Abstract
In the present review article, the definitions and the most advanced findings within Process Intensification are collected and discussed. The intention is to give the readers the basic concepts, fixing the syllabus, as well as some relevant application examples of a discipline that is well-established and considered a hot topic in the chemical reaction engineering field at present.
1. Introduction
Process intensification (PI) is defined as an innovative principle applied in chemical reaction engineering and process design. The first definitions were given in 2000, when the pioneering work of Stankiewicz and Moulijn began this means of novel enthusiastic definition of chemical processes [1], which can bring significant benefits in terms of process and efficiency, higher quality of products, lower capital and operating expenses, less waste, and improved process safety. Starting from that moment, several roadmaps [2,3,4,5], books [6,7,8,9], and scientific papers [1,10,11,12,13,14,15] were published in the field, focused on PI in the chemical industry. As more papers have been published in the field, there is a growing need to summarizing all the efforts published in the literature in order to provide a starting point for researchers in both the academic and the industrial arena to begin investigating the different options available within Process Intensification. For this reason, the authors have decided to write this dedicated review article.
2. Overview of Strategies for PI
Efficiency in terms of atoms, mass and energy, along with process profitability, mainly depend on the selection of a proper chemical transformation, a suitable catalyst, and a favorable reactor type. More active and long-term stable catalysts are one route for significant improvement. For a synthesis with an already optimized catalyst, the productivity and selectivity of the process can be remarkably affected by the choice of the reactor type and operating conditions, because thermodynamic equilibrium as well as reaction kinetic properties are fixed in this case. Although the reactor typically represents only 5% to 15% of the capital and operating costs of the plant, it mainly dictates the number of up- and downstream process units, and therefore the costs and efficiency of the whole process [16,17]. For improvements in reactor technology, chemical reaction engineers have focused on the integration of multiple unit operations in one apparatus, enhanced transport properties, and alternative process fluids and energy sources [14].
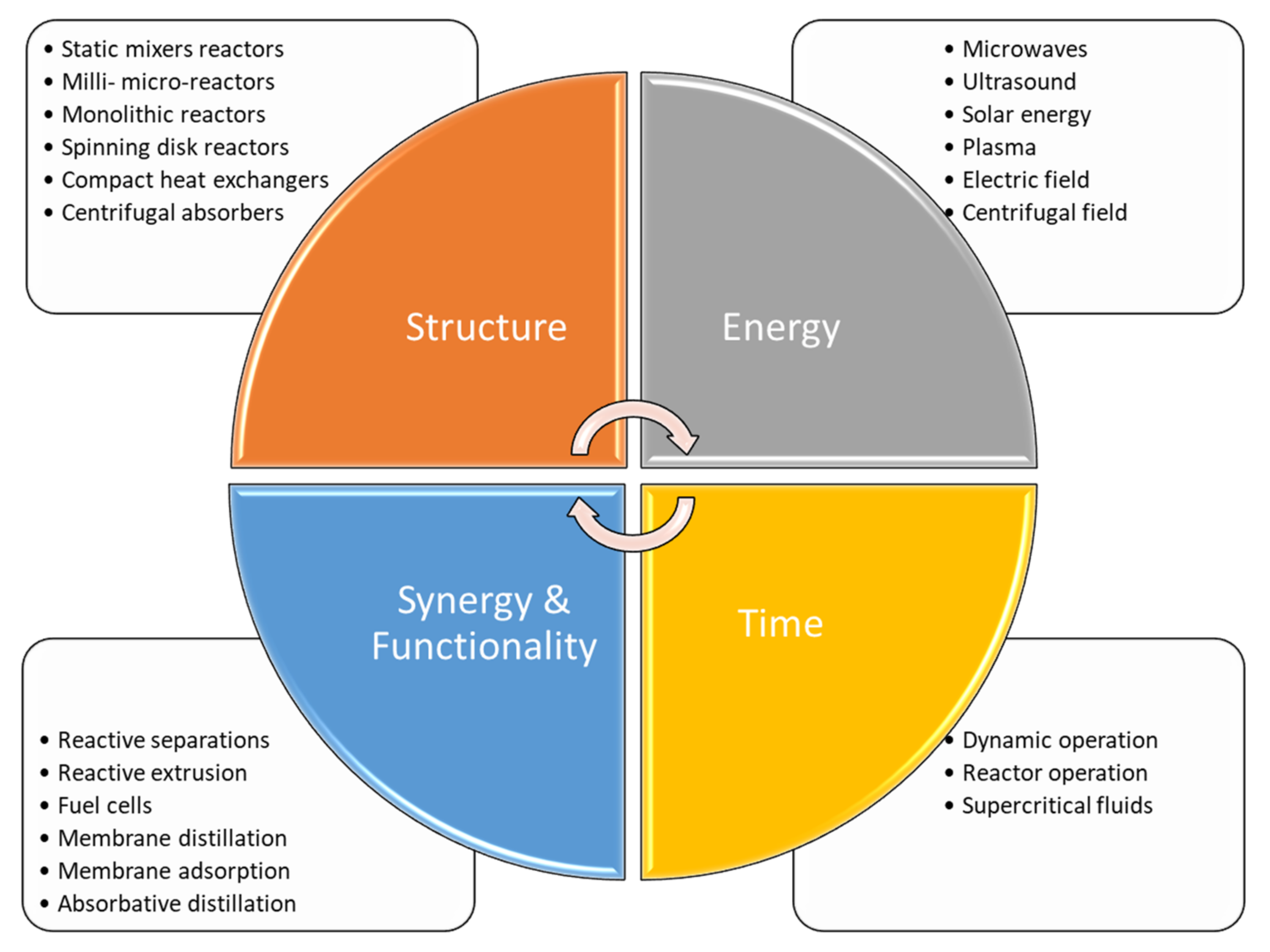
Van Gerven and Stankiewicz (2009) [11] provide four guiding principles for PI:
- Maximize the effectiveness of intramolecular and intermolecular events (example: dynamically changing conditions to attain kinetic regimes with higher conversion and selectivity).
- Provide all molecules the same process experience (example: plug flow reaction with uniform, gradientless heating).
- Optimize driving forces at all scales and maximize the specific surface areas to which they apply (example: increase transfer surface area through microchannel designs).
- Maximize synergistic effects from partial processes (example: multifunctional reactors).
The principles and the main concepts are accepted worldwide, and are today considered common practice and theory. The four principles above lead to practical application. Changing the structure of a conventional reactor means focusing both on the catalyst and on the reactor’s shape and dimension. The synergy between chemical reaction and separation unit leads to the design of more compact and cheaper plants, reducing the amount of equipment and thus simplifying the control systems of the chemical plant. The pursuit of novel energy sources leads to more efficient mixing and heating systems, allowing for optimal heat and mass transfer properties. Alternative time use and optimization can be reached by implementing dynamic operations or using alterative fluids that enhance the global conversion of a reaction network.
A summary of the main idea, including the four principles and their means of application, is shown in Figure 1.
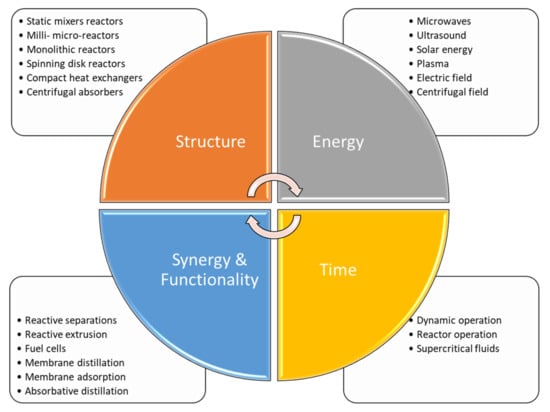
Figure 1.
Principles of Process Intensification: definition of the main concepts of PI with application examples, adopted from [11,18].
Starting from these concepts, Process Intensification allowed for the opening of Novel Process Windows, making possible the achievement of process performances in terms of conversion, selectivity, and safety issues [19]. As reviewed by Illg et al. [19], it is possible to achieve good performances for reactions characterized by strong limits (e.g., high exothermicity, low miscibility of the reactants) by going beyond the classical protocols and applying PI concepts, permitting excellent mixing and thermal control when using milli- and microreactors. This aspect clearly led to the possibility of working in harsh conditions with respect to the element of safety; for instance, explosive conditions that cannot be achieved in a conventional apparatus (e.g., Kolbe–Schmitt synthesis or the bromination of 3-nitrotoluene [19]), as well as the development of one-pot processes, reducing waste compared to classical methods for the synthesis of chemical intermediates (e.g., synthesis of phenyl boronic acid [20]). Fast reactions can be handled and studied, as residence times can be on the order of magnitude of milliseconds; thus, it is possible to achieve very precise kinetic information to optimize the chemical process.
Writing a review article on PI is a rather difficult task today, as several books and reviews have been published already. Therefore, the aim of this paper is to give the reader both the basic tools of PI, including definitions and the explanation of the primary strategies. Moreover, an evaluation of intensification potential, including guidelines to help a chemist to choose an intensification pathway, are be reported here as well. The review is accompanied with PI examples; it must be remembered that the application examples reported within this review article are only a small selection of chemical processes, as the volume of PI literature is exploding in the most recent years. The main trends can be seen in the Scival Analysis shown in Figure 2.

Figure 2.
Scival Analysis trends obtained using Process Intensification as keyword.
As revealed, the trends are very clear, suggesting that researchers are exploring PI alternatives in every field of chemical reaction engineering, starting with reactor design and including, among others, mixing science, catalysis, unit operation design, and alternative energy sources.
3. Detailed Description of Selected Strategies
3.1. Equipment Design
In this section, the newest efforts published in the different areas of process intensification dealing with equipment design are described. Different levels for the process intensification of a reactive unit can be identified as:
- (a)
- miniaturization, reactor structure, and catalysts;
- (b)
- integration of mixing elements and static mixers;
- (c)
- combination of unit operations with multifunctional reactors.
The basic concepts of each technology area along with examples applications are given in detail within this section.
3.1.1. Structuring and Miniaturization of the Reaction Chamber
The integration of regular structures into the contact apparatus is a successful approach to intensifying the transport of mass and energy. By structuring on the catalyst level, the pore system inside the catalyst is designed in such a way that diffusive transport is maximized or diffusion limitations are used to promote desired reactions and suppress undesired side-reactions. By structuring on the reactor level, random packed beds of particles, pellets, or extrudates inside reactor columns are replaced by a regular catalyst structure. This results in larger surface areas for solid–fluid contact and in higher porosities, which reduce flow resistance. Furthermore, the reactor void volume is partly separated into smaller reaction chambers which provoke thinner boundary layers, enhancing mass transfer as well as enabling precise control of the fluid flow, affecting sharper residence time distributions, among other things [21,22].
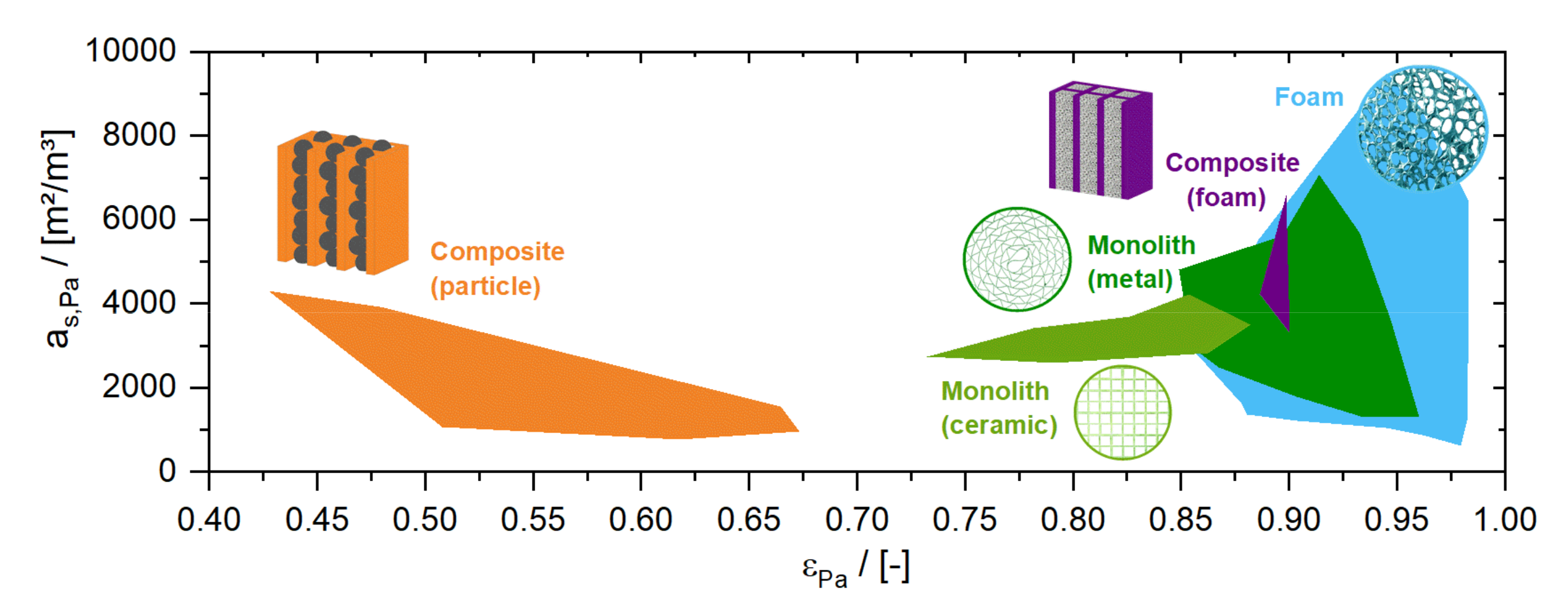
A well-known example of PI through structuring of the apparatus is the implementation of structured packings in continuous distillation columns [23]. These packings are composed of vertical crimped sheets, increasing the packing porosity from 40% to more than 80% in comparison to a random particle packing in similar geometric surface areas [24].
In chemical reactors, structured monolithic packings (known as honeycomb packings) have been used in exhaust gas treatment since the 1970s. Monolithic packings consist of an array of parallel flow channels, and combine large specific surface areas of several 1000 m2/m3 with high porosities of 80% or more [25] (see Figure 3). Usually, monolithic packings are manufactured by extrusion of a ceramic paste (hydraulic channel diameters down to 0.7 mm [26]) or by wrapping a set of straight and corrugated metal sheets (hydraulic channel diameters down to 0.6 mm [27]). Even smaller channel diameters, of 100 µm or less, can be generated by mechanical processing; by milling, for example, or by chemical processing, e.g., etching of foils which are afterwards stapled and connected by welding or bolted fastenings. The latter structures are commonly known as microreactors. By stapling of the foils guiding the reactants and the heat transfer fluids (a principle known from plate heat exchangers), reactors with excellent temperature control can be built, which may open novel process windows for so-called flow chemistry devices [28,29].
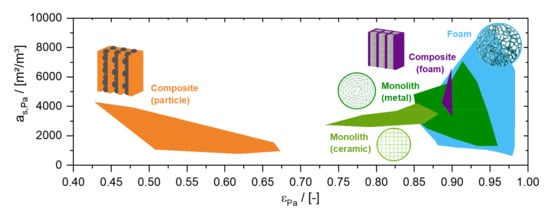
Figure 3.
Comparison of bed porosity () and solid surface area () for different structured packings.
Beginning in 2003, open-celled foam structures have become more and more the focus of research due to their extremely high porosity and surface area [24,30]. Furthermore, such structures also provoke a radial transfer of matter which is not present in monolithic packings, making them less sensitive to initial liquid distribution. However, an efficient and reliable method to deposit industrial catalysts is still a central challenge in the application of these novel catalyst structures. An alternative could be the integration of industrial catalyst particles into structures which guide the flow in so-called composite structured catalysts [31,32,33]. Particles, pellets, extrudates and even foams are suggested as internals.
Due to the advancements in additive manufacturing methods, the manufacture of process-tailored structures seems to be the next step in further exploiting the full potential of structuration and miniaturization, as addressed in a recent special issue of Process Intensification of Chemical Engineering and Processing [34,35,36,37]. It should be mentioned that there are many other strategies for structuring and miniaturisation, such as fibre-based structures [38], capillaries with slurry flows [39,40], or micro-packed bed reactors [41,42], to name a number of areas which were not addressed here.
3.1.2. Integration of Mixing Elements and Static Mixers
Mixing plays a very important role in industrial processes as the way in which reagents are mixed affects the selectivity of reactions, and consequently the process efficiency. Static mixers have become widely used in the process industry thanks to their favorable characteristics [1,18,43,44]. Static mixers, together with heat exchangers, are very important in the process industry as mixing, chemical reactions and heat transfer occur within the same equipment. Static mixers are used as an alternative to classical mixers because similar results can be obtained with lower costs. Several advantages make them suitable to replace the classical equipment, among which are lower space requirements and energy demand thanks to the absence of moving parts. Further benefits include low operational costs, increased process safety, and better selectivity with respect to the reduction of byproducts reduction thanks to mixing intensification [43,45,46]. This has led to various applications in several fields, e.g., chemistry, pharmaceuticals, food, polymers, and water treatment. A static mixer is made by fixed inserts placed in pipes with a specific geometrical form; they can be made from different materials, making them suitable even in difficult operating conditions.
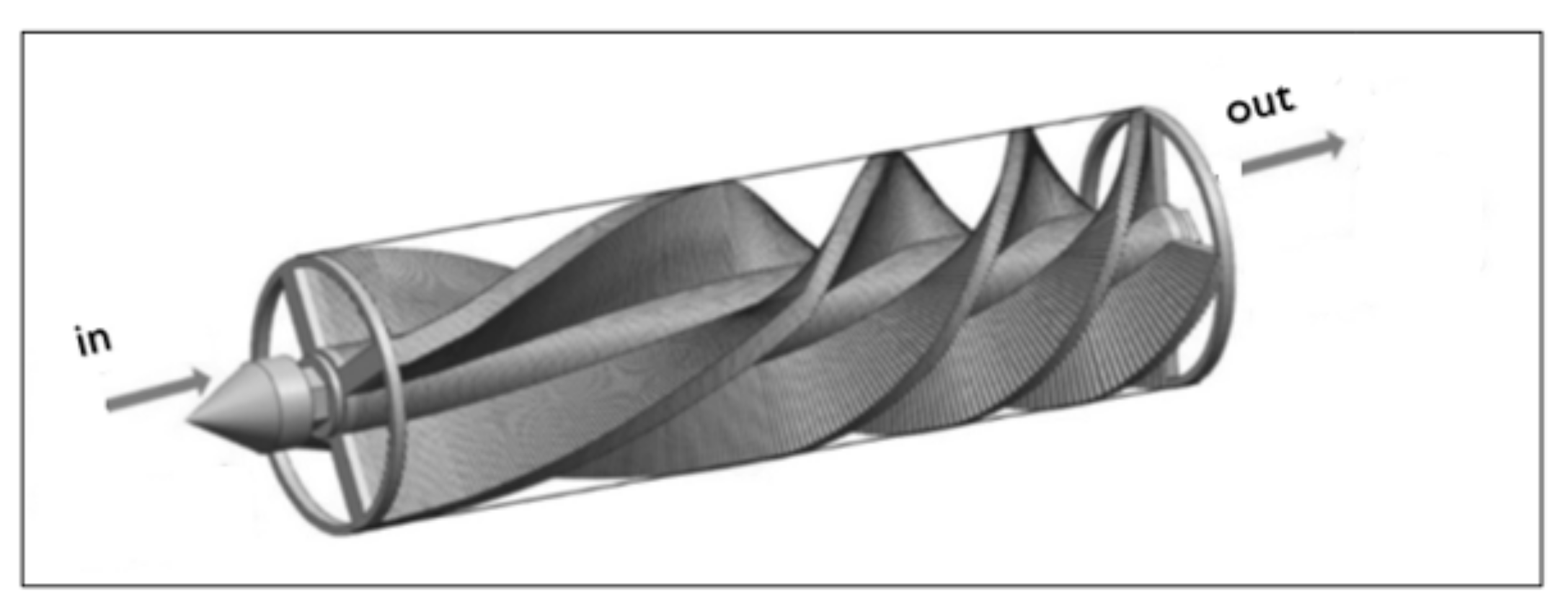
Static mixers have a wide variety of geometries, which can be classified into five groups: open designs with helices, open designs with blades, corrugated plates, multi-layer designs, and closed designs with channels or holes [43,45,47]. Figure 4 shows a generic representation of a static mixer.
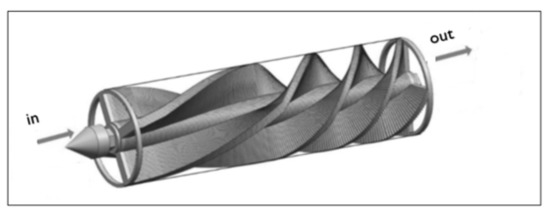
Figure 4.
Representation of a static mixer with helix elements for installation in a conventional pipe; adapted from [46].
The industrial application influences the choice of mixer, as the inserts have different effects on the fluid flow. In this way the improvement of mass and heat transfer can be realized.
Static mixers are used in several applications including liquid–liquid, gas–liquid, solid–liquid and solid–solid systems [45]. They lead to better homogenization of the feed and lower residence time. Originally, static mixers were developed to mix fluid in laminar flow, and successive applications for heat transfer in turbulent systems were improved [45]. Mixing mechanisms are different in laminar and turbulent flows. Laminar flows are typical of high viscosity fluids, for example in the food, cosmetics, polymer and varnish industries [43]. A low mixing level in the laminar flow leads to spatial and temporal non-homogeneity in composition, as molecules that leave the pipe at the same time enter at different moments in turbulent flows; static mixers promote a high radial mixing leading to heat and mass transfer increase, particularly in the case of laminar flow, while the improved effect is smaller in turbulent flows [43,48]. Important applications of static mixers are gas mixing and mixing of aqueous solutions in turbulent flow, particularly for water treatment and mixing of polymeric solutions. They are used also as reactors for polymerization. Static mixers are useful for mixing gases and liquid fuel before a reaction to improve the yield. Furthermore, they can reduce NO emissions in combustors, and can be used in catalyst tubes in a reformer furnace, where inserts increase the heat transfer coefficient and avoid cocking, which prevents the catalyst degradation caused by hot spots [43].
3.1.3. Multifunctional Reactors
Multifunctional reactors represent the frontier of Chemical Reaction Engineering science and the intensification of chemical systems. The term ‘multifunctional’ clearly indicates that a single unit consists of a smart combination of more than one unit operation, including a chemical reactor. The main advantage of this approach consists of realizing optimized equipment where both the kinetics and transport phenomena are optimized, obtaining the desired target product specimen (i.e., conversion and selectivity) while at the same time separating the desired product from the reaction mixture (usually composed of byproducts and unreacted reagents). The idea behind this concept is not novel, as many articles on the topic have appeared in both the scientific and technical literature. Some applications are even running at the industrial level, such as FCC, wherein a single piece of equipment combines several units: (i) the riser, serving as the chemical reactor; (ii) an initial fluidized bed to separate the products from the spent catalyst; and (iii) a third fluidized bed to regenerate the catalyst via coke oxidation.
The rationalization of the concept in terms of process intensification, along with a related classification, was published by Dautzenberg [10], individuating four main classes including the multifunctionality of the following:
- The catalyst, combining catalytic properties with an engineered catalyst structure;
- The reaction inter-phase, in which a chemical reaction is improved by interphase mass transfer;
- The intra-reactor level, combining a chemical reaction with an intra-reactor unit, e.g., heat transfer or separation;
- The inter-reactor level, combining two reactors using recirculation of solids.
The latter two categories are well-framed in this review article in the context of the process intensification of chemical plant units.
Coupling a chemical reaction with an intra-reactor process is a valid procedure in process intensification. The integration of the unit process (e.g., chromatographic separation, distillation) with the chemical reaction occurs by combining the two operations within the same equipment [49,50]. In every case, both operating costs and investment are lower than for plants designed with conventional reaction followed by the operation unit.
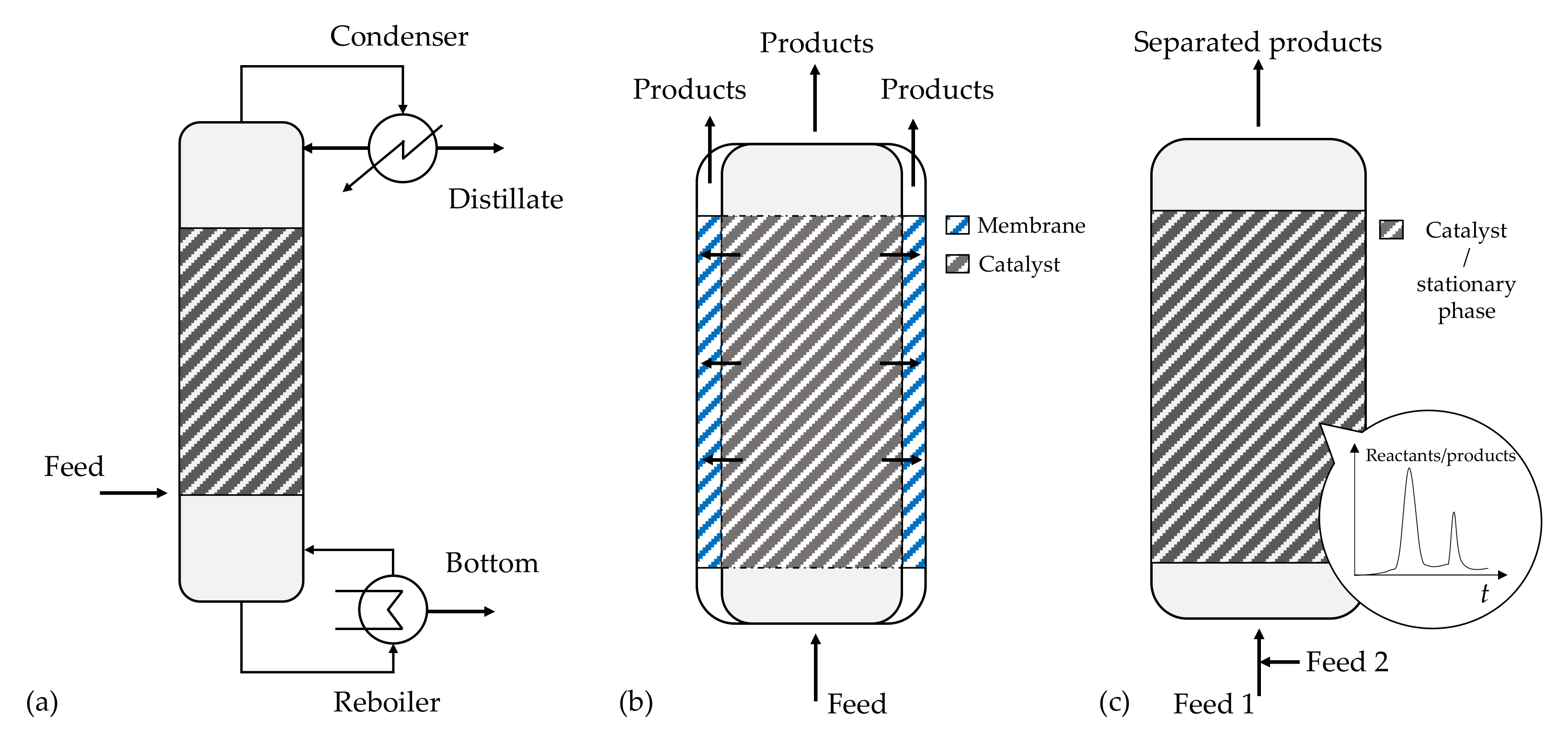
Reactive distillation represents a combination of chemical reaction and distillation, normally conducted using heterogeneous catalysts packed within the equipment (Figure 5a). The unit works at a constant pressure, ensuring precise temperature control in the catalyst zone as set at the boiling point of the mixture, using the reaction heat to vaporize the products. In the case of reversible reactions, catalytic distillation permits working over the chemical equilibrium, as the products are separated during the distillation once they are formed, switching the reaction to the products side.
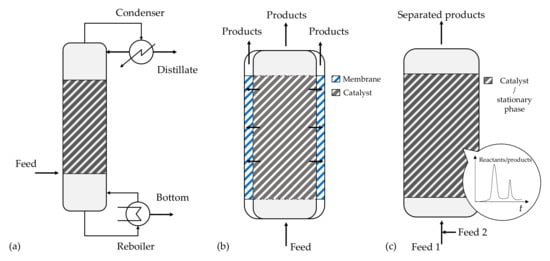
Figure 5.
Sketch of multifunctional reactor units: (a) reactive distillation column; (b) membrane reactor; (c) chromatographic reactor.
Selective extraction combines chemical reaction and liquid–liquid extraction, where a solvent is fed either in co-current or in counter-current to a packed bed reactor, where the packing is a heterogeneous catalyst. Even in this case, a reversible reaction can be switched to the correct side by extracting the products from the reaction mixture.
Another interesting case is the use of membrane reactors, where membranes are used to selectively separate one of the products to shift the chemical equilibrium. The main idea is sketched in Figure 5b, where the membrane can be placed next to the catalytic bed to allow the selective separation of one of the components.
Reactive chromatography implies the combination of reaction and chromatographic separation in the same unit, where the packing material must act both as catalyst and stationary phase (Figure 5c). For a reversible reaction, the chromatographic separation permits working over the chemical equilibrium, offering different advantages compared with classical packed bed reactors: (i) conversion enhancement, overcoming equilibrium limitations; (ii) separation of the products; and (iii) enhancement of the selectivity of complex reaction networks [51,52]. This system is well suited for esterification or ketalization reactions, where water must be removed on-stream to achieve full conversion of the reactant [52,53,54,55]. Its industrial application is unrealistic, as it is in effect a semi-batch system; therefore, the analogy of the simulated moving bed reactor was developed, where the feed/extract/raffinate position of a packed bed is switched automatically in different positions of the packed bed in order to simulate the motion of the catalytic bed with the operation time [56,57], leading to a continuous operation.
3.2. Alternative Energy Sources
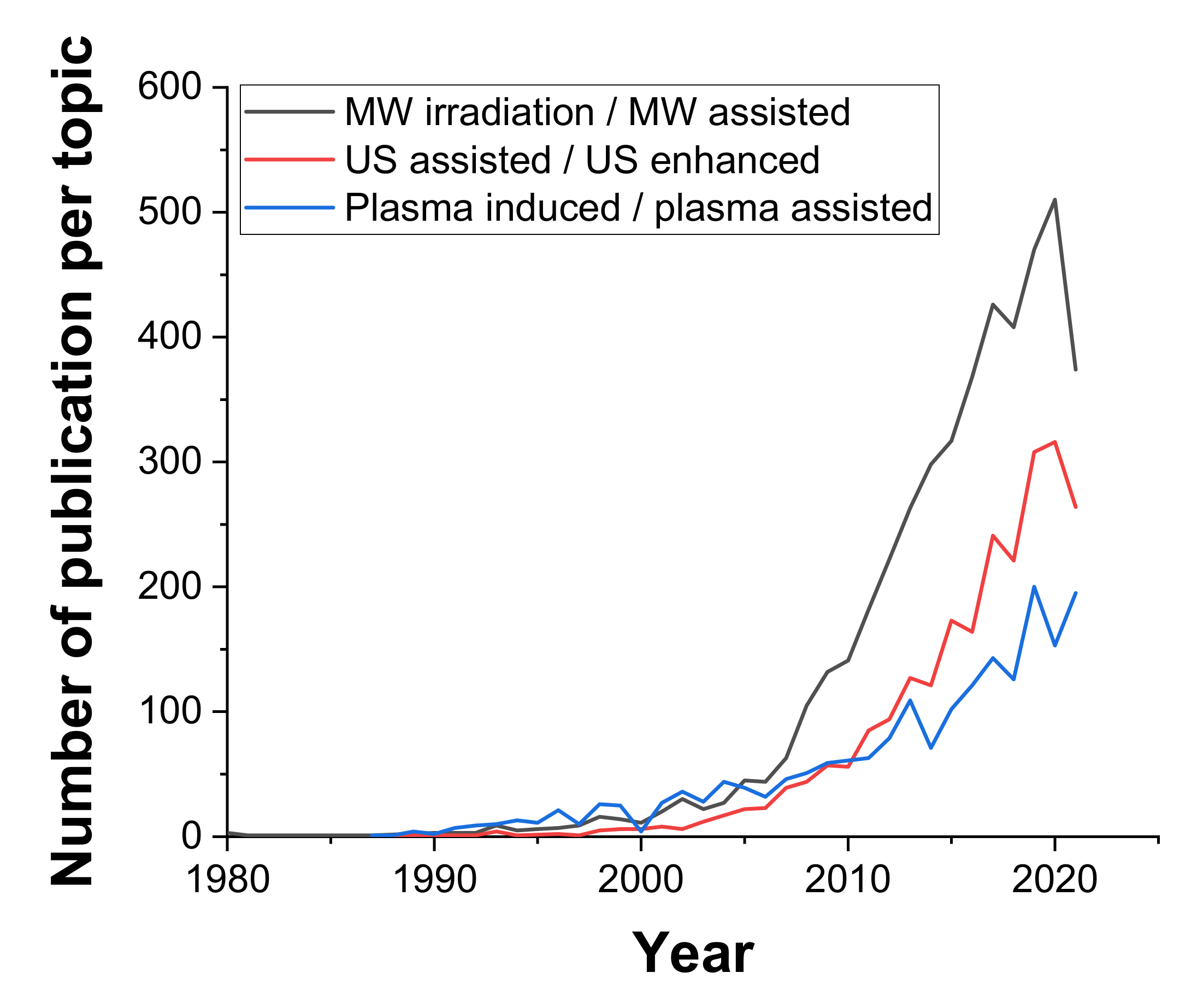
There are several different types of irradiation which can be used for process intensification due to their ability to produce intense localized heating, turbulence, and chemical effects [58]. Here, we will briefly describe the most popular types, namely, microwave, ultrasound, and plasma irradiation, and highlight some of the most recent scientific progress using these techniques in the context of chemical engineering. As seen in Figure 6, among these three technologies, using microwaves to enhance chemical systems has been the most commonly reported in scientific research, with an annual output on this topic of over 500 publications in the chemical engineering field at present.
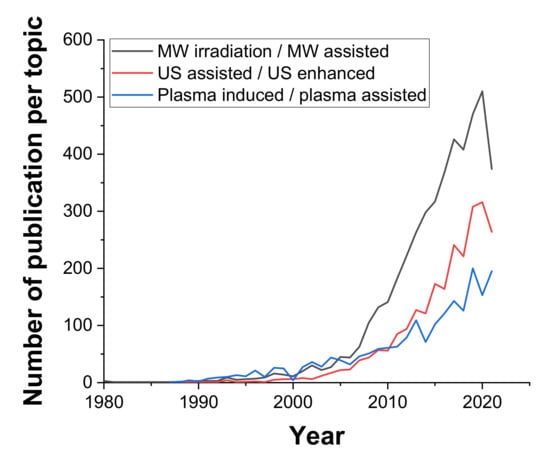
Figure 6.
Number of annual articles on the specified topics (MW/US/Plasma) in the field of Chemical engineering; source: Web of knowledge 18.10.2021.
3.2.1. Microwave
One of the greatest benefits of using microwave irradiation is selective, targeted heating. Microwaves are frequencies in the gigahertz (0.1–300 GHz) range, falling between the infrared and radio wave regions; typically, microwave ovens operate at 2.45 GHz. Here, different molecules behave differently depending on their absorbance. More polar substances absorb better than less polar ones; hence, for example, water is rapidly heated by MW radiation, while oil is heated much slower and a ceramic plate underneath is not directly heated at all when preparing some food in a microwave oven. In terms of energy saving, using microwave heating can save energy consumption; however, other benefits can be found as well. There are many examples of this in chemical engineering, where this kind of selective heating can be beneficial, such as the extraction processes of various components, as well as in actual reactions such as transesterification reactions.
Extraction of natural substances from one phase to another is one of the oldest applications. Typically, conventional extraction is carried out with a solvent which captures the desired compound. This is the most-developed type of technique utilizing microwave technology at the moment, as it offers such important advantages over traditional solvent extraction [59] as reduced use of solvents, improved extraction kinetics, and higher yields in shorter extraction times due to the benefit of localized heating under microwave radiation, which can disrupt cell walls and plant tissues and result in the release of solutes from the material [60]. Recent advances in extraction using MW include using supercritical conditions. The idea is to apply higher pressure over the system in order to maintain the solvent in its liquid state even at higher temperatures, which increases solubility, extraction, and extraction velocity [61]. Other new strategies in achieving microwave enhanced extraction include so-called negative pressure cavitation, utilizing nitrogen for increasing turbulence in liquid–solid systems [62].
Reaction intensification using microwaves is perhaps the most-debated topic in process intensification over the last decade. The discussion has focused on whether microwaves truly enhance the kinetic reaction rate itself, or if the enhancement is due to the fact that the temperature is locally increased such that reactions occur faster due to the Arrhenius effect. However, the observed enhancements are attributed to selective heating and generation of local hotspots. Regarding the debate on this matter, attention has focused on the accuracy of temperature measurements. A worthy critical review on this topic is given by Priecel and Lopez-Sanchez [63]. Selective heating is accomplished when at least two substances with different absorption responses to MW are present. Often, one of the substances is a catalyst and becomes much hotter than the bulk temperature around it, and therefore the reaction rate is increased. This property can be successfully enhanced by, for instance, adding a specific metal or carbon material, i.e., Pd on active carbon, which strongly absorbs MW irradiation [64]. However, care must be taken in the selection of the MW field strength, as overheating can have negative effects such as deactivation of the catalyst. Therefore, it is recommended to carefully measure the true temperature applied to the particles which are heated by MW, for instance, by using fiber optic probes/thermocouples along with thermal cameras [65].
Additionally, microwave irradiation in chemical reaction systems has found an important place in materials processing due to the different behaviors of materials when affected by microwaves. Non-magnetic materials experience dipolar and conduction losses, whereas magnetic material behave in much more complex ways. These properties have led to new research in various areas of composites, such as gas sensor nanomaterials, photocatalytic materials, and graphene, to name a few. An excellent overview of current applications of microwave processing of materials is available from Mishra et al. (2016) [66].
Recently, Kappe et al. rationalized the effect of microwaves in organic synthesis, underlining that the main effects of microwaves can be explained as bulk thermal phenomena [67]. In detail, the authors critically defined and analyzed the phenomenon, discussing cases in which microwave irradiation is very effective (e.g., the synthesis of 2-methylbenzimidazole) or in which it is inefficient, concluding that nonthermal microwave effects do not exist.
3.2.2. Ultrasound
Ultrasonification is a term describing the use of ultrasound in a process to enhance either the efficiency of a reaction or to improve mass transfer or dissolution of solid reactants. The effect of ultrasound originates from the extremely high local temperatures and pressures which are attained inside of cavitating “bubbles” when they are about to collapse, leading to the formation of reactive intermediates. The first description of cavitations originating from ultrasound was reported by two shipbuilders in Britain (Thornycroft and Barnaby) as early as 1895, who noted strange erosion on a propeller. The first study of the relevant chemical reactions was reported by Richards and Loomis in 1927 [68] in a study on the hydrolysis of Dimethyl Sulfate. Later, so-called cavitations were described in 1949 by Plesset [69], defining the phenomenon for the first time. However, the growth in the field of sonochemistry started on a large scale in the late 1990s. Both phenomena, enhancing of reactivity and mass transfer, have subsequently been widely studied on the lab scale; however, the adoption of this technology in industry has been difficult, particularly due to issues with microfluidic clogging, numbering up, and scaling up [15]. Taking a closer look at where most of the research dealing with ultrasound is being carried out, it is not surprising to see the medical research category clearly in the top position, with the food processing and multidisciplinary chemistry areas more or less tied in the second place based on a count of the review articles over the recent five years containing the keyword ‘ultrasound’ (source: web of science).
Chemistry-related research dealing with ultrasound has grown steadily during the last 20 years. Currently, the leading journal in this field, Ultrasonics Sonochemistry (Elsevier, IF 6.5 at date), yields around 500 publications per year. When inspecting the type of research that is currently done, relating primarily to chemical reactions and reactors induced by ultrasonic waves, a breakdown of review articles can be made: food related utilization applications are dominant, while on the pure chemistry side, degradation of different aquatic pollutants as well as nano-related reviews stand out (source: reviews in Ultrasonics Sonochemistry, 2020). We now take a closer look at what kinds of ultrasound-related research relating to chemical modification have been performed in the past several years. Web of Science offers a filtration of search results within “Hot Papers”. In order to be considered a “Hot Paper”, a publication must have been published within the past two years and received enough citations as of May/June 2020 to place it in the top 0.1% of papers in the academic field of its section (here, the field of Engineering). The hottest paper today, cited 38 times to date, (18 October 2020, keyword: ultrasound, field: engineering) is a paper studying photocatalytic degradation, where ultrasound combined with photocatalysis is used to assist in the removal of the pharmaceutical drug sulfadiazine [70]. In sono-photocatalysis, US waves can refresh the surface of photocatalysts continuously and create a fresh catalyst for further reactions. Water dissociation and subsequent dissolving of oxygen molecules take place in hot spots to form free radicals (), leading to the destruction of organic compounds. In the study of Hayati, a clear improvement in the reaction kinetics was found when increasing the effect of UV intensity, as well as when increasing the effect of US power. Radical scavengers were used to prove that radicals are formed; additionally, they found that the most active radicals for sulfadiazine degradation were hydroxyl radicals, and that the second-most active were positively-charged holes.
A review by Sancheti et al. [71] summarized the current engineering aspects in terms of chemical synthesis using ultrasound technology. In their study, the reaction system was classified depending on the phases involved: homogeneous reactions benefit from US only in radical mechanisms, whereas heterogeneous reactions are influenced by the physical effects of cavitation such as shrinking of particles and improved mass transfer. If combined (radical reaction and several phases), there is a high probability of enhanced intensification. Moreover, in this review, recent reactions assisted with US were well-summarized: benefits were found from oxidation reactions i.e., the cyclohexanone reaction time can be shortened from 60 to 15 min while using less energy [72]. Shorter reaction times were noticed with acetylation reactions [73], Mannich-type reactions [74], Aza–Michael reactions [75], and catalytic coupling reactions [76,77]. Significantly higher yields were reported for bromination of aromatic compounds [78]. A cleaner product and shorter reaction time was reported for synthesis of lutein disuccinate [79], as well as for esterification of carboxylic acids [80] and enzymatically catalyzed transesterification of glycerol [81]. Other reported benefits using US were reported, such as avoiding the formation of unwanted side products in synthesis of dichloroaziridines [82]. The guidelines for ultrasound operating parameters, such as the effects of power and frequency, duty cycle, and temperature on performance were thoroughly discussed in the review by Sancheti [71], highlighting how crucial it is to take all these into account when designing a new ultrasonificated reactor system.
3.2.3. Plasma
Plasma-assisted process intensification is the third non-conventional energy source explored in this review. Plasma can be defined as the fourth state of matter, where an ionized substance becomes highly electrically conductive such that long-range electric and magnetic fields dominate its behavior. Using plasma reactors is a rather new and exciting technology compared to MW and US. However, it is finding grounds for use in a vast range of applications, from decomposition of CO2 and other volatile organics and removal of aquatic pollutants to naphtha-cracking, steam reforming of ethanol, and biomass pyrolysis. Indeed, plasma technologies are now widely studied for their application in CO2 reduction in fuels and chemicals due to the potential to enable methane activation to more reactive intermediates [83]. It may be worthwhile here to distinguish between different plasma types. Plasma is usually associated with a very hot fluid; however, plasmas can occur in a wide range of temperatures depending on their energy level, temperature, electronic density, and whether the plasma state is classified either as a high temperature (thermal) plasma or a cold (non-thermal or non-equilibrium) plasma. Very hot temperatures can have a negative or ‘scary’ chime and many reactions cannot be performed at high temperatures. Therefore, authors have recently adopted the term non-thermal plasma (NTP) technology for cases when the process occurs at ambient temperatures. NTP finds applications in the removal of volatile organic compounds (VOCs), where typically control of VOC emissions has relied on technologies such as adsorption, thermal and catalytic oxidation, membrane separation, bioreaction and photocatalysis [84]. NTP has proven to be more efficient and less energy-intensive than most of the traditional gas treatment technologies, and is capable of ionizing compounds in gaseous form, thus leading to future innovations in many chemical synthesis reactions, especially in the field pollution control [85]. A wide variety of plasma technologies has emerged lately, including dielectric barrier discharge (DBD), corona, microwave, radio frequency (RF), glow discharge and gliding arc discharge technologies [86].
So-called cold plasma has gained attention in food processing due to the ability of plasma to inactivate microbial growth [87]. Food science thus tops the current research trends in plasma; however, this is outside of the chemical engineering scope of this review.
Chemical engineering, especially reaction engineering research, has been focusing recently on non-thermal plasma technology, as the low temperatures used lead the process in a more sustainable direction. Using NTP for synthesis of ammonia has been a recent trend [88], as ammonia could be a clean, sustainable fuel source and an efficient medium to store energy due to its power a hydrogen carrier molecule. The Haber–Bosch process is known as a major non-green energy consumer, and here NTP would be an excellent candidate to reduce the losses and emissions of VOCs. At this point, researchers have made smaller developments in catalyst synthesis, providing stronger plasma synergistic activities, and reactors designs have been optimized for more rapid separation of ammonia after being synthesized. As for the catalyst type, it has been found that powder is unsuitable as it tends to spread under plasma conditions; therefore, pellets are preferred [89].
In addition to ammonia, other chemical processes with lower pressures and temperatures have been reported, such as hydrocarbon reforming, for instance, for hydrogen production in fuel cells [90]. These processes, assisted by non-thermal plasmas for hydrogen production, have been much studied in recent years. In the review of Petitpas [90], many different reforming reactor technologies which have emerged in the non-thermal plasmatic field were discussed. Most recently, Abiev et al. [83] reviewed the latest discoveries in NTP-assisted dry reforming of methane (DRM) fields, focusing on renewable feedstock. The leading vector in this field is the combination of metal doped Ni-based catalysts and post-plasma photocatalysis. Photocatalysis seems to be an important step towards further developments in the plasma catalysis field. In summary, plasma and catalysis have different reaction mechanisms and might complement each other; thus, combining them can be a promising technique to improve DRM performance. Electron density can be increased with a catalyst, resulting in higher energy efficiency, whereas plasma interacts with the catalyst to restructure active metals and supports, leading to enhanced catalyst activity. Combining them can provide many benefits via synergistic effects [91].
Carbon dioxide is a hot topic in thermal and non-thermal plasma research. It has been found that CO2 can be activated in a pulsed discharge field. Vibrational levels of CO2 molecules can be excited by plasma, and the kinetic energy of electron impact dissociation can consequent be reduced from 11 eV to 5.5 eV [91].
3.3. Dynamic Operation Modes
Dynamic or unsteady operation modes of chemical reactors may improve reactant conversion, selectivity to the target molecule, energy efficiency, and catalyst lifetime, among many others. By tuning the dynamically changing conditions at the catalyst surface, the catalyst can operate in another kinetic regime or in multiple regimes; affecting the yield and selectivity and/or mass transfer of the limiting species can significantly elevate reactor productivity. Furthermore, the yield of intermediates in consecutive–competitive reaction networks can be elevated by time-dependent acceleration of forming reactions and throttling of decomposition reactions due to variable dosing of the educts. The thermal inertia of the catalytic bed may be used as a recuperator to preheat educt streams and to homogenise temperature profiles [8,92,93,94,95,96].
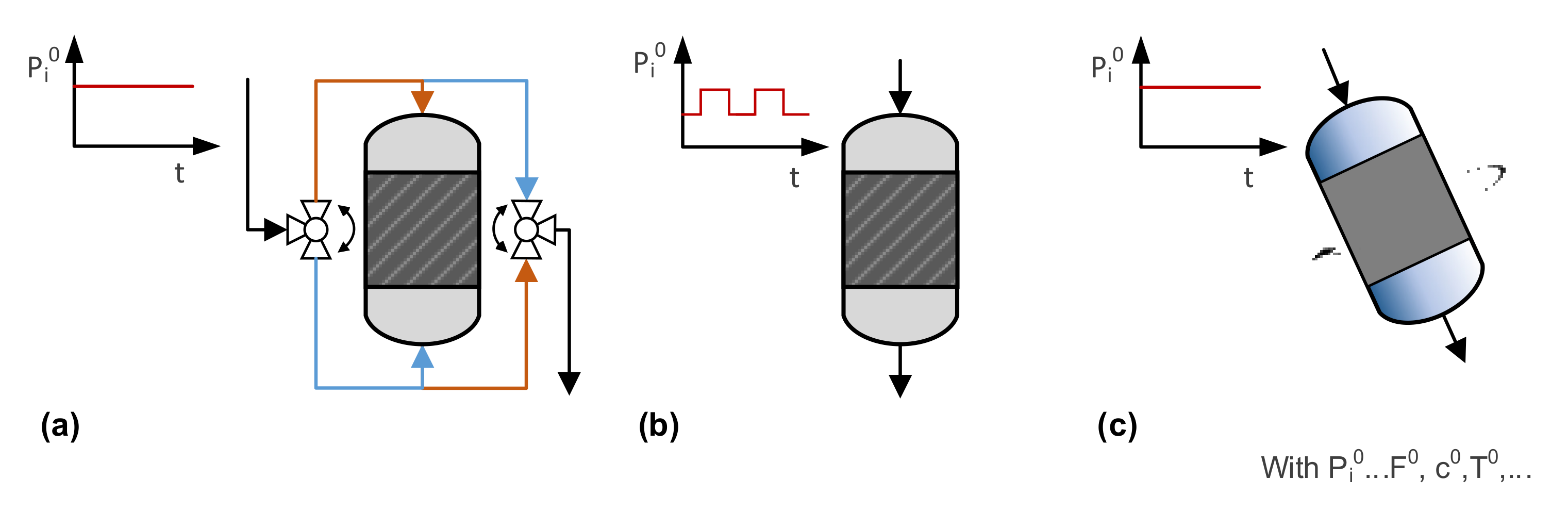
In dynamic operation modes, process parameters such as flow direction, flow rate, concentration, temperature, and pressure are periodically modulated, which may be achieved by (for example) flow reversal, modulation of inlet parameters, and movement of the reactor, as illustrated in Figure 7. It must be mentioned that there are many other strategies for benefiting from process dynamics, such as in discontinuously operated reactors, apparatuses combining reaction and separation (e.g., chromatographic, membrane or swing reactors), and systems with oscillating reactions, which will not be addressed here. Important characteristics of the dynamics include (a) the period , defining the time between the repetitions of changes; (b) the split , giving the duration ratio of a partial cycle; and (c) the amplitude , describing the intensity of the change. These additional degrees of freedom provide further tuning parameters for process optimization. On the other hand, identification of favorable operation modes and reliable comparison with steady-state modes is very challenging, and usually requires extensive reactor simulation.
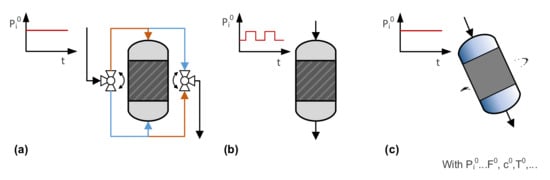
Figure 7.
Selected strategies for dynamic operation modes of fixed-bed reactors: (a) reverse flow reactors, (b) reactors with inlet modulation, and (c) rotating reactors.
Reverse flow reactors have been investigated for exothermic reactions since the 1960s. Such an apparatus efficiently traps the reaction heat of the chemical reaction in the fixed catalyst bed. By switching between the two flow directions, the thermal inertia of the catalytic bed is used to quickly elevate fluid temperatures at the reactor inlet, to adjust optimal temperature profiles for reversible and selective reactions, and to exploit dynamic properties of the catalyst [94,95].
Exhaust gas treatment converters for cars were introduced in the 1970s, and are a typical example of a reactor operated under varying inlet conditions. However, the change in the amount and composition of the inflowing gas is not on purpose because it is affected by the operation mode of the upstream engine. A systematic variation of inlet parameters was extensively studied in trickle-bed reactors, that is, reactors with a fixed solid catalyst and gas–liquid downflow. In the so-called forced periodic operation, mostly liquid flow rates are modulated to enhance reactor performance. The liquid-rich pulses modify flow regimes to intensify mixing and mass transfer as well as to improve catalyst wetting by flushing and restructuring the flow in the catalyst bed. The low-liquid or dry phase provokes a thinning of liquid films, which enhances the transport of the gaseous compound to the solid catalyst and reduces heat removal, elevating the bed temperature [96]. Other typical modulation mode use changes include: (a) the feed composition, to adjust selectivity [97,98,99]; (b) the system pressure, to enhance mass transfer inside the porous catalyst as well as to benefit from adsorption/desorption phenomena [100]; and (c) temperature, which is rather challenging because of the thermal reactor mass [101]. It should be mentioned that reactors with feed modulation will play a major role in the implementation of power-to-X processes with fluctuating energy supplies [102].
Another way to provoke periodic changes in chemical reactors is to move parts of the reactor, or even the whole reactor. Spinning disk reactors are a typical example, and were introduced in the final years of the last century [103]. In these contact apparatuses, thin liquid films are created on a rotating disk inside the reactor, which provides large and tunable mass transfer rates and favors applications in absorption, mixing, and reaction processes [104]. Härting et al. [105,106] presented the concept of a rotating, inclined packed-bed reactor for gas–liquid reactions. The inclination and gravity lead to radial gas and liquid separation inside the reactor, i.e., parts of the catalyst bed are completely wetted, whereas others are surrounded by the gas phase. By applying rotation, the catalyst bed is periodically immersed in the liquid phase, which provokes adjustable contact times for gas and liquid with the solid catalyst [107]. Enhancement factors of 80% have been reported for a hydrogenation reaction [108]. Even more complex movements of the whole reactor have been investigated by the group of Prof. Larachi using a hexapod ship motion simulator [109,110]. The setup mimics the rolling and heaving motions in marine applications, which supports the development of offshore reactor installations. In addition, the potential of inlet modulation for reduction of maldistribution was analyzed [110]. Recent reviews summarize important findings in this area [111,112,113].
The review article published recently by Marin et al. is a particularly useful reference [114], as the technology involved was screened in all its aspects, ranging from modeling to application examples to practical considerations. The authors guide readers in designing and modelling the correct reactor for specific chemical applications.
3.4. Alternative Fluids
Great attention is focused at present on the development of solvents to replace volatile organic compounds, improving processes using environmentally friendly solvents such as water, supercritical fluids, and ionic liquids [115]. Ionic liquids are salts melting at a temperature lower than 100 °C, resulting in liquids composed of cations and anions [116]. The growing interest in ionic liquids can be attributed to the wide range of their applications, such as in catalytic reactions, where the solubility of components and the interactions between solvents and solutes are of crucial interest due to their influencing the reactivity of the solutes themselves, as electrolytes in batteries and capacitors and to replace conventional solvents [115,116,117]. The miscibility of a substance with an ionic liquid depends on the ions which compose the ionic liquid itself and the properties of the solute. In particular, miscibility with water is very interesting. Some ionic liquids are miscible with water in all compositions, while in some other cases two different layers are formed. This phenomenon is due to the strong hydrogen bonds between water and the ionic liquid anions; the solubility of water in the ionic liquid can be modified by adding short chain alcohol to the biphasic systems to increase mixing [116]. Interesting properties are common among ionic liquids: they are non-flammable, which is very important when they are employed as solvents in exothermic reactions; their vapor pressure is low as well, a characteristic which allows their use in separation techniques such as distillation or sublimation, where low-boiling solvents cannot be used. Furthermore, thermal stability in a wide temperature range is typical of ionic liquids, allowing good kinetic control of chemical processes as well as applications in temperature-dependent processes such as extraction or crystallization [115].
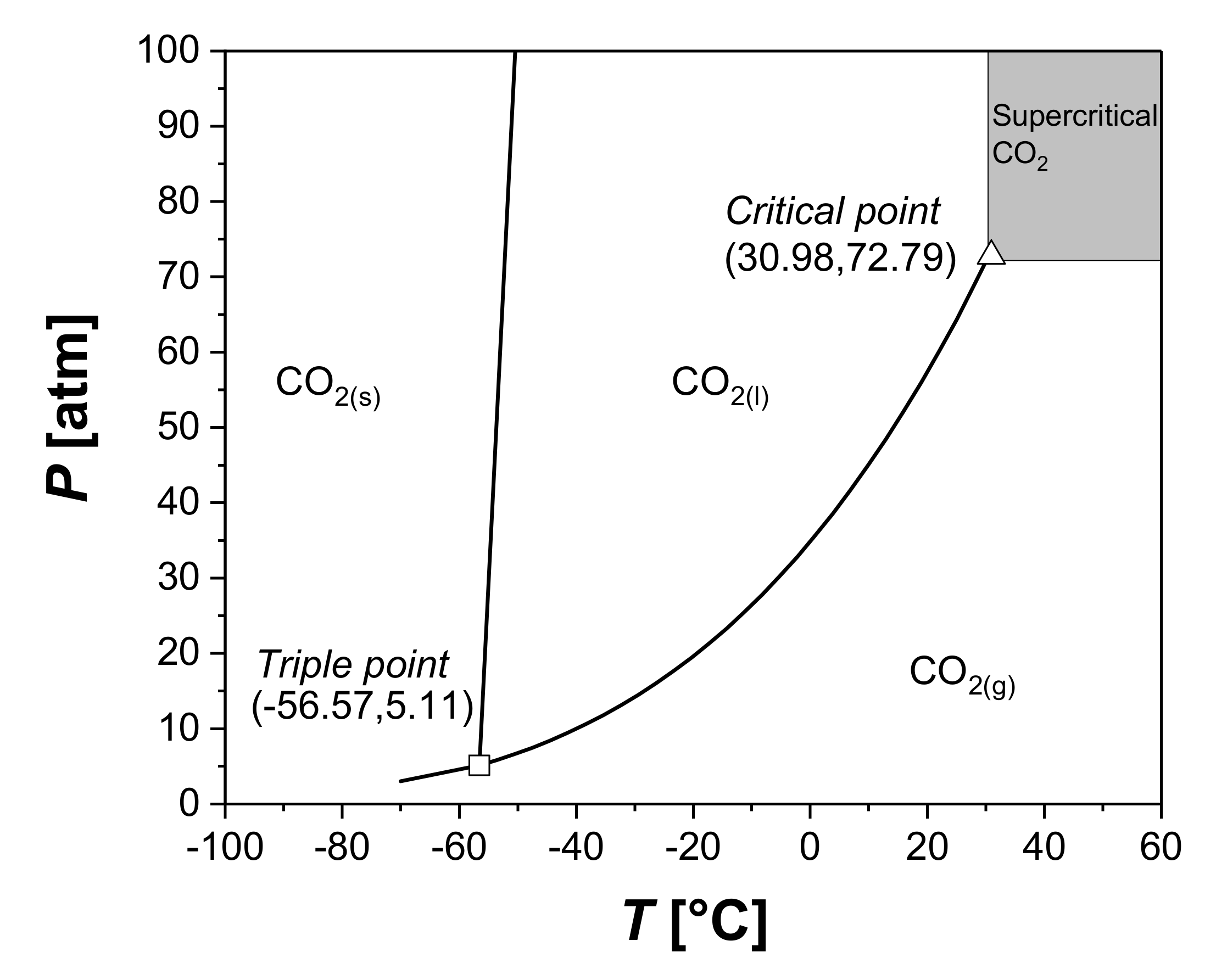
Another interesting alternative is represented by the supercritical fluids (Figure 8). A fluid is said to be supercritical when its temperature and pressure overcome the critical temperature and pressure. Supercritical fluids are characterized by intermediate properties between gases and liquids. They show a density similar to liquids with a viscosity and diffusivity similar to gases, and these properties can be modified by varying pressure and temperature [118].
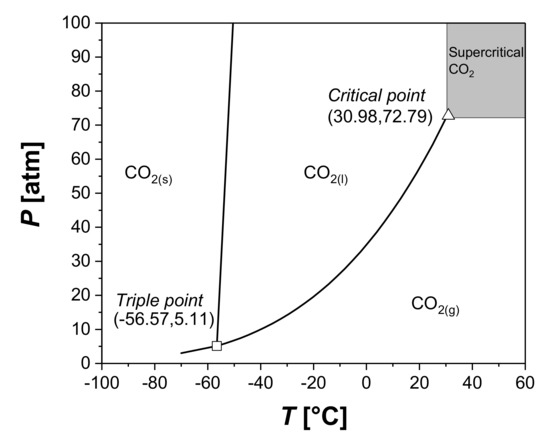
Figure 8.
CO2 phase diagram, highlighting the region of supercritical fluids and both the triple and critical points.
Consequently, a supercritical fluid can diffuse in a matrix faster than a liquid solvent; however, at the same time it displays the solvent strength of a liquid to extract a component from the matrix itself [119]. The main advantage of the use of supercritical fluids in industrial processes is the possibility of separating the product and reusing the gas without any other purification steps. These benefits are particularly evident in the use of the most important supercritical fluids, CO2 and water. They are nontoxic, non-flammable, and thermodynamically stable. Furthermore, they show excellent heat transfer properties, and thanks to this characteristic they are considered a valid alternative to the fluids used in refrigeration systems [120]. Great attention has been paid to the use of CO2 as a solvent, as there are no additional costs for waste treatment and the process residual can be considered a highly value-added byproduct; however, the use of other solvents such as propane, butane, hexane, and ethanol in supercritical condition recently is drawing interest as well [121]. Supercritical fluids can be used in many applications, such as for the synthesis of new materials, supports for new catalysts, as a reaction medium in several reactions (examples of reactions which can be conducted supercritically with CO2 include Friedel–Crafts alkylation, hydroformylation and transesterification [122]), and in separation techniques such as chromatography and extraction processes [120]. Supercritical fluid extraction and chromatography are important purification techniques in the pharmaceutical and food industries [121]. One example is the extraction process of some natural compounds, such as vitamins and flavors, which are soluble in supercritical fluids. The classical method occurs in the presence of organic solvents to dissolve and separate the compounds from the solid, after which the product can be separated from the solvent by increasing the temperature. The advantage of using supercritical fluids lies in the possibility of removing the solvent from the product through depressurization, which lowers the temperature of the process and can be carried out in the absence of organic solvents [120]. Supercritical fluid extraction can be performed by bringing into contact the solid substrate and the supercritical solvent. Usually, the solid substrate is in a fixed bed and the supercritical gas flows through the fixed bed itself, extracting the product components [122].
4. Process Intensification: Potentials and Guidelines
In this paragraph, the potential of the PI strategies described in the previous section will be described and discussed. Moreover, some guidelines are provided to help the researchers choose between the different available options for intensification of a given chemical process.
In Table 1, the main benefits and drawbacks of the different PI strategies are reported, together with some common application areas. This table may be of interest for a reader who is starting his or her scientific journey in the field of PI.

Table 1.
Main benefits and drawbacks of different PI strategies and potential application areas.
The main benefits were already discussed in the previous paragraph; thus, it may be worthwhile here to underline some important drawbacks of these operations.
Miniaturized and structured systems seem to be the best alternative in PI, as they allow to work in ideal conditions in terms of fluid dynamics, allowing achievement of high selectivity and low chemical risks and reducing operation volumes. Therefore, operation is difficult to scale at industrial level, as numbering up is an expensive procedure due to the high price of a single reactor. Moreover, there is an elevated blocking risk as microchannels can be blocked by even small traces of microparticles contained in the reactant vessels.
Static mixers are easy to be set up, as the operation consists of packing a standard pipe. Therefore, even if good mixing can be achieved the reactor volume can decrease dramatically, leading long pipes to achieve the reasonable residence times needed for operation.
Multifunctional reactors are very efficient when selectivity problems are the main issue in the process; however, their operation can be expensive and sophisticated in terms of process control. Membranes are usually expensive, and must be maintained and replaced frequently overall when the reaction leads to heavy byproducts. A simulated moving bed needs a sophisticated control logic to control the switch between the feed and withdrawal positions.
MW, US and Plasma are very good options when thermal control is an issue; therefore, the equipment is expensive and characterized by an intrinsic risk, i.e., exposure to irradiation, which requires additional attention to the management of the chemical plant.
Dynamic operations are useful to optimize product selectivity; however, complex process control is required to ensure reproducibility; thus, phase composition may vary at the reactor outlet from test to test, which must be avoided during the operation of the industrial plant.
Alternative fluids are well-suited to replace standard solvents, and ensure high mixing of reactant and products as well as their subsequent separation. Therefore, these liquids are often very expensive, and their recovery and relayed purification is a major issue.
A second question to be answered is how to choose between the different PI strategies. In Table 2, the most frequently encountered process limitations and the suitability of different PI strategies are reported. This table can be considered a conceptual map where the reader should detect the correct PI strategy once the limitations of the chemical process of interest are defined.

Table 2.
Commonly-encountered process limitations and suitability of different PI strategies.
When the main limitation, and thus the investigation area, is the reaction kinetics, most of the options are valid. Miniaturized systems can be useful to suppress internal mass transfer aspects and both concentration and temperature gradients along the axial and radial direction of the reactor, allowing the achievement of ideal fluid dynamics. Thus, it is possible to obtain the intrinsic kinetics of the system. When surface reactions are under investigation, transient operations can be of high impact, as they allow for collection of a large amount of steady-state data in one experiment [133,134] in order to distinguish between reaction mechanisms.
When working with continuous devices, very often the problem involves fluid–solid external mass transfer resistance [135,136]. In these cases, millireactors, microreactors, and static mixers can be the best option due to their high local turbulence, which can be achieved even at a low Reynolds number.
Product selectivity can be enhanced by using miniaturized reactors, static mixers, multifunctional reactors, and dynamic operations. There is a great deal of published literature in this field, demonstrating that when working at low residence times and for consecutive reaction networks, a high selectivity towards the intermediate can easily be achieved. For example, microreactors and transient-state packed beds can be adopted to enhance selectivity to ethylene oxide in the ethylene partial oxidation process [124,133].
Different options can be selected when the main idea is to decrease the process complexity; for example, reducing the amount of operation units using multifunctional reactors [52,55], or working with supercritical fluids to easily remove solvent from the reaction medium without the necessity of designing a sophisticated separation unit [119].
Finally, thermal and mixing control can be achieved using any of the selected options, as already discussed in the dedicated sections in the previous paragraph. The main idea is either to increase the specific surface area, namely the contact area between the different phases (milli- or microreactors, static mixers), use alternative energy sources (MW, US, plasma), or use a novel solvent which can be easily separated and acting as a thermal medium.
5. Conclusions
There is no a single choice, nor a simple choice, to intensify a chemical process. The PI strategy must be tailored to the chemical and physical application by investing time, passion, and energy in trying to find the best solution to achieve the PI goal. Possible alternatives, routes, and strategies are numerous, and new technologies will definitely emerge along with the need to reducing energy and loss of resources. In the present review article, we have summarized the main definitions and the strategies for PI in an attempt to provide order to the enormous literature published on this topic to date.
Author Contributions
Conceptualization, V.R., S.H. and P.T.; methodology, V.R. and S.H; formal analysis, V.R., S.H. and P.T.; data curation, S.H. and P.T.; writing—original draft prepara-tion, V.R., S.H. and P.T.; writing—review and editing, V.R., S.H. and P.T.; project administration, V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Francesco Taddeo is acknowledged for helping to realize this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stankiewicz, A.; Moulijn, J.A. Process intensification. Ind. Eng. Chem. Res. 2002, 41, 1920–1924. [Google Scholar] [CrossRef]
- Klipstein, D.H.; Robinson, S. Vision 2020: Reaction Engineering Roadmap; Office of Energy Efficiency and Renewable Energy (EERE): Washington, DC, USA, 2001; pp. 1–91. [Google Scholar]
- Ly, A.T.A. Roadmap for Catalysis Research in Germany; Society for Chemical Engineering and Biotechnology: Frankfurt, Germany, 2010. [Google Scholar]
- Schubert, M.; Bauer, T.; Agar, D.W.; Bertau, M.; Busch, M.; Claus, P.; Demtröder, D. Roadmap der chemischen Reaktionstechnik. Dechema Ges. Chem. Tech. 2010, 1, 41. [Google Scholar]
- IChemE. A Roadmap for 21st Century Chemical Engineering; The Institution of Chemical Engineers: Rugby, UK, 2007. [Google Scholar]
- Keil, F.J. Modeling of Process Intensification-An Introduction and Overview. In Modeling of Process Intensification; Wiley-VCH: Weinheim, Germany, 2007; pp. 1–7. ISBN 9783527311439. [Google Scholar] [CrossRef] [Green Version]
- Tonkovich, A.L.; Daymo, E. Process Intensification. In Handbook of Thermal Science and, Engineering; Kulacki, F., Ed.; Springer: Berlin, Germany, 2018; ISBN 9783319266954. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Gerven, T.V.; Stefanidis, G. The Fundamentals of Process Intensification; Wiley-VCH: Weinheim, Germany, 2019; ISBN 3527327835. [Google Scholar]
- Reay, D.; Ramshaw, C.; Harvey, A. Process Intensification: Engineering for Efficiency, Sustainability and Flexibility, 2nd ed.; Butterwoth-Heinemann: Oxford, UK, 2013; ISBN 9780080983042. [Google Scholar] [CrossRef]
- Dautzenberg, F.M.; Mukherjee, M. Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001, 56, 251–267. [Google Scholar] [CrossRef]
- Van Gerven, T.; Stankiewicz, A. Structure, energy, synergy, time-the fundamentals of process intensification. Ind. Eng. Chem. Res. 2009, 48, 2465–2474. [Google Scholar] [CrossRef]
- Grützner, T.; Ziegenbalg, D.; Güttel, R. Process Intensification—An Unbroken Trend in Chemical Engineering. Chem. Ing. Tech. 2018, 90, 1823–1831. [Google Scholar] [CrossRef]
- Hüther, A.; Geißelmann, A.; Hahn, H. Prozessintensivierung-Eine strategische option für die chemische industrie. Chem. Ing. Tech. 2005, 77, 1829–1837. [Google Scholar] [CrossRef]
- Keil, F.J. Process intensification. Rev. Chem. Eng. 2018, 34, 135–200. [Google Scholar] [CrossRef] [Green Version]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef] [Green Version]
- Dudukovic, M.P. Reaction engineering: Status and future challenges. Chem. Eng. Sci. 2010, 65, 3–11. [Google Scholar] [CrossRef]
- Salmi, T.; Mikkola, J.-P.; Wärnå, J. Chemical Reaction Engineering and Reactor Technology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Moulijn, J.A. Process intensification: Transforming chemical engineering. Chem. Eng. Prog. 2000, 96, 22–33. [Google Scholar]
- Illg, T.; Löb, P.; Hessel, V. Flow chemistry using milli- and microstructured reactors—From conventional to novel process windows. Bioorg. Med. Chem. 2010, 18, 3707–3719. [Google Scholar] [CrossRef]
- Hessel, V.; Hofmann, C.; Löwe, H.; Meudt, A.; Scherer, S.; Schönfeld, F.; Werner, B. Selectivity gains and energy savings for the industrial phenyl boronic acid process using micromixer/tubular reactors. Org. Process Res. Dev. 2004, 8, 511–523. [Google Scholar] [CrossRef]
- Gascon, J.; Van Ommen, J.R.; Moulijn, J.A.; Kapteijn, F. Structuring catalyst and reactor—An inviting avenue to process intensification. Catal. Sci. Technol. 2015, 5, 807–817. [Google Scholar] [CrossRef]
- Güttel, R.; Turek, T. Improvement of Fischer-Tropsch Synthesis through Structuring on Different Scales. Energy Technol. 2016, 4, 44–54. [Google Scholar] [CrossRef]
- Kockmann, N. 200 Jahre Entwicklung in Der Kontinuierlichen Destillation. Chem. Ing. Tech. 2013, 85, 1815–1823. [Google Scholar] [CrossRef]
- Stemmet, C.P. Gas-Liquid Solid Foam Reactors: Hydrodynamics and Mass Transfer. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Tomašić, V. Application of the monoliths in DeNOx catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Knon, H.; Brenscheidt, T.; Flörchinger, P. Keramische Ultradünnwandträger für zukünftige Emissionsanforderungen. MTZ 2001, 9, 662–666. [Google Scholar] [CrossRef]
- Brueck, R.; Mueller-Haas, K.; Breuer, J.; Webb, C. Advanced performance of metallic converter systems demonstrated on a production V8 engine. SAE Tech. Pap. 2002, 100, 54-3. [Google Scholar] [CrossRef] [Green Version]
- Hessel, V. Novel process windows—Gates to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 2009, 32, 1641. [Google Scholar] [CrossRef]
- Jensen, K.F. Flow chemistry—Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Lali, F.; Pahner, F.A.; Lange, R. Modeling and Simulation of the Hydrogenation of α-Methylstyrene on Catalytically Active Metal Foams as Tubular Reactor Packing. Int. J. Chem. Eng. 2016, 2016, 7082381. [Google Scholar] [CrossRef] [Green Version]
- Kallinikos, L.E.; Papayannakos, N.G. Intensification of hydrodesulphurization process with a structured bed spiral mini-reactor. Chem. Eng. Process. Process Intensif. 2010, 49, 1025–1030. [Google Scholar] [CrossRef]
- Shao, N.; Gavriilidis, A.; Angeli, P. Mass transfer during Taylor flow in microchannels with and without chemical reaction. Chem. Eng. J. 2010, 160, 873–881. [Google Scholar] [CrossRef]
- Langsch, R.; Haase, S.; Lange, R. Hydrodynamik und Stofftransport in einem Perlschnurreaktor für Gas/Flüssig/Fest-Reaktionen. Chem. Ing. Tech. 2013, 85, 642–655. [Google Scholar] [CrossRef]
- Vernuccio, S.; Dempfle, D.; Goy, R.; Medlock, J.; Rudolf von Rohr, P. External mass transfer in a laser sintered structured reactor for continuous hydrogenation of alkynes. Chem. Eng. Process. Process Intensif. 2018, 126, 74–80. [Google Scholar] [CrossRef]
- Lämmermann, M.; Horak, G.; Schwieger, W.; Freund, H. Periodic open cellular structures (POCS) for intensification of multiphase reactors: Liquid holdup and two-phase pressure drop. Chem. Eng. Process.-Process Intensif. 2018, 126, 178–189. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Snijkers, F.; Bouwen, W.; Bengaouer, A.; Marty, P. Innovative 3D-manufacture of structured copper supports post-coated with catalytic material for CO2 methanation. Chem. Eng. Process.-Process Intensif. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Biswas, P.; Mamatha, S.; Varghese, K.; Johnson, R.; Vijay, R.; Kumar, R. 3D printing of high surface area ceramic honeycombs substrates and comparative evaluation for treatment of sewage in Phytorid application. J. Water Process Eng. 2020, 37, 101503. [Google Scholar] [CrossRef]
- Reichelt, E.; Heddrich, M.P.; Jahn, M.; Michaelis, A. Fiber based structured materials for catalytic applications. Appl. Catal. A Gen. 2014, 476, 78–90. [Google Scholar] [CrossRef]
- Peng, Z.; Gai, S.; Barma, M.; Rahman, M.M.; Moghtaderi, B.; Doroodchi, E. Experimental study of gas-liquid-solid flow characteristics in slurry Taylor flow-based multiphase microreactors. Chem. Eng. J. 2021, 405, 126646. [Google Scholar] [CrossRef]
- Liedtke, A.K.; Bornette, F.; Philippe, R.; De Bellefon, C. Gas-liquid-solid “slurry Taylor” flow: Experimental evaluation through the catalytic hydrogenation of 3-methyl-1-pentyn-3-ol. Chem. Eng. J. 2013, 227, 174–181. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.N.; Larachi, F. Reactions, hydrodynamics and mass transfer in micro-packed beds—Overview and new mass transfer data. Chem. Eng. Process. Process Intensif. 2016, 110, 80–96. [Google Scholar] [CrossRef]
- Yang, C.; Teixeira, A.R.; Shi, Y.; Born, S.C.; Lin, H.; Li Song, Y.; Martin, B.; Schenkel, B.; Peer Lachegurabi, M.; Jensen, K.F. Catalytic hydrogenation of: N-4-nitrophenyl nicotinamide in a micro-packed bed reactor. Green Chem. 2018, 20, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Lobry, E.; Theron, F.; Gourdon, C.; Le Sauze, N.; Xuereb, C.; Lasuye, T. Turbulent liquid-liquid dispersion in SMV static mixer at high dispersed phase concentration. Chem. Eng. Sci. 2011, 66, 5762–5774. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.K.; Vial, C.; Nigam, K.D.P.; Nauman, E.B.; Djelveh, G. Static mixers in the process industries—A review. Chem. Eng. Res. Des. 2003, 81, 787–826. [Google Scholar] [CrossRef]
- Yuan, F.; Cui, Z.; Lin, J. Experimental and Numerical Study on Flow Resistance and Bubble Transport in a Helical Static Mixer. Energies 2020, 13, 1228. [Google Scholar] [CrossRef] [Green Version]
- Anxionnaz, Z.; Cabassud, M.; Gourdon, C.; Tochon, P. Heat exchanger/reactors (HEX reactors): Concepts, technologies: State-of-the-art. Chem. Eng. Process. Process Intensif. 2008, 47, 2029–2050. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Habchi, C.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Energy efficiency in process industry—High-efficiency vortex (HEV) multifunctional heat exchanger. Renew. Energy 2013, 56, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Agar, D.W. Multifunctional reactors: Old preconceptions and new dimensions. Chem. Eng. Sci. 1999, 54, 1299–1305. [Google Scholar] [CrossRef]
- Krishna, R. Reactive Separations: More Ways to Skin a Cat. Chemie Ing. Tech. 2001, 73, 766. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Rossano, C.; Vitiello, R.; Turco, R.; Salmi, T.; Di Serio, M. Chromatographic reactor modelling. Chem. Eng. J. 2019, 377, 119692. [Google Scholar] [CrossRef]
- Rossano, C.; Pizzo, C.L.; Tesser, R.; Di Serio, M.; Russo, V. Reactive chromatography applied to ethyl levulinate synthesis: A proof of concept. Processes 2021, 9, 1684. [Google Scholar] [CrossRef]
- Moreira, M.N.; Corrêa, I.; Ribeiro, A.M.; Rodrigues, A.E.; Faria, R.P.V. Solketal Production in a Fixed Bed Adsorptive Reactor through the Ketalization of Glycerol. Ind. Eng. Chem. Res. 2020, 59, 2805–2816. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Gomes, P.S.; Gandi, G.K.; Silva, V.M.T.M.; Rodrigues, A.E. Multifunctional Reactor for the Synthesis of Dimethylacetal. Ind. Eng. Chem. Res. 2007, 47, 3515–3524. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pereira, C.S.M.; Santos, J.C. Chromatographic Reactors. Chem. Eng. Technol. 2012, 35, 1171–1183. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Li, Y.; Xu, J.; Yu, W.; Ray, A.K. Multi-Objective Optimizations of Non-Isothermal Simulated Moving Bed Reactor: Parametric Analyses. Process 2021, 9, 360. [Google Scholar] [CrossRef]
- Spitters, J.; Gonçalves, J.C.; Faria, R.P.V.; Rodrigues, A.E. Optimization of the Production of 1,1-Diethoxybutane by Simulated Moving Bed Reactor. Process 2021, 9, 189. [Google Scholar] [CrossRef]
- Stankiewicz, A. Energy matters: Alternative sources and forms of energy for intensification of chemical and biochemical processes. Chem. Eng. Res. Des. 2006, 84, 511–521. [Google Scholar] [CrossRef]
- Martín, Á.; Navarrete, A. Microwave-assisted process intensification techniques. Curr. Opin. Green Sustain. Chem. 2018, 11, 70–75. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, Á.; Mato, R.B.; Cocero, M.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crops Prod. 2015, 74, 83–94. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, D.Y.; Luo, M.; Jin, S.; Zu, Y.G.; Efferth, T.; Fu, Y.J. Negative pressure cavitation-microwave assisted preparation of extract of Pyrola incarnata Fisch. rich in hyperin, 2′-O-galloylhyperin and chimaphilin and evaluation of its antioxidant activity. Food Chem. 2015, 169, 270–276. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2018, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, S.; Osawa, A.; Abe, M.; Serpone, N. On the generation of hot-spots by microwave electric and magnetic fields and their impact on a microwave-assisted heterogeneous reaction in the presence of metallic Pd nanoparticles on an activated carbon support. J. Phys. Chem. C 2011, 115, 23030–23035. [Google Scholar] [CrossRef]
- Gangurde, L.S.; Sturm, G.S.J.; Devadiga, T.J.; Stankiewicz, A.I.; Stefanidis, G.D. Complexity and Challenges in Noncontact High Temperature Measurements in Microwave-Assisted Catalytic Reactors. Ind. Eng. Chem. Res. 2017, 56, 13379–13391. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave-material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave Effects in Organic Synthesis: Myth or Reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef]
- Richards, W.T.; Loomis, A.L. The chemical effects of high frequency sound waves I. A preliminary survey. J. Am. Chem. Soc. 1927, 49, 3086–3100. [Google Scholar] [CrossRef]
- Plesset, M.S. The Dynamics of Cavitation Bubbles. J. Appl. Mech. 1949, 16, 277–282. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-assisted photocatalytic degradation of sulfadiazine using MgO@CNT heterojunction composite: Effective factors, pathway and biodegradability studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochem. 2017, 36, 527–543. [Google Scholar] [CrossRef]
- Chatel, G.; Monnier, C.; Kardos, N.; Voiron, C.; Andrioletti, B.; Draye, M. Green, selective and swift oxidation of cyclic alcohols to corresponding ketones. Appl. Catal. A Gen. 2014, 478, 157–164. [Google Scholar] [CrossRef]
- Gholap, A.R.; Venkatesan, K.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Ultrasound promoted acetylation of alcohols in room temperature ionic liquid under ambient conditions. Green Chem. 2003, 5, 693–696. [Google Scholar] [CrossRef]
- Zeng, H.; Li, H.; Shao, H. One-pot three-component Mannich-type reactions using Sulfamic acid catalyst under ultrasound irradiation. Ultrason. Sonochem. 2009, 16, 758–762. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Mukherjee, S.; Turrubiartes, L.C.; Banik, B.K. Ultrasound-assisted aza-Michael reaction in water: A green procedure. Ultrason. Sonochem. 2012, 19, 969–973. [Google Scholar] [CrossRef]
- Domini, C.E.; Silbestri, G.F.; Fernández Band, B.; Chopa, A.B. Ultrasound-assisted synthesis of unsymmetrical biaryls by Stille cross-coupling reactions. Ultrason. Sonochem. 2012, 19, 410–414. [Google Scholar] [CrossRef]
- De Souza, A.L.F.; da Silva, L.C.; Oliveira, B.L.; Antunes, O.A.C. Microwave- and ultrasound-assisted Suzuki-Miyaura cross-coupling reactions catalyzed by Pd/PVP. Tetrahedron Lett. 2008, 49, 3895–3898. [Google Scholar] [CrossRef]
- Lévêque, J.M.; Fujita, M.; Bosson, A.; Sohmiya, H.; Pétrier, C.; Komatsu, N.; Kimura, T. Secondary sonochemical effect on Mo-catalyzed bromination of aromatic compounds. Ultrason. Sonochem. 2011, 18, 753–756. [Google Scholar] [CrossRef]
- Li, D.J.; Song, J.F.; Xu, A.Q.; Liu, C.Q. Optimization of the ultrasound-assisted synthesis of lutein disuccinate using uniform design. Ultrason. Sonochem. 2014, 21, 98–103. [Google Scholar] [CrossRef]
- Dange, P.N.; Kulkarni, A.V.; Rathod, V.K. Ultrasound assisted synthesis of methyl butyrate using heterogeneous catalyst. Ultrason. Sonochem. 2015, 26, 257–264. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Vetal, M.D.; Rathod, V.K. Ultrasound assisted enzyme catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Ultrason. Sonochem. 2015, 22, 311–316. [Google Scholar] [CrossRef]
- Rabiei, K.; Naeimi, H. Ultrasonic assisted synthesis of gem-dichloroaziridine derivatives using Mg/CCl4 under neutral conditions. Ultrason. Sonochem. 2015, 24, 150–154. [Google Scholar] [CrossRef]
- Abiev, R.S.; Sladkovskiy, D.A.; Semikin, K.V.; Murzin, D.Y.; Rebrov, E.V. Non-Thermal Plasma for Process and Energy Intensification in Dry Reforming of Methane. Catal 2020, 10, 1358. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Schultheis, S.E. Non-Thermal Plasma Techniques for Pollution Control; Springer Science & Business Media, Springer: Berlin, Germany, 1993. [Google Scholar] [CrossRef]
- Du, C.; Li, H.; Zhang, L.; Wang, J.; Huang, D.; Xiao, M.; Cai, J.; Chen, Y.; Yan, H.; Xiong, Y.; et al. Hydrogen production by steam-oxidative reforming of bio-ethanol assisted by Laval nozzle arc discharge. Int. J. Hydrogen Energy 2012, 37, 8318–8329. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Schiappacasse, C.; Zhou, N.; Anderson, E.; Chen, D.; Liu, J.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; et al. A review on the non-thermal plasma-assisted ammonia synthesis technologies. J. Clean. Prod. 2018, 177, 597–609. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 2017, 14, 1600157. [Google Scholar] [CrossRef]
- Petitpas, G.; Rollier, J.D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Chung, W.C.; Chang, M.B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Lange, R. Modeling and Simulation of Unsteady-State-Operated Trickle-Flow Reactors. In Modeling of Process Intensification; Wiley-VCH: Weinheim, Germany, 2007; ISBN 9783527311439. [Google Scholar] [CrossRef]
- Hudgins, R.R.; Silveston, P.L.; Renken, A.; Matros, Y.S. Introduction. In Periodic Operation of Reactors; Silveston, P.L., Hudgins, R.R., Eds.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 1–22. ISBN 9780123918543. [Google Scholar] [CrossRef]
- Matros, Y.S.H.; Bunimovich, G.A. Reverse-flow operation in fixed bed catalytic reactors. Catal. Rev.-Sci. Eng. 1996, 38, 1–68. [Google Scholar] [CrossRef]
- Bunimovich, G.; Sapoundjiev, H. Periodic flow reversal. In Periodic Operation of Reactors; Silveston, P.L., Hudgins, R.R., Eds.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 495–542. ISBN 9780123918543. [Google Scholar] [CrossRef]
- Lange, R.; Hanika, J.; Stradiotto, D.; Hudgins, R.R.; Silveston, P.L. Investigations of periodically operated trickle-bed reactors. Chem. Eng. Sci. 1994, 49, 5615–5621. [Google Scholar] [CrossRef]
- Lange, R.; Schubert, M.; Dietrich, W.; Grünewald, M. Unsteady-state operation of trickle-bed reactors. Chem. Eng. Sci. 2004, 59, 5355–5361. [Google Scholar] [CrossRef]
- Silveston, P.L.; Hanika, J. Periodic operation of three—Phase catalytic reactors. Can. J. Chem. Eng. 2004, 82, 1105–1142. [Google Scholar] [CrossRef]
- Atta, A.; Roy, S.; Larachi, F.; Nigam, K.D.P. Cyclic operation of trickle bed reactors: A review. Chem. Eng. Sci. 2014, 115, 205–214. [Google Scholar] [CrossRef]
- Silveston, P.L.; Hudgins, R.R. Pressure modulation. In Periodic Operation of Reactors; Silveston, P.L., Hudgins, R.R., Eds.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 415–434. ISBN 9780123918543. [Google Scholar] [CrossRef]
- Brandner, J.D.J.; Silveston, P.L.; Hudgins, R.R. Temperature modulation. In Periodic Operation of Reactors; Silveston, P.L., Hudgins, R.R., Eds.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 435–462. ISBN 9780123918543. [Google Scholar] [CrossRef]
- Matthischke, S.; Roensch, S.; Güttel, R. Start-up Time and Load Range for the Methanation of Carbon Dioxide in a Fixed-Bed Recycle Reactor. Ind. Eng. Chem. Res. 2018, 57, 6391–6400. [Google Scholar] [CrossRef]
- Jachuck, R.J.; Ramshaw, C.; Boodhoo, K.; Dalgleish, J.C. Process intensification: The opportunity presented by spinning disc reactor technology. Inst. Chem. Eng. Symp. Ser. 1997, 141, 417–424. [Google Scholar]
- Pask, S.D.; Nuyken, O.; Cai, Z. The spinning disk reactor: An example of a process intensification technology for polymers and particles. Polym. Chem. 2012, 3, 2698–2707. [Google Scholar] [CrossRef]
- Härting, H.U.; Bieberle, A.; Lange, R.; Larachi, F.; Schubert, M. Hydrodynamics of co-current two-phase flow in an inclined rotating tubular fixed bed reactor—Wetting intermittency via periodic catalyst immersion. Chem. Eng. Sci. 2015, 128, 147–158. [Google Scholar] [CrossRef]
- Dashliborun, A.M.; Larachi, F.; Hamidipour, M. Cyclic operation strategies in inclined and moving packed beds—Potential marine applications for floating systems. AIChE J. 2016, 62, 4157–4172. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Luo, Y.; Chu, G.W.; Liu, W.; Shao, L.; Chen, J.F. Liquid holdup and wetting efficiency in a rotating trickle-bed reactor. AIChE J. 2019, 65, e16618. [Google Scholar] [CrossRef]
- Härting, H.U.; Lange, R.; Larachi, F.; Schubert, M. A novel inclined rotating tubular fixed bed reactor concept for enhancement of reaction rates and adjustment of flow regimes. Chem. Eng. J. 2015, 281, 931–944. [Google Scholar] [CrossRef]
- Assima, G.P.; Motamed-Dashliborun, A.; Larachi, F. Emulation of gas-liquid flow in packed beds for offshore floating applications using a swell simulation hexapod. AIChE J. 2015, 61, 2354–2367. [Google Scholar] [CrossRef]
- Motamed Dashliborun, A.; Larachi, F. Hydrodynamics of gas-liquid cocurrent downflow in floating packed beds. Chem. Eng. Sci. 2015, 137, 665–676. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Hydrodynamics and reaction performances of multiphase reactors for marine applications—A review. Int. J. Chem. React. Eng. 2019, 17, 20180178. [Google Scholar] [CrossRef]
- Motamed Dashliborun, A.; Larachi, F.; Schubert, M. Offshore Floating Packed-Bed Reactors: Key Challenges and Potential Solutions. Chem. Eng. Technol. 2017, 40, 1975–1984. [Google Scholar] [CrossRef] [Green Version]
- Iliuta, I.; Larachi, F. Fischer-Tropsch synthesis in vertical, inclined and oscillating trickle-bed reactors for offshore floating applications. Chem. Eng. Sci. 2018, 177, 509–522. [Google Scholar] [CrossRef]
- Marín, P.; Díez, F.V.; Ordóñez, S. Reverse flow reactors as sustainable devices for performing exothermic reactions: Applications and engineering aspects. Chem. Eng. Process.-Process Intensif. 2019, 135, 175–189. [Google Scholar] [CrossRef]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids—An overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Gordon, C.M. New developments in catalysis using ionic liquids. Appl. Catal. A Gen. 2001, 222, 101–117. [Google Scholar] [CrossRef]
- Cansell, F.; Aymonier, C.; Loppinet-Serani, A. Review on materials science and supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 331–340. [Google Scholar] [CrossRef]
- Ramsey, E.D.; Guo, W.; Liu, J.Y.; Wu, X.H. Supercritical Fluids. Compr. Biotechnol. Second Ed. 2011, 2, 1007–1026. [Google Scholar] [CrossRef]
- Knez, Z.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Brunner, G. Applications of supercritical fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- Russo, V.; Protasova, L.; Turco, R.; De Croon, M.H.J.M.; Hessel, V.; Santacesaria, E. Hydrogen peroxide decomposition on manganese oxide supported catalyst: From batch reactor to continuous microreactor. Ind. Eng. Chem. Res. 2013, 52, 7668–7676. [Google Scholar] [CrossRef]
- Russo, V.; Kilpiö, T.; Hernandez Carucci, J.; Di Serio, M.; Salmi, T.O. Modeling of microreactors for ethylene epoxidation and total oxidation. Chem. Eng. Sci. 2015, 134, 563–571. [Google Scholar] [CrossRef]
- Khan, Y.; Kilpiö, T.; Marin, M.; Russo, V.; Lehtonen, J.; Karinen, R.; Salmi, T. Modelling of a microreactor for the partial oxidation of 1-butanol on a titania supported gold catalyst. Chem. Eng. Sci. 2020, 221, 115695. [Google Scholar] [CrossRef]
- Santacesaria, E.; Russo, V.; Tesser, R.; Di Serio, M. A kinetic biphasic approach to biodiesel process intensification. Chem. Eng. Trans. 2019, 74, 1339–1344. [Google Scholar] [CrossRef]
- Santacesaria, E.; Turco, R.; Tortorelli, M.; Russo, V.; Di Serio, M.; Tesser, R. Biodiesel process intensification by using static mixers tubular reactors. Ind. Eng. Chem. Res. 2012, 51, 8777–8787. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Serio, M.D.; Russo, V.; Turco, R. A new simple microchannel device to test process intensification. Ind. Eng. Chem. Res. 2011, 50, 2569–2575. [Google Scholar] [CrossRef]
- Vu, T.D.; Seidel-Morgenstern, A. Quantifying temperature and flow rate effects on the performance of a fixed-bed chromatographic reactor. J. Chromatogr. A 2011, 1218, 8097–8109. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Yin, L.S.; Cao, S.W.; Gu, L.N.; Xu, G.S.; Du, P.; Chai, H.; Liao, Y.S.; Xue, C. Microwave-assisted heating synthesis: A general and rapid strategy for large-scale production of highly crystalline g-C3N4 with enhanced photocatalytic H2 production. Green Chem. 2014, 16, 4663–4668. [Google Scholar] [CrossRef]
- Mason, J.B.; Sun, Y. Microwave-Assisted Production of 5-Hydroxymethylfurfural from Glucose. ChemistrySelect 2021, 6, 10582–10586. [Google Scholar] [CrossRef]
- Shinde, K.; Kaliaguine, S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering 2019, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Alvear, M.; Fortunato, M.E.; Russo, V.; Salmi, T.; Di Serio, M. Modelling of transient kinetics in trickle bed reactors: Ethylene oxide production via hydrogen peroxide. Chem. Eng. Sci. 2022, 248, 117156. [Google Scholar] [CrossRef]
- Alvear, M.; Fortunato, M.E.; Russo, V.; Eränen, K.; Di Serio, M.; Lehtonen, J.; Rautiainen, S.; Murzin, D.; Salmi, T. Continuous Liquid-Phase Epoxidation of Ethylene with Hydrogen Peroxide on a Titanium-Silicate Catalyst. Ind. Eng. Chem. Res. 2021, 60, 9429–9436. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Rossano, C.; Cogliano, T.; Vitiello, R.; Leveneur, S.; Di Serio, M. Kinetic study of Amberlite IR120 catalyzed acid esterification of levulinic acid with ethanol: From batch to continuous operation. Chem. Eng. J. 2020, 401, 126126. [Google Scholar] [CrossRef]
- Salmi, T.; Russo, V.; Freites Aguilera, A. Modelling of the interaction of kinetics and external transport phenomena in structured catalysts: The effect of reaction kinetics, mass transfer and channel size distribution in solid foams. Chem. Eng. Sci. 2021, 244, 116815. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).