Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment
Abstract
:1. Introduction
2. Incorporated MOFs Synthesis Method
3. Application of Incorporated MOFs
3.1. Gas Separation Applications
3.2. Catalysis Applications
3.3. Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2021, 230, 109532. [Google Scholar] [CrossRef]
- Seth, S.; Matzger, A.J. Metal–organic frameworks: Examples, counterexamples, and an actionable definition. Cryst. Growth Des. 2017, 17, 4043–4048. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Anumah, A.; Louis, H.; Zafar, S.-U.; Hamzat, A.T.; Amusan, O.O.; Pigweh, A.I.; Akakuru, O.U.; Adeleye, A.T.; Magu, T.O. Metal-Organic Frameworks (MOFs): Recent Advances in Synthetic Methodologies and Some Applications. Chem. Methodol. 2018, 3, 283–305. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced adsorption properties. Mater. Res. Bull. 2018, 109, 124–133. [Google Scholar] [CrossRef]
- Cota, I.; Martinez, F.F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Coord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Pu, S.; Wang, J.; Li, L.; Zhang, Z.; Bao, Z.; Yang, Q.; Yang, Y.; Xing, H.; Ren, Q. Performance Comparison of Metal–Organic Framework Extrudates and Commercial Zeolite for Ethylene/Ethane Separation. Ind. Eng. Chem. Res. 2018, 57, 1645–1654. [Google Scholar] [CrossRef]
- Yao, L.; Tang, Y.; Cao, W.; Cui, Y.; Qian, G. Highly Efficient Encapsulation of Doxorubicin Hydrochloride in Metal–Organic Frameworks for Synergistic Chemotherapy and Chemodynamic Therapy. ACS Biomater. Sci. Eng. 2021, 7, 4999–5006. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, L.; Newby, R.; Han, X.; Morris, C.G.; Savage, M.; Krap, C.P.; Easun, T.L.; Frogley, M.D.; Cinque, G.; Murray, C.A. Binding and separation of CO2, SO2 and C2H2 in homo-and hetero-metallic metal–organic framework materials. J. Mater. Chem. A 2021, 9, 7190–7197. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kumar, V.; Kim, K.-H.; Kukkar, D. Metal–organic frameworks (MOFs): Potential and challenges for capture and abatement of ammonia. J. Mater. Chem. A 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M.J. High CO2 adsorption capacity and CO2/CH4 selectivity by nanocomposites of MOF-199. Energy Fuels 2017, 31, 5376–5384. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.J. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Jiang, H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3, 2740–2748. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Ribeiro, R.P.; Mota, J.P.; Rebelo, L.P.; Esperança, J.M.; Esteves, I.A. Ionic Liquid-Impregnated Metal–Organic Frameworks for CO2/CH4 Separation. ACS Appl. Nano Mater. 2019, 2, 7933–7950. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Xu, W.; Cao, J.-F.; Shu, Y.; Wang, J.-H. Ionic liquid modification of metal-organic framework endows high selectivity for phosphoproteins adsorption. Anal. Chim. Acta 2021, 1147, 144–154. [Google Scholar] [CrossRef]
- Dao, X.; Nie, M.; Sun, H.; Dong, W.; Xue, Z.; Li, Q.; Liao, J.; Wang, X.; Zhao, X.; Yang, D.; et al. Electrochemical performance of metal-organic framework MOF(Ni) doped graphene. Int. J. Hydrogen Energy 2022, 47, 16741–16749. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Sun, J.; Abednatanzi, S.; Van Der Voort, P.; Liu, Y.-Y.; Leus, K. POM@MOF Hybrids: Synthesis and Applications. Catalysts 2020, 10, 578. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.-J. Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef] [PubMed]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Co-ord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal−organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Vera, A.T.; De Moura, B.A.; Esteves, L.M.; Tariq, M.; Esperança, J.M.; Esteves, I.A. Paramagnetic ionic liquid/metal organic framework composites for CO2/CH4 and CO2/N2 separations. Front. Chem. 2020, 8, 590191. [Google Scholar] [CrossRef]
- Ming, M.; Yin, S.; Shi, J. Poly(ionic liquids)-Impregnated UiO-66 composites for efficient sequestration of dichromate. J. Solid State Chem. 2022, 310, 123091. [Google Scholar] [CrossRef]
- Abbasi, Z.; Cseri, L.; Zhang, X.; Ladewig, B.P.; Wang, H. Metal–Organic Frameworks (MOFs) and MOF-Derived Porous Carbon Materials for Sustainable Adsorptive Wastewater Treatment. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–194. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Co-ord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Xu, L.; Yan, S.; Choi, E.-Y.; Lee, J.Y.; Kwon, Y.-U. Product control by halide ions of ionic liquids in the ionothermal syntheses of Ni–(H)BTC metal–organic frameworks. Chem. Commun. 2009, 23, 3431–3433. [Google Scholar] [CrossRef]
- Lu, K.; Peláez, A.L.; Wu, L.-C.; Cao, Y.; Zhu, C.-H.; Fu, H. Ionothermal Synthesis of Five Keggin-Type Polyoxometalate-Based Metal–Organic Frameworks. Inorg. Chem. 2019, 58, 1794–1805. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Yoo, D.K.; Woo, H.C.; Jhung, S.H. Removal of particulate matter with metal–organic framework-incorporated materials. Co-ord. Chem. Rev. 2020, 422, 213477. [Google Scholar] [CrossRef]
- Kalaj, M.; Cohen, S.M. Postsynthetic modification: An enabling technology for the advancement of metal–organic frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Otal, E.H.; Kim, M.L.; Hattori, Y.; Kitazawa, Y.; Hinestroza, J.P.; Kimura, M. Versatile Covalent Postsynthetic Modification of Metal Organic Frameworks via Thermal Condensation for Fluoride Sensing in Waters. Bioengineering 2021, 8, 196. [Google Scholar] [CrossRef]
- Sosa, J.D.; Bennett, T.F.; Nelms, K.J.; Liu, B.M.; Tovar, R.C.; Liu, Y. Metal–Organic Framework Hybrid Materials and Their Applications. Crystals 2018, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Kiang, Y.-H.; Gardner, G.B.; Lee, S.; Xu, A.Z.; Lobkovsky, E.B. Variable Pore Size, Variable Chemical Functionality, and an Example of Reactivity within Porous Phenylacetylene Silver Salts. J. Am. Chem. Soc. 1999, 121, 8204–8215. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. Energy Chem. 2019, 1, 100006. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-organic frameworks as a platform for CO2 capture and chemical processes: Adsorption, membrane separation, catalytic-conversion, and electrochemical reduction of CO2. Catalysts 2020, 10, 1293. [Google Scholar] [CrossRef]
- Rufford, T.; Smart, S.; Watson, G.; Graham, B.; Boxall, J.; da Costa, J.D.; May, E. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar] [CrossRef]
- Grande, C.A.; Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Singh, P.; Kemper, J. CO2 Capture in Natural Gas Production by Adsorption Processes. Energy Procedia 2017, 114, 2259–2264. [Google Scholar] [CrossRef]
- Zeeshan, M.; Nozari, V.; Yagci, M.B.; Isık, T.; Unal, U.; Ortalan, V.; Keskin, S.; Uzun, A. Core–shell type ionic liquid/metal organic framework composite: An exceptionally high CO2/CH4 selectivity. J. Am. Chem. Soc. 2018, 140, 10113–10116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezginel, K.B.; Keskin, S.; Uzun, A. Tuning the Gas Separation Performance of CuBTC by Ionic Liquid Incorporation. Langmuir 2016, 32, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Clarizia, G. 30 years of membrane technology for gas separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar]
- Chen, Z.; Hong, Z.; Wu, H.; Li, C.; Jiang, Z. Tröger’s Base Polyimide Hybrid Membranes by Incorporating UiO-66-NH2 Nanoparticles for Gas Separation. Ind. Eng. Chem. Res. 2022, 61, 3418–3427. [Google Scholar] [CrossRef]
- Castarlenas, S.; Téllez, C.; Coronas, J. Gas separation with mixed matrix membranes obtained from MOF UiO-66-graphite oxide hybrids. J. Membr. Sci. 2017, 526, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Nabais, A.R.; Martins, A.P.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly (ionic liquid)-based engineered mixed matrix membranes for CO2/H2 separation. Sep. Purif. Technol. 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Pascanu, V.; Miera, G.G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Hu, X.; Lam, F.L.-Y. Towards a recyclable MOF catalyst for efficient production of furfural. Catal. Today 2018, 314, 129–136. [Google Scholar] [CrossRef]
- Gangu, K.K.; Jonnalagadda, S.B. A review on metal-organic frameworks as congenial heterogeneous catalysts for potential organic transformations. Front. Chem. 2021, 9, 747615. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, H.; Vardhan, H.; Aguila, B.; Zhong, C.; Perman, J.A.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation. ACS Appl. Mater. Interfaces 2018, 10, 27124–27130. [Google Scholar] [CrossRef]
- Veisi, H.; Abrifam, M.; Kamangar, S.A.; Pirhayati, M.; Saremi, S.G.; Noroozi, M.; Tamoradi, T.; Karmakar, B. Pd immobilization biguanidine modified Zr-UiO-66 MOF as a reusable heterogeneous catalyst in Suzuki–Miyaura coupling. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Xu, S.; Zhang, J.; Zhu, Y.; Gao, L.; Xiao, G. 2-Methylimidazole Modified Co-BTC MOF as an Efficient Catalyst for Chemical Fixation of Carbon Dioxide. Catal. Lett. 2019, 149, 2575–2585. [Google Scholar] [CrossRef]
- Ten, S.; Torbina, V.V.; Zaikovskii, V.I.; Kulinich, S.A.; Vodyankina, O.V. Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid. Materials 2020, 13, 5471. [Google Scholar] [CrossRef]
- Muralikrishna, I.V.; Manickam, V. Industrial wastewater treatment technologies, recycling, and reuse. Environ. Manag. 2017, 295–336. [Google Scholar]
- Muralikrishna, I.V.; Manickam, V.J.E.M. Chapter One—Introduction. In Environmental Management; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–4. ISBN 978-0-12-811989-1. [Google Scholar]
- Shojaei, S.; Shojaei, S. Optimization of process. In Soft Computing Techniques in Solid Waste and Wastewater Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–392. [Google Scholar]
- Bhadra, B.N.; Lee, J.K.; Cho, C.-W.; Jhung, S.H. Remarkably efficient adsorbent for the removal of bisphenol A from water: Bio-MOF-1-derived porous carbon. Chem. Eng. J. 2018, 343, 225–234. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.-S.; Di Serio, M. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 581487. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2017, 119, 157–184. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M.; Gupta, A. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–25. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Kavak, S.; Durak, Ö.; Kulak, H.; Polat, H.M.; Keskin, S.; Uzun, A. Enhanced Water Purification Performance of Ionic Liquid Impregnated Metal–Organic Framework: Dye Removal by [BMIM][PF6]/MIL-53 (Al) Composite. Front. Chem. 2021, 8, 622567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.; Dilbaghi, N. Nanotechnology-Based Water Treatment Strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. In Heterogeneous Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–264. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Balaguru, S.; Arthanareeswaran, G.; Das, D.B. Removal of hazardous material from wastewater by using metal organic framework (MOF) embedded polymeric membranes. Sep. Sci. Technol. 2018, 54, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Younis, S.A.; Serp, P.; Nassar, H.N. Photocatalytic and biocidal activities of ZnTiO2 oxynitride heterojunction with MOF-5 and g-C3N4: A case study for textile wastewater treatment under direct sunlight. J. Hazard. Mater. 2020, 410, 124562. [Google Scholar] [CrossRef] [PubMed]
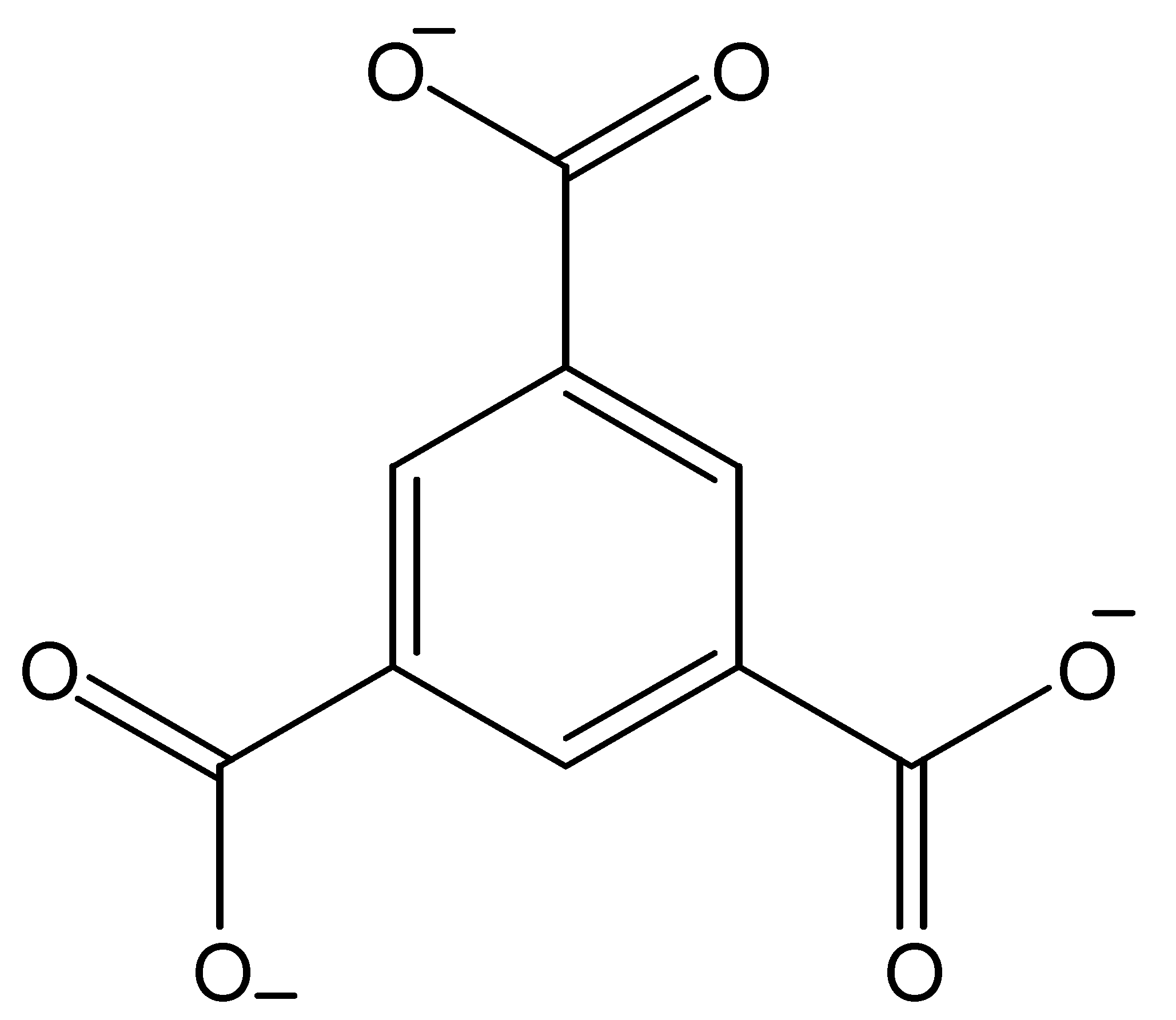

| Hybrid Materials | Synthesis Method | Separation | Efficiency | Reference |
|---|---|---|---|---|
| ZIF-8/[HEMIM] [DCA] | Wet impregnation | CO2, CH4 | Increase in CO2 uptake and decrease in CH4 uptake. | [44] |
| ZIF-8/[C2MIM] [NTf2] ZIF-8/[C2OHMIM] [NTf2] ZIF-8/[C6MIM] [NTf2] ZIF-8/[BzMIM] [NTf2] ZIF-8/[C10MIM] [NTf2] ZIF-8/[P6 6 6 14] [NTf2] ZIF-8/[C6MIM] [N(CN)2] ZIF-8/[C6MIM] [C(CN)3] ZIF-8/[C6MIM] [Cl] ZIF-8/[C2MIM] [Ac] | Wet impregnation | CO2/CH4 | Better ideal selectivity between CO2/CH4. | [18] |
| CuBTC/[BMIM] [BF4] | Wet impregnation | CO2/CH4, CO2/N2, CO2/H2, CH4/N2, CH4/H2, N2/H2 | CH4 selectivity over CO2, H, and N2 gases increase by 1.5-fold. | [45] |
| UiO-66_GO | Hydrothermal | H2/CH4, CO2/CH4 | UiO-GO hybrids produced the best separation results in the case of the CO2/CH4 separation. | [48] |
| UiO-66-NH2/Tröger’s Base Polyimide Hybrid Membranes | Solution casting method | CO2/CH4, CO2/N2, O2/N2 | Exhibits ∼166% increases in CO2 and O2 permeabilities. CO2 separation performance shows a trend in approaching the 2008 Robeson upper bound, showing a potential application prospect for CO2 removal from NG. | [47] |
| MIL-53(Al)/poly[Pyr11] [Tf2N] + [C4mpyr] [Tf2N] (MMM) Cu3(BTC)2/MMM ZIF-8/MMM | Solvent evaporation | CO2/H2 | Improvement in both CO2 permeability and CO2/H2 ideal selectivity. | [49] |
| Hybrid Materials | Synthesis Method | Application | Efficiency | Reference |
|---|---|---|---|---|
| MIL-101−SO3H/IL | Acid−base attraction | CO2 Chemical Fixation | High catalytic activity for cyclizing CO2 with epichlorohydrin under atmospheric pressure | [55] |
| UiO-66-biguanidine/Pd | Hydrothermal | Suzuki–Miyaura coupling | Better recyclability | [56] |
| Co-BTC/2MEIm | Solvothermal | CO2 Chemical Fixation | Higher selectivity and higher conversion | [57] |
| UiO-66/Bimetallic AgPd | Incipient Wetness Impregnation Double solvent impregnation | PG into LA | Higher PG conversion | [58] |
| Hybrid Materials | Synthesis Method | Pollutant Removal | Efficiency | Reference |
|---|---|---|---|---|
| Bio-MOF-1-derived carbons (BMDC) | Carbonization | Bisphenol A(BPA) | BMDC-12h adsorbent showed an efficiency ∼5 times that of a commercial activated carbon in BPA capture. | [62] |
Embedded polymeric membrane
| Immersion precipitation | Metal ions (copper Cu (II) and cobalt Co (II)) | More than 50% rejection efficiency for Cu(II). CA/MOF-5 showed 77% rejection efficiency on Co(II). | [71] |
| Al-MIL-53/[BMIM] [PF6] | Wet impregnation | Dye removal | increased dye removal efficiency and the maximum dye adsorption capacity for both methylene blue (MB) and methyl orange (MO). | [68] |
| MOF-5/Zn0.05TiOxNy | Microwave | Organic dye and microbial contaminants | Shows highest ability in inhibit microbial growth. Can be recyled up to five times. | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffar, Z.; Yunus, N.M.; Shaharun, M.S.; Allim, M.F.; Rahim, A.H.A. Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment. Processes 2022, 10, 2368. https://doi.org/10.3390/pr10112368
Jaffar Z, Yunus NM, Shaharun MS, Allim MF, Rahim AHA. Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment. Processes. 2022; 10(11):2368. https://doi.org/10.3390/pr10112368
Chicago/Turabian StyleJaffar, Zahirah, Normawati M. Yunus, Maizatul Shima Shaharun, Muhammad Faizadmesa Allim, and Asyraf Hanim Ab Rahim. 2022. "Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment" Processes 10, no. 11: 2368. https://doi.org/10.3390/pr10112368
APA StyleJaffar, Z., Yunus, N. M., Shaharun, M. S., Allim, M. F., & Rahim, A. H. A. (2022). Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment. Processes, 10(11), 2368. https://doi.org/10.3390/pr10112368






