Biomass-Derived Carbon/Sulfur Composite Cathodes with Multiwalled Carbon Nanotube Coatings for Li-S Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of RC
2.2. Preparation of RCS
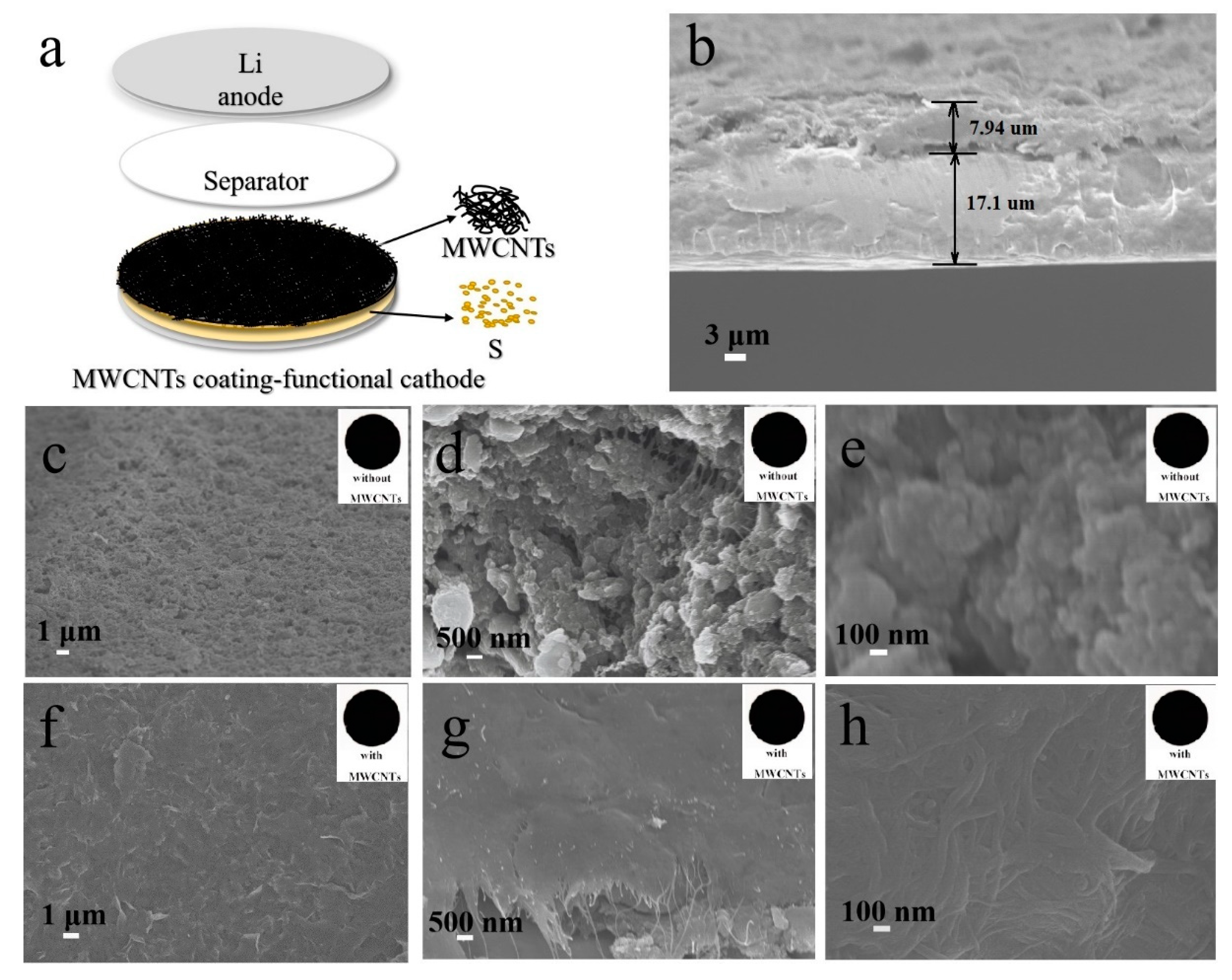
2.3. Preparation of Cathodes with MWCNT Protective Coatings
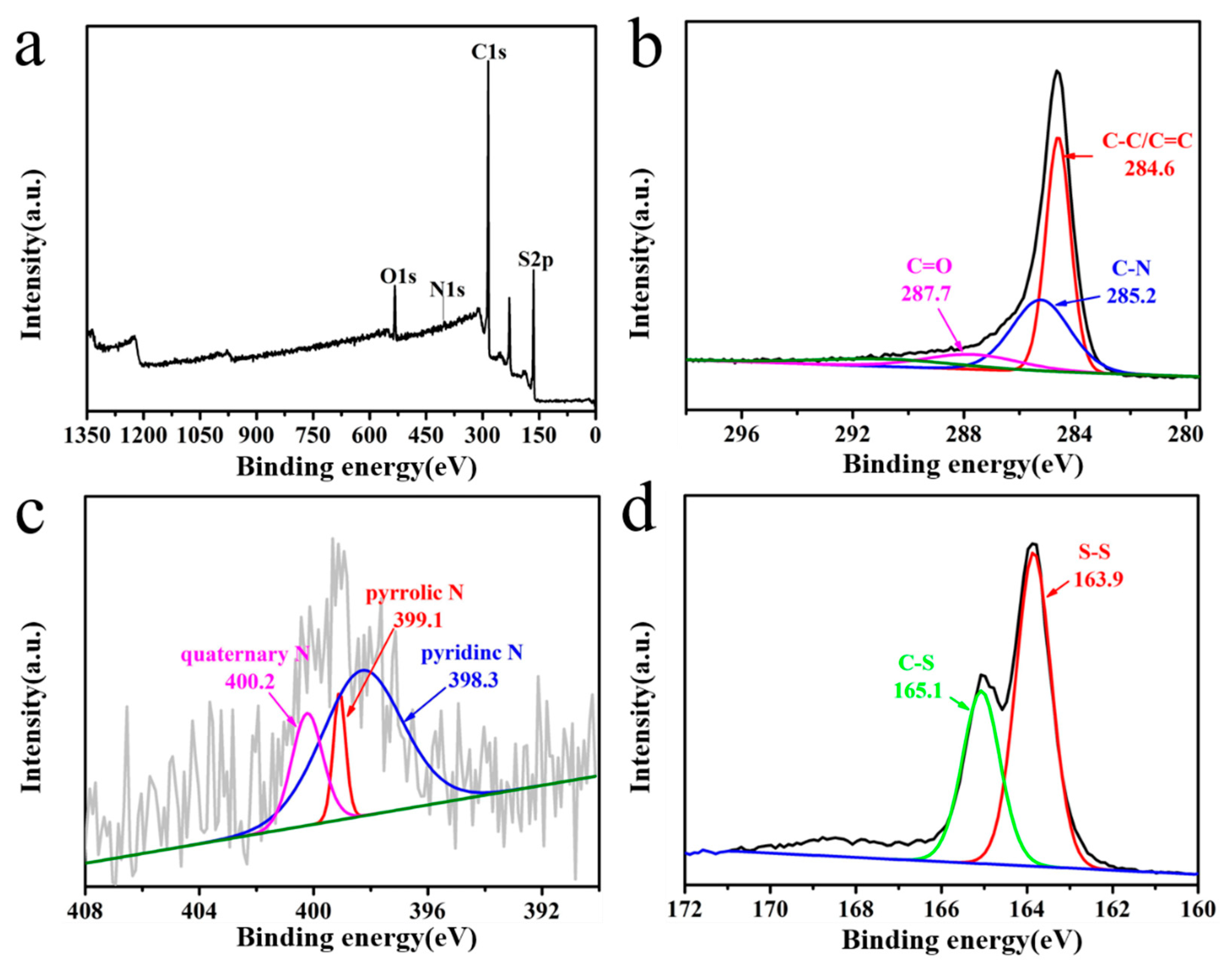
2.4. Material Characterizations
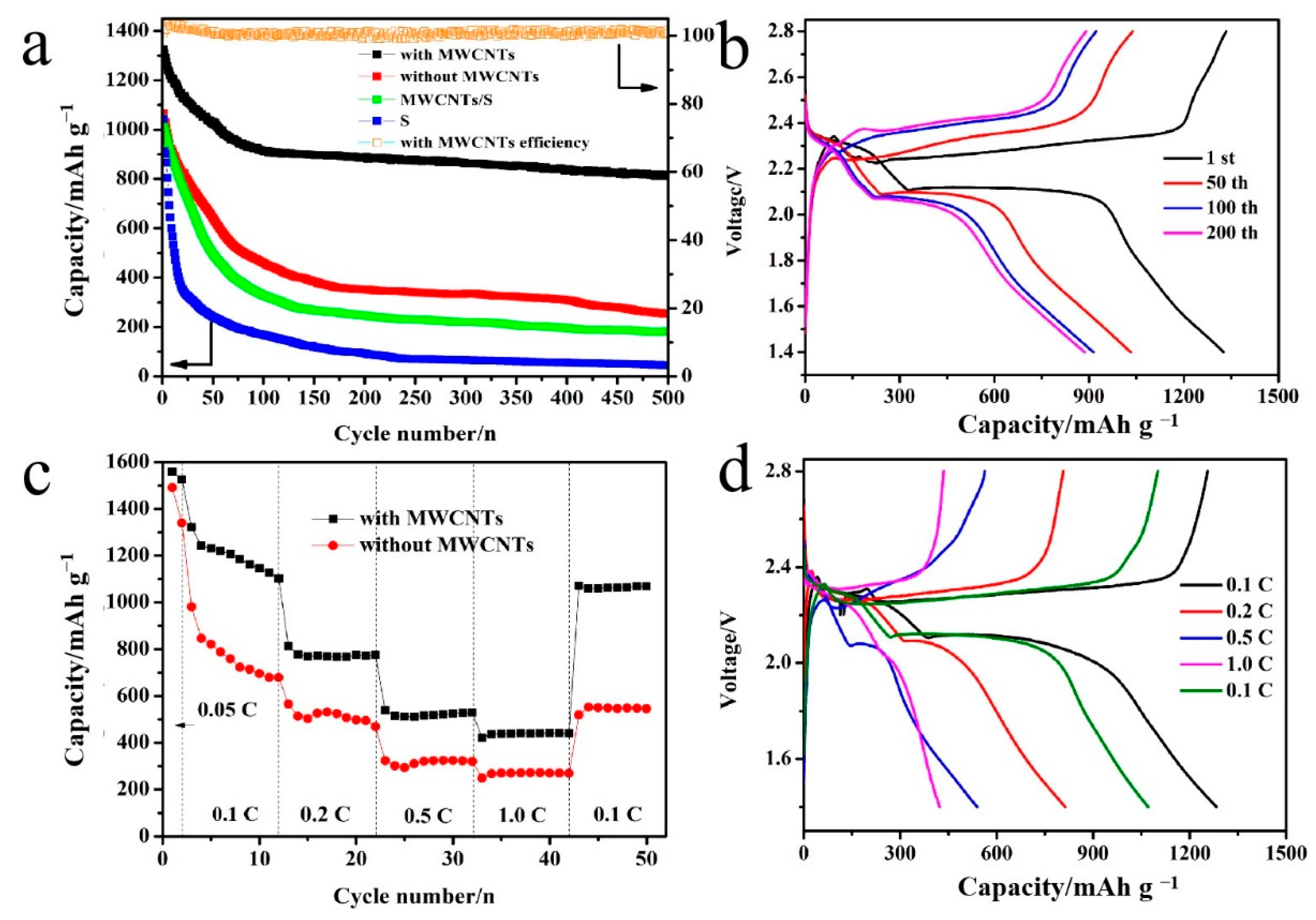
2.5. Electrochemical Measurements
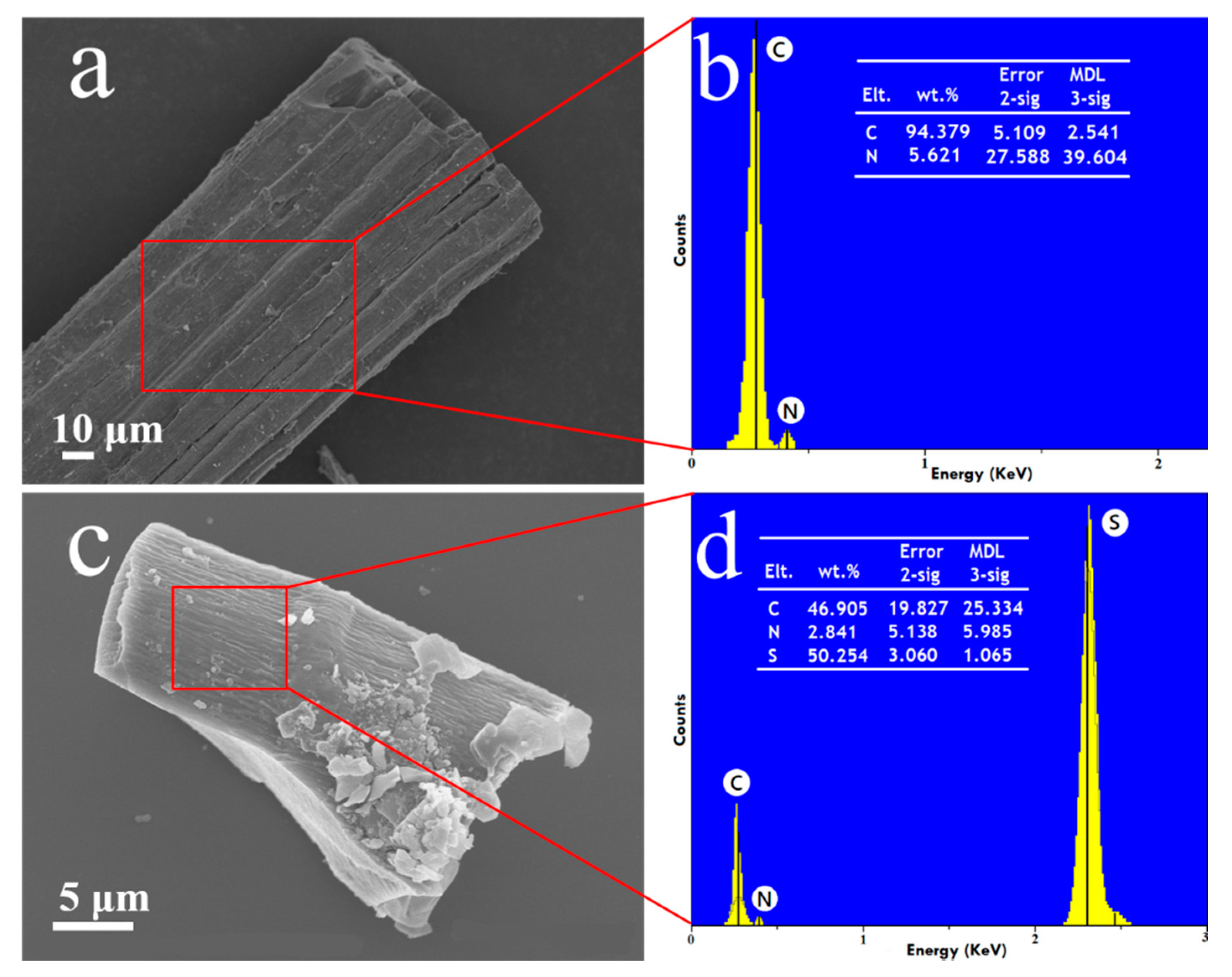
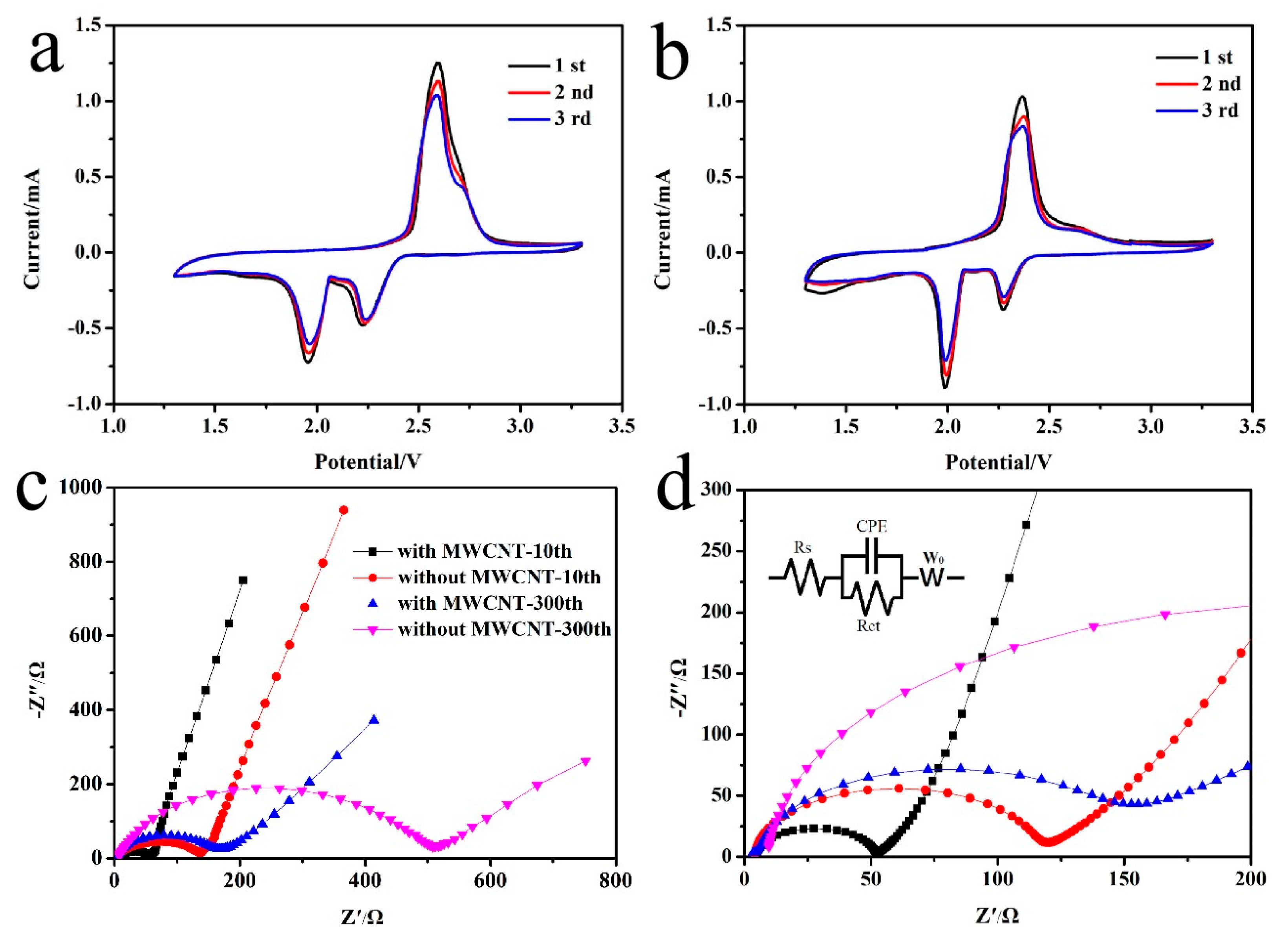
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, B.; Chen, Y.; Wang, Z.; Chen, D.; Wang, X.; Zhang, W.; He, J.; He, W. 1T-MoS2 nanotubes wrapped with N-doped graphene as highly-efficient absorbent and electrocatalyst for Li–S batteries. J. Power Sources 2020, 447, 227364. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Wu, F.; Lee, J.T.; Zhao, E.; Zhang, B.; Yushin, G. Graphene–Li2S–carbon nanocomposite for lithium–sulfur batteries. ACS Nano 2016, 10, 1333–1340. [Google Scholar] [CrossRef]
- Gu, X.; Li, H.; Wen, H.; Zhou, Y.; Kang, H.; Liao, H.; Gao, M.; Wang, Y.; Deng, L.; Yi, X. From agaric hydrogel to nitrogen-doped 3D porous carbon for high-performance Li–S batteries. J. Mater. Sci. 2020, 55, 1136–1147. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C. One dimensional nanostructures contribute better Li–S and Li–Se batteries: Progress, challenges and perspectives. Energy Storage Mater. 2019, 23, 190–224. [Google Scholar] [CrossRef]
- Wu, T.-H.; Liang, W.-Y. Reduced intercalation energy barrier by rich structural water in spinel ZnMn2O4 for high-rate zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 23822–23832. [Google Scholar] [CrossRef]
- Wu, T.-H.; Lin, W.-S. Boosting proton storage in layered vanadium oxides for aqueous zinc-ion batteries. Electrochim. Acta 2021, 394, 139134. [Google Scholar] [CrossRef]
- Li, G.; Lu, F.; Dou, X.; Wang, X.; Luo, D.; Sun, H.; Yu, A.; Chen, Z. Polysulfide regulation by the zwitterionic barrier toward durable lithium-sulfur batteries. J. Am. Chem. Soc. 2020, 142, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, C.P.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Sulfur encapsulated in graphitic carbon nanocages for high-rate and long-cycle lithium–sulfur batteries. Adv. Mater. 2016, 28, 9539–9544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Wang, Z.; Liang, Y.; Cui, Z.; Zhao, J.; Li, X.; Ren, L. Multistimuli-Responsive microstructured superamphiphobic surfaces with large-range, reversible switchable wettability for oil. ACS Appl. Mater. Interfaces 2019, 11, 28478–28486. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.-Y.; Xiao, R.-J.; Gu, L.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Interfacial mechanism in lithium-sulfur batteries: How salts mediate the structure evolution and dynamics. J. Am. Chem. Soc. 2018, 140, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chi, C.; Wu, M.; Liu, K.; Zhao, T. A long-life Li–S battery enabled by a cathode made of well-distributed B4C nanoparticles decorated activated cotton fibers. J. Power Sources 2020, 451, 227751. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, K.; Zhu, Z.; Zhang, R.; Li, N.; Zhao, C. CoP/C nanocubes-modified separator suppressing polysulfide dissolution for high-rate and stable lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 12, 2497–2504. [Google Scholar] [CrossRef]
- Lu, K.; Liu, Y.; Chen, J.; Zhang, Z.; Cheng, Y. Redox catalytic and quasi-solid sulfur conversion for high-capacity lean lithium sulfur batteries. ACS Nano 2019, 13, 14540–14548. [Google Scholar] [CrossRef]
- Sevilla, M.; Carro-Rodríguez, J.; Díez, N.; Fuertes, A.B. Straightforward synthesis of Sulfur/N, S-codoped carbon cathodes for Lithium-Sulfur batteries. Sci. Rep. 2020, 12, 2497–2504. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Cui, B.; Ye, B.; Wang, W.; Ding, J.; Wang, G. Poly (ionic liquid)-Based quasi-solid-state copolymer electrolytes for dynamic-reversible adsorption of Lithium polysulfides in Lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 38136–38146. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, L.; Jiang, H.; Ruan, W.; Chen, Q.; Zhao, T. Rational design of spontaneous reactions for protecting porous lithium electrodes in lithium-sulfur batteries. Nat. Commun. 2019, 10, 3249. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Cheng, M.; Wu, J.; Zhang, Q.; Liu, X.; Wang, C.; Wang, J.; Li, K.; Wang, J. N-doped porous carbon-graphene cables synthesized for self-standing cathode and anode hosts of Li–S batteries. Electrochim. Acta 2020, 349, 136231. [Google Scholar] [CrossRef]
- Cha, E.; Patel, M.D.; Park, J.; Hwang, J.; Prasad, V.; Cho, K.; Choi, W. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries. Nat. Nanotech. 2018, 13, 337–344. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; An, Y.; Chen, R.; Sun, N.; Wu, F.; Xu, B. A 3D conductive carbon interlayer with ultrahigh adsorption capability for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 440, 770–777. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhai, Y.-P.; Xu, G.-L.; Jiang, Y.-X.; Zhao, D.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium-sulfur battery. Electrochim. Acta 2011, 56, 9549–9555. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Hong, X.J.; Song, C.L.; Yang, Y.; Tan, H.C.; Li, G.H.; Cai, Y.P.; Wang, H. Cerium based metal-organic frameworks as an efficient separator coating catalyzing the conversion of polysulfides for high performance lithium-sulfur batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Sung, S.; Kim, B.H.; Lee, S.; Choi, S.; Yoon, W.Y. Increasing sulfur utilization in lithium-sulfur batteries by a Co-MOF-74@ MWCNT interlayer. J. Energy Chem. 2021, 60, 186–193. [Google Scholar] [CrossRef]
- Ahn, W.; Kim, K.B.; Jung, K.N.; Shin, K.H.; Jin, C.S. Synthesis and electrochemical properties of a sulfur-multi walled carbon nanotubes composite as a cathode material for lithium sulfur batteries. J. Power Sources 2012, 202, 394–399. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Chien, Y.C.; Caballero, Á.; Morales, J.; Brandell, D. Recent advances in lithium-sulfur batteries using biomass-derived carbons as sulfur host. Renew. Sust. Energ. Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.; Yu, J.; Li, D.; Du, J.; Yang, B.; Li, C.; Zhu, C.; Xu, J. N-doped foam flame retardant polystyrene derived porous carbon as an efficient scaffold for lithium-selenium battery with long-term cycling performance. Chem. Eng. J. 2018, 350, 411–418. [Google Scholar] [CrossRef]
- Yao, M.; Zhu, J.; Meng, W.; Li, C.; Li, C.; Wang, L.; Jiang, Z.; He, Z.; Li, Y.; Meng, W. Enhanced lithium storage performance of nanostructured NaTi2(PO4)3 decorated by nitrogen-doped carbon. Electrochim. Acta 2019, 294, 226–232. [Google Scholar] [CrossRef]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced sulfur cathode enabled by highly crumpled nitrogen-doped graphene sheets for high-energy-density lithium-sulfur batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Recent advances in hollow porous carbon materials for lithium-sulfur batteries. Small 2019, 15, 1804786. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, E.; Wang, J.; Liu, Z.; Ge, J.; Yu, X.; Yang, H.; Lu, B. Potato derived biomass porous carbon as anode for potassium ion batteries. Electrochim. Acta 2019, 293, 364–370. [Google Scholar] [CrossRef]
- Ren, M.; Lu, X.; Chai, Y.; Zhou, X.; Ren, J.; Zheng, Q.; Lin, D. A three-dimensional conductive cross-linked all-carbon network hybrid as a sulfur host for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2019, 552, 91–100. [Google Scholar] [CrossRef]
- Li, B.; Xie, M.; Yi, G.; Zhang, C. Biomass-Derived activated carbon/sulfur composites as cathode electrodes for Li–S batteries by reducing the oxygen content. RSC Adv. 2020, 10, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, L.; Li, Z.; Qi, Y.; Wu, C.; Liu, J.; Huang, Y. Improving the electrochemical performance of a lithium-sulfur battery with a conductive polymer-coated sulfur cathode. RSC Adv. 2015, 5, 44160–44164. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Meng, C. In-situ grown manganese silicate from biomass-derived heteroatom-doped porous carbon for supercapacitors with high performance. J. Colloid Interface Sci. 2019, 534, 142–155. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.; Xiao, J.; Wang, Y.; Lin, L. Moss-derived mesoporous carbon as bi-functional electrode materials for lithium-sulfur batteries and supercapacitors. Nanomaterials 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-Impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A. Core-Shell structured sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. RSC Adv. 2012, 2, 5927–5929. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Zhang, Q.; Huang, J.-Q.; Tian, G.-L.; Nie, J.-Q.; Peng, H.-J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nature Commun. 2014, 5, 3410. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, X.; Chen, L.; Zhu, W. New insight into the discharge process of sulfur cathode by electrochemical impedance spectroscopy. J. Power Sources 2009, 189, 127–132. [Google Scholar] [CrossRef]
- Holzapfel, M.; Martinent, A.; Alloin, F.; Le Gorrec, B.; Yazami, R.; Montella, C. First lithiation and charge/discharge cycles of graphite materials, investigated by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2003, 546, 41–50. [Google Scholar] [CrossRef]


| Sample | Formula | Providers | Purities |
|---|---|---|---|
| Sulfur | S | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. | CP |
| Ramie | - | Tmall, Hangzhou, China. | - |
| Conductive Carbon | C | Alfa Aesar, Shanghai, China. | AR |
| MWCNTs | C | Alfa Aesar, Shanghai, China. | AR |
| NMP | C5H9NO | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. | AR |
| PVDF | -(CH2-CF2)n- | Yuxiang Plastic Material Factory, Dalian, China. | AR |
| Electrolyte | LiTFSI+DME/DOL | Beijing Institute of Chemical Reagents, Beijing, China. | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Li, Z.; Feng, Y.; Wang, L.; Li, B.; Lei, Z.; Wang, W.; Huang, W. Biomass-Derived Carbon/Sulfur Composite Cathodes with Multiwalled Carbon Nanotube Coatings for Li-S Batteries. Processes 2022, 10, 136. https://doi.org/10.3390/pr10010136
Han L, Li Z, Feng Y, Wang L, Li B, Lei Z, Wang W, Huang W. Biomass-Derived Carbon/Sulfur Composite Cathodes with Multiwalled Carbon Nanotube Coatings for Li-S Batteries. Processes. 2022; 10(1):136. https://doi.org/10.3390/pr10010136
Chicago/Turabian StyleHan, Lina, Zemin Li, Yang Feng, Lijiang Wang, Bowen Li, Zijie Lei, Wenyan Wang, and Weiwei Huang. 2022. "Biomass-Derived Carbon/Sulfur Composite Cathodes with Multiwalled Carbon Nanotube Coatings for Li-S Batteries" Processes 10, no. 1: 136. https://doi.org/10.3390/pr10010136





