCT03205501 | Colorectal cancer, Thyroid cancer, oesophageal cancer |
| Cetuximab-IRDye800CW * | Targeted | EGFR | Phase I-II | NCT02855086, NCT02736578 (terminated) | Glioma, pancreatic cancer |
| cRGDY-PEG-Cy5.5 nanoparticles * | Targeted | Integrins | Phase I–II | NCT02106598 | Head and neck cancer, melanoma |
| PARPi-FL * | Targeted | PARP | Phase I–II | NCT03085147, NCT03631017 | Oral SCC, head and neck cancer |
| VST-1001 (dilute fluorescein) | Non-specific | - | Phase I–II | NCT02294565 | Breast cancer |
| [111In]In-DOTA-Labetuzumab-IRDye800CW | Targeted | CEA | Phase I–II | NCT03699332 | Colorectal cancer |
| Nimotuzumab-IRDye800CW * | Targeted | EGFR | Phase I–II | NCT04459065 | Lung cancer |
| VST-1001 (dilute fluorescein) | Non-specific | - | Phase I–II | NCT02294565 | Breast cancer |
| [111In]In-DOTA-Labetuzumab-IRDye800CW | Targeted | CEA | Phase I–II | NCT03699332 | Colorectal cancer |
| VGT-309 * | Activatable | Cathepsin | Phase I | NCT05400226, NCT06145048, NCT06034197 | Lung cancer, colorectal cancer |
| 111In-DOTA-girentuximab IRDye800Cw * | Targeted | CAIX | Phase I | NCT02497599 | Renal cell carcinoma |
| FluoAB | Non-specific | - | Phase I | NCT05394246 | Liver cancer |
| 68Ga-BBN-IRDye800CW * | Targeted | GRPR | Phase I | NCT03407781, NCT02910804 | Glioma, glioblastoma |
| QRHKPRE-Cy5 * | Targeted | EGFR | Phase I | NCT02574858 | N/A–healthy adults |
| RD0Cy7 fluorophore | Targeted | ITGA6 | Phase I | NCT06204835 | Hepatocellular carcinoma |
| Author | Year | Study Design | Type of Sample | Fluorescent Dye/Probe | Target | Reference |
|---|---|---|---|---|---|---|
| Papers Investigating Probe Accumulation Within Tumour Tissue | ||||||
| Abdelhafeez et al. | 2021 | Clinical | Patients | ICG | Untargeted | [8] |
| Abdelhafeez et al. | 2023 | Clinical | Patients | ICG | Untargeted | [9] |
| Ak et al. | 2015 | Preclinical | Cell lines | ICG | Untargeted | [10] |
| Di Giulio et al. | 2021 | Preclinical | Cell lines | PARPi | PARP | [16] |
| Harris et al. | 2023 | Clinical | Patients | ICG | Untargeted | [20] |
| King et al. | 2020 | Preclinical | Cell lines | PARPi | PARP | [24] |
| Keller et al. | 2023 | Preclinical | Genomics of Drug Sensitivity in Cancer database; cell lines | PARPi | PARP | [22] |
| Lai et al. | 2024 | Preclinical | Cell lines | PARPi | PARP | [48] |
| McNeil et al. | 2013 | Preclinical | Cell lines | PARPi | PARP | [28] |
| Norris et al. | 2014 | Preclinical | Cell lines, xenograft models | PARPi | PARP | [32] |
| Takagi et al. | 2017 | Preclinical | Tissue samples, cell lines, xenograft models | PARPi | PARP | [38] |
| Takagi et al. | 2022 | Clinical | Patients | PARPi | PARP | [39] |
| Watanabe et al. | 2022 | Preclinical | Cell lines | 5-ALA | Untargeted | [42] |
| Papers Investigating Target Expression Within Tumour Samples | ||||||
| Ameis et al. | 2015 | Preclinical | Tissue samples, cell lines | 111In-DOTA-girentuximab IRDye800Cw | CAIX | [11] |
| Ara et al. | 1998 | Preclinical | Tissue samples | BLZ-100, AVB-620 | MMP-2 | [12] |
| Ashok et al. | 2021 | Preclinical | Cancer genetics web database | Bevacizumab IRDye800CW | VEGF/VEGFR | [13] |
| Bjornland et al. | 2001 | Preclinical | Cell lines | BLZ-100, AVB-620 | MMP-2 | [14] |
| Colicchia et al. | 2017 | Preclinical | Cell lines | PARPi-FL | PARP | [15] |
| Du et al. | 2022 | Preclinical | Cell lines, xenograft models | Lumicell (LUM015), VGT-309 | Cathepsin | [17] |
| Favrot et al. | 1991 | Preclinical | Tissue samples | cRGD-ZW800-1, cRGDY-PEG-Cy5.5 nanoparticles, RD0Cy7 fluorophore | Integrins | [18] |
| Gallwitz et al. | 2024 | Preclinical | Cell lines | Lumicell (LUM015), VGT-309 | Cathepsin | [47] |
| Gangoda et al. | 2015 | Preclinical | Cell lines | Lumicell (LUM015), VGT-309 | Cathepsin | [19] |
| Izycka-Swieszewska et al. | 2010 | Preclinical | Tissue samples | Panitumumab-IRDye800CW, Cetuximab-IRDye800CW, Nimotuzumab-IRDye800CW, QRHKPRE-Cy5 | EGFR | [21] |
| Kim et al. | 2002 | Preclinical | Cell lines, tissue samples | 68Ga-BBN-IRDye800CW | GRP/GRPR | [23] |
| Langer et al. | 2000 | Preclinical | Cell lines, tissue samples | Bevacizumab IRDye800CW | VEGF/VEGFR | [25] |
| Makvandi et al. | 2019 | Preclinical | Tissue samples | PARPi-FL | PARP-1 | [26] |
| Marcus et al. | 2005 | Preclinical | Cell lines, xenograft models, tissue samples | Bevacizumab IRDye800CW | VEGF/VEGFR | [27] |
| Meister et al. | 1999 | Preclinical | Cell lines, tissue samples | Bevacizumab IRDye800CW | VEGF/VEGFR | [29] |
| Meyer et al. | 2004 | Preclinical | Cell lines | cRGD-ZW800-1, cRGDY-PEG-Cy5.5 nanoparticles, RD0Cy7 fluorophore | Integrins | [30] |
| Navarro et al. | 2022 | Preclinical | Cell lines, xenograft models | cRGD-ZW800-1, cRGDY-PEG-Cy5.5 nanoparticles, RD0Cy7 fluorophore | Integrins | [31] |
| Qiao et al. | 2008 | Preclinical | Cell lines, xenograft models | 68Ga-BBN-IRDye800CW | GRPR | [33] |
| Ramani et al. | 2012 | Preclinical | Tissue samples | Bevacizumab IRDye800CW | VEGFR | [34] |
| Sagulenko et al. | 2008 | Preclinical | Cell lines | Lumicell (LUM015), VGT-309 | Cathepsin | [35] |
| Sebesta et al. | 2001 | Preclinical | Tissue samples | 68Ga-BBN-IRDye800CW | GRP/GRPR | [36] |
| Secomandi et al. | 2022 | Preclinical | Cell lines | Lumicell (LUM015), VGT-309 | Cathepsin D | [37] |
| Secomandi et al. | 2024 | Preclinical | Cell lines | Lumicell (LUM015), VGT-309 | Cathepsin D | [49] |
| Wang et al. | 2017 | Preclinical | Cell lines | BLZ-100 | Annexin A2 | [40] |
| Wang et al. | 2020 | Preclinical | Tissue samples | BLZ-100, AVB-620 | MMP2 | [41] |
| Yuan et al. | 2021 | Preclinical | Cell lines, tissue samples | BLZ-100, AVB-620 | MMP-2 | [43] |
| Zhang et al. | 2017 | Preclinical | Cell lines, tissue samples, xenograft mouse models | Lumicell (LUM015), VGT-309 | Cathepsin | [44] |
| Zhang et al. | 2023 | Preclinical | Patient venous blood samples, cell lines | BLZ-100 | Annexin A2 | [45] |
| Zheng et al. | 2016 | Preclinical | Cell lines, tissue samples | Panitumumab-IRDye800CW, Cetuximab-IRDye800CW, Nimotuzumab-IRDye800CW, QRHKPRE-Cy5 | EGFR | [46] |
| First Author and Year | Study Design | Probe Investigated | Neuroblastoma Patient Cohort | Age Range (Years) | Reference |
|---|---|---|---|---|---|
| Abdelhafeez, 2021 | Retrospective cohort study | ICG | 6 | <1–21 | [8] |
| Abdelhafeez, 2023 | Phase I trial | ICG | 1 | <1–23 | [9] |
| Harris, 2023 | Phase I trial | ICG | 3 | <1–4.1 | [20] |
| Takagi, 2022 | Phase I trial | Olaparib (PARPi) | 6 | 3–18 | [39] |
| First Author and Year | Study Design | ROB Assessment Tool | ROB Assessment Grading | Reference |
|---|---|---|---|---|
| Abdelhafeez, 2021 | Retrospective cohort study | ROBINS-I | Serious | [8] |
| Abdelhafeez, 2023 | Single-centre, open-label, nonrandomised, prospective clinical trial | ROBINS-I | Serious | [9] |
| Harris, 2023 | Single-centre, open-label, nonrandomised, prospective clinical trial | ROBINS-I | Serious | [20] |
| Takagi, 2022 | Multi-centre, open-label, nonrandomised, prospective clinical trial | ROBINS-I | Serious | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennessy, M.; Neville, J.J.; Privitera, L.; Sedgwick, A.; Anderson, J.; Giuliani, S. Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review. Children 2025, 12, 550. https://doi.org/10.3390/children12050550
Hennessy M, Neville JJ, Privitera L, Sedgwick A, Anderson J, Giuliani S. Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review. Children. 2025; 12(5):550. https://doi.org/10.3390/children12050550
Chicago/Turabian StyleHennessy, Megan, Jonathan J. Neville, Laura Privitera, Adam Sedgwick, John Anderson, and Stefano Giuliani. 2025. "Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review" Children 12, no. 5: 550. https://doi.org/10.3390/children12050550
APA StyleHennessy, M., Neville, J. J., Privitera, L., Sedgwick, A., Anderson, J., & Giuliani, S. (2025). Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review. Children, 12(5), 550. https://doi.org/10.3390/children12050550







