Construction and Optimization of Malonyl-CoA Sensors in Saccharomyces cerevisiae by Combining Promoter Engineering Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Medium
2.2. Plasmid and Strain Construction
2.3. Analysis of Malonyl-CoA Sensors
3. Results and Discussion
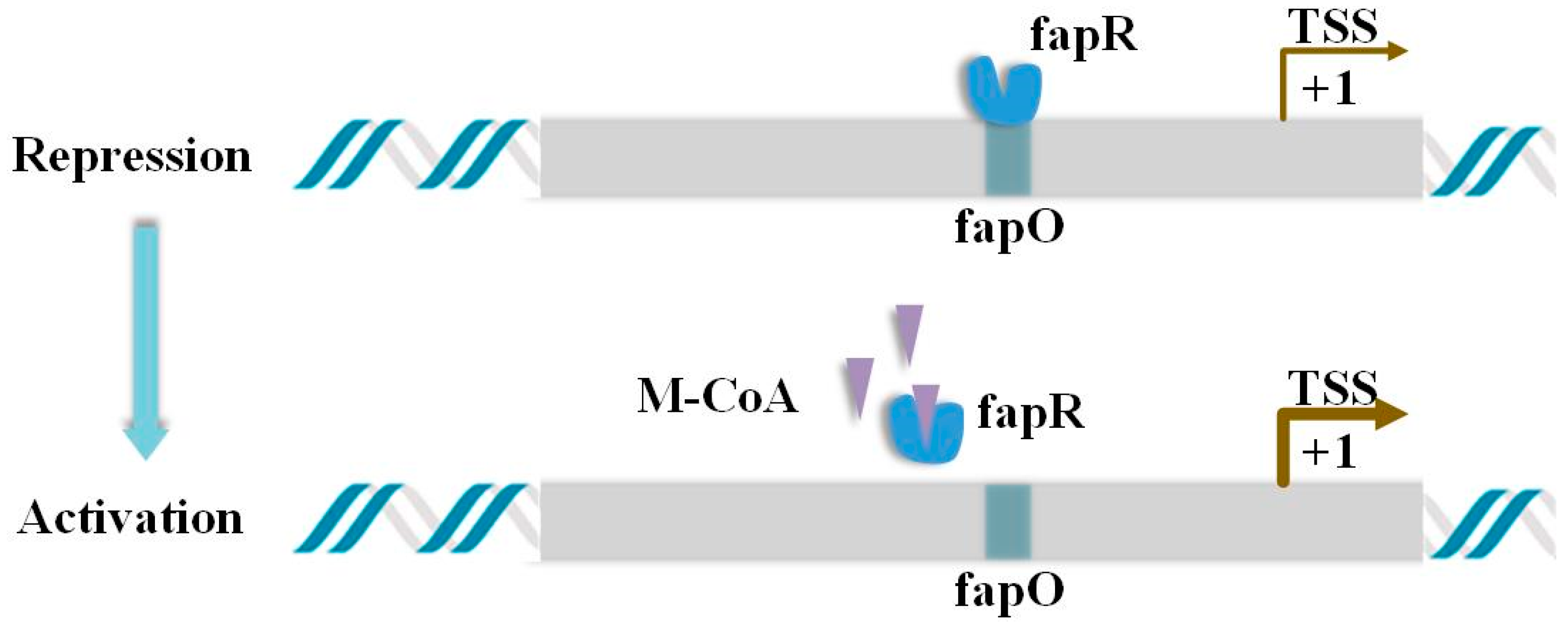
3.1. Construction of Malonyl-CoA Sensor
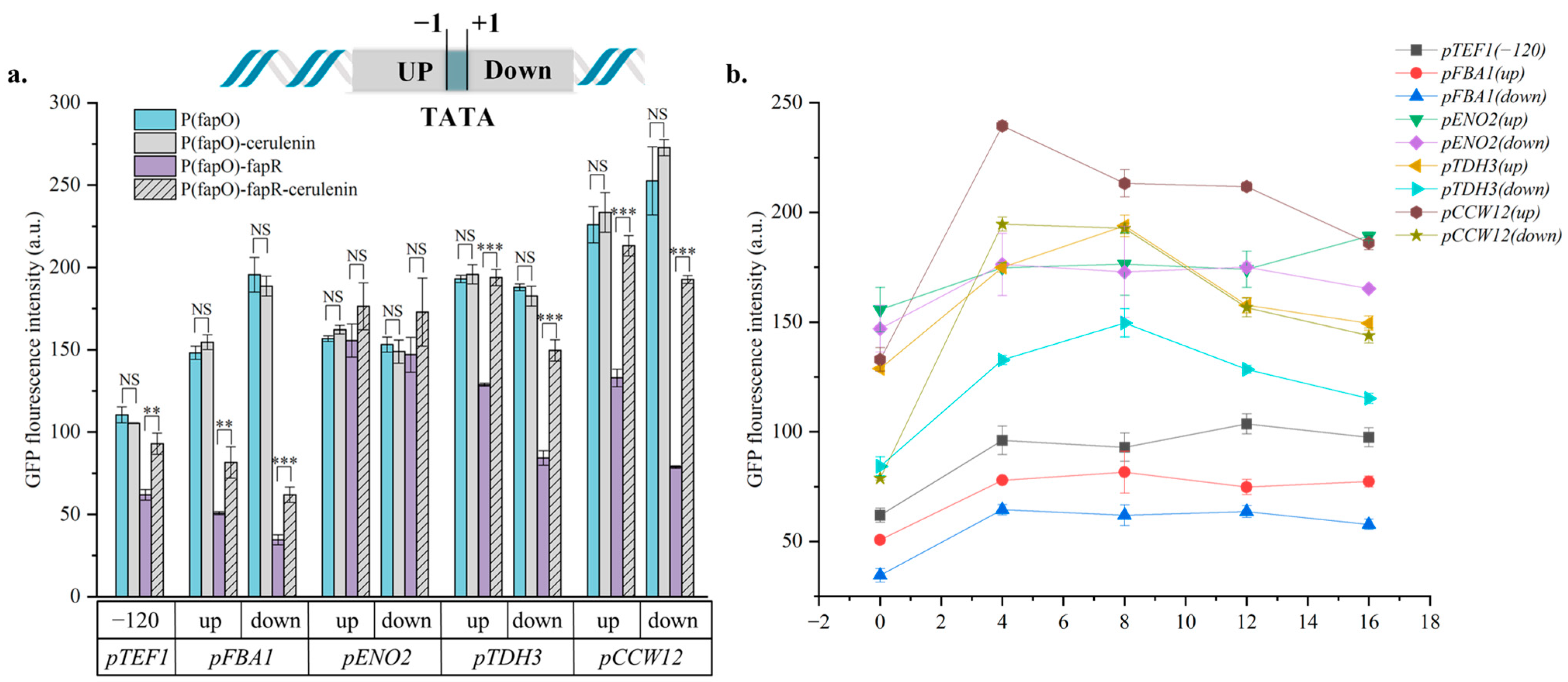
3.2. Optimization of Malonyl-CoA Sensors by Choosing Different fapO Inserting Sites
3.3. Broaden the Regulation Range of Malonyl-CoA Sensors by Combining Promoter Engineering Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Ding, Y.; Xian, M.; Liu, M.; Liu, H.; Ma, Q.; Zhao, G. Malonyl-CoA pathway: A promising route for 3-hydroxypropionate biosynthesis. Crit. Rev. Biotechnol. 2017, 37, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Li, L.; Linhardt, R.J.; Yan, Y. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab. Eng. 2015, 29, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xie, C.; Hong, R. Bioinspired iterative synthesis of polyketides. Front. Chem. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Schujman, G.E.; Guerin, M.; Buschiazzo, A.; Schaeffer, F.; Llarrull, L.; Reh, G.; Vila, A.; Alzari, P.M.; De Mendoza, D. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J. 2006, 25, 4074–4083. [Google Scholar] [CrossRef]
- Xu, P.; Wang, W.; Li, L.; Bhan, N.; Zhang, F.; Koffas, M.A.G. Design and kinetic analysis of a hybrid promoter–regulator system for malonyl-CoA sensing in Escherichia coli. ACS Chem. Biol. 2013, 9, 451–458. [Google Scholar] [CrossRef]
- Xu, P.; Li, L.; Zhang, F.; Stephanopoulos, G.; Koffas, M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl. Acad. Sci. USA 2014, 111, 11299–11304. [Google Scholar] [CrossRef]
- Wen, J.; Tian, L.; Xu, M.; Zhou, X.; Zhang, Y.; Cai, M. A Synthetic Malonyl-CoA Metabolic Oscillator in Komagataella phaffii. ACS Synth. Biol. 2020, 9, 1059–1068. [Google Scholar] [CrossRef]
- Wen, J.; Tian, L.; Liu, Q.; Zhang, Y.; Cai, M. Engineered dynamic distribution of malonyl-CoA flux for improving polyketide biosynthesis in Komagataella phaffii. J. Biotechnol. 2020, 320, 80–85. [Google Scholar] [CrossRef]
- Li, S.; Si, T.; Wang, M.; Zhao, H. Development of a Synthetic Malonyl-CoA Sensor in Saccharomyces cerevisiae for Intracellular Metabolite Monitoring and Genetic Screening. ACS Synth. Biol. 2015, 4, 1308–1315. [Google Scholar] [CrossRef]
- David, F.; Nielsen, J.; Siewers, V. Flux Control at the Malonyl-CoA Node through Hierarchical Dynamic Pathway Regulation in Saccharomyces cerevisiae. ACS Synth. Biol. 2016, 5, 224–233. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Shen, Y.; Hou, J.; Bao, X. Screening Phosphorylation Site Mutations in Yeast Acetyl-CoA Carboxylase Using Malonyl-CoA Sensor to Improve Malonyl-CoA-Derived Product. Front. Microbiol. 2018, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Dabirian, Y.; Li, X.; Chen, Y.; David, F.; Nielsen, J.; Siewers, V. Expanding the Dynamic Range of a Transcription Factor-Based Biosensor in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-Q.; Jin, W.-R.; Ma, Z.-C.; Shen, Q.; Cai, X.; Liu, Z.-Q.; Zheng, Y.-G. Promoter engineering strategies for the overproduction of valuable metabolites in microbes. Appl. Microbiol. Biotechnol. 2019, 103, 8725–8736. [Google Scholar] [CrossRef] [PubMed]
- Blazeck, J.; Garg, R.; Reed, B.; Alper, H.S. Controlling promoter strength and regulation in Saccharomyces cerevisiae using synthetic hybrid promoters. Biotechnol. Bioeng. 2012, 109, 2884–2895. [Google Scholar] [CrossRef]
- Blazeck, J.; Miller, J.; Pan, A.; Gengler, J.; Holden, C.; Jamoussi, M.; Alper, H.S. Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl. Microbiol. Biotechnol. 2014, 98, 8155–8164. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, H.; Rexida, R.; Shen, Y.; Hou, J.; Bao, X. Developing synthetic hybrid promoters to increase constitutive or diauxic shift-induced expression in Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18, foy098. [Google Scholar] [CrossRef]
- Guarente, L.; Yocum, R.R.; Gifford, P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc. Natl. Acad. Sci. USA 1982, 79, 7410–7414. [Google Scholar] [CrossRef]
- Feng, X.; Marchisio, M. Novel S. cerevisiae Hybrid Synthetic Promoters Based on Foreign Core Promoter Sequences. Int. J. Mol. Sci. 2021, 22, 5704. [Google Scholar] [CrossRef]
- Purvis, I.J.; Chotai, D.; Dykes, C.W.; Lubahn, D.B.; French, F.S.; Wilson, E.M.; Hobden, A.N. An androgen-inducible expression system for Saccharomyces cerevisiae. Gene 1991, 106, 35–42. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Nagawa, F. Effect of artificially inserted intron on gene expression in Saccharomyces cerevisiae. DNA Cell Biol. 1994, 13, 51–58. [Google Scholar] [CrossRef]
- Hoshida, H.; Kondo, M.; Kobayashi, T.; Yarimizu, T.; Akada, R. 5´-UTR introns enhance protein expression in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 241–251. [Google Scholar] [CrossRef]
- Cui, X.; Ma, X.; Prather, K.L.; Zhou, K. Controlling protein expression by using intron-aided promoters in Saccharomyces cerevisiae. Biochem. Eng. J. 2021, 176, 108197. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Huq, E.; Tepperman, J.M.; Quail, P.H. A light-switchable gene promoter system. Nat. Biotechnol. 2002, 20, 1041–1044. [Google Scholar] [CrossRef]
- Louvion, J.-F.; Havaux-Copf, B.; Picard, D. Fusion of GAL4-VP16 to a steroid-binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene 1993, 131, 129–134. [Google Scholar] [CrossRef]
- McIsaac, R.S.; Silverman, S.J.; McClean, M.N.; Gibney, P.A.; Macinskas, J.; Hickman, M.J.; Petti, A.A.; Botstein, D. Fast-acting and nearly gratuitous induction of gene expression and protein depletion in Saccharomyces cerevisiae. Mol. Biol. Cell 2011, 22, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Alper, H.; Fischer, C.; Nevoigt, E.; Stephanopoulos, G. Tuning genetic control through promoter engineering. Proc. Natl. Acad. Sci. USA 2005, 102, 12678–12683. [Google Scholar] [CrossRef] [PubMed]
- Nevoigt, E.; Kohnke, J.; Fischer, C.R.; Alper, H.; Stahl, U.; Stephanopoulos, G. Engineering of promoter replacement cassettes for fine-tuning of gene expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2006, 72, 5266–5273. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, H. Directed evolution of a highly efficient cellobiose utilizing pathway in an industrial Saccharomyces cerevisiae strain. Biotechnol. Bioeng. 2013, 110, 2874–2881. [Google Scholar] [CrossRef]
- Ruohonen, L.; Penttilä, M.; Keränen, S. Optimization of Bacillus α-amylase production by Saccharomyces cerevisiae. Yeast 1991, 7, 337–346. [Google Scholar] [CrossRef]
- Ruohonen, L.; Aalto, M.K.; Keränen, S. Modifications to the ADH1 promoter of Saccharomyces cerevisiae for efficient production of heterologous proteins. J. Biotechnol. 1995, 39, 193–203. [Google Scholar] [CrossRef]
- Raveh-Sadka, T.; Levo, M.; Shabi, U.; Shany, B.; Keren, L.; Lotan-Pompan, M.; Zeevi, D.; Sharon, E.; Weinberger, A.; Segal, E. Manipulating nucleosome disfavoring sequences allows fine-tune regulation of gene expression in yeast. Nat. Genet. 2012, 44, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Redden, H.; Alper, H.S. The development and characterization of synthetic minimal yeast promoters. Nat. Commun. 2015, 6, 7810. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Characterization of Promoters from Saccharomyces cerevisiae and Escherichia coli and Application in Metabolic Engineering. Beijing Institute of Technology, 2015. Available online: https://www.ckcest.cn/default/es3/detail/1003/dw_thesis_copy/e387acd6d014df8e2a43f2206b20b7df (accessed on 20 April 2022).
- Partow, S.; Siewers, V.; Bjørn, S.; Nielsen, J.; Maury, J. Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 2010, 27, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Aref, R.; Schüller, H.-J. Functional analysis of Cti6 core domain responsible for recruitment of epigenetic regulators Sin3, Cyc8 and Tup1. Curr. Genet. 2020, 66, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Tagoj, M.N.; Comino, A.; Komel, R. Fluorescence based assay of GAL system in yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2005, 244, 105–110. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Zhang, J.; Shen, Y.; Qi, Q.; Bao, X.; Hou, J. Quorum sensing-mediated protein degradation for dynamic metabolic pathway control in Saccharomyces cerevisiae. Metab. Eng. 2021, 64, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Averesch, N.; Winter, G.; Plan, M.; Vickers, C.; Nielsen, L.; Krömer, J. Quorum-sensing linked RNA interference for dynamic metabolic pathway control in Saccharomyces cerevisiae. Metab. Eng. 2015, 29, 124–134. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhang, Z.; Zhang, C.; Lu, W. Construction and Optimization of Malonyl-CoA Sensors in Saccharomyces cerevisiae by Combining Promoter Engineering Strategies. Processes 2022, 10, 2660. https://doi.org/10.3390/pr10122660
He S, Zhang Z, Zhang C, Lu W. Construction and Optimization of Malonyl-CoA Sensors in Saccharomyces cerevisiae by Combining Promoter Engineering Strategies. Processes. 2022; 10(12):2660. https://doi.org/10.3390/pr10122660
Chicago/Turabian StyleHe, Shifan, Zhanwei Zhang, Chuanbo Zhang, and Wenyu Lu. 2022. "Construction and Optimization of Malonyl-CoA Sensors in Saccharomyces cerevisiae by Combining Promoter Engineering Strategies" Processes 10, no. 12: 2660. https://doi.org/10.3390/pr10122660
APA StyleHe, S., Zhang, Z., Zhang, C., & Lu, W. (2022). Construction and Optimization of Malonyl-CoA Sensors in Saccharomyces cerevisiae by Combining Promoter Engineering Strategies. Processes, 10(12), 2660. https://doi.org/10.3390/pr10122660






