Research on the Application of Coal Gasification Slag in Soil Improvement
Abstract
:1. Introduction
2. Overview of the Case Study Area
3. Sample Collection and Treatment
3.1. Sample Collection
3.2. Sample Treatment and Analysis
4. Testing Results of CCGS
4.1. Microscopic Characterization
4.2. Pollutant Content
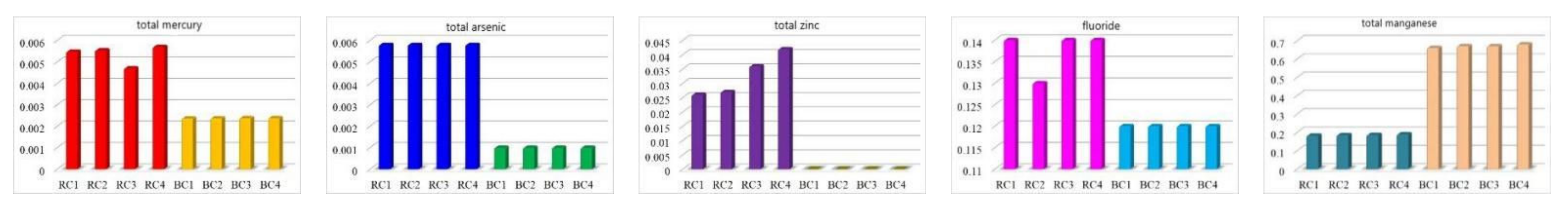
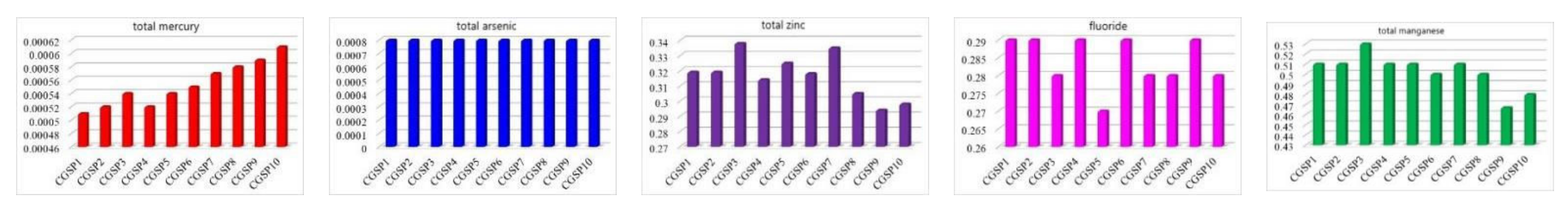
4.2.1. Raw Coal Analysis
4.2.2. Contents of Characteristic Pollutants in CCGS
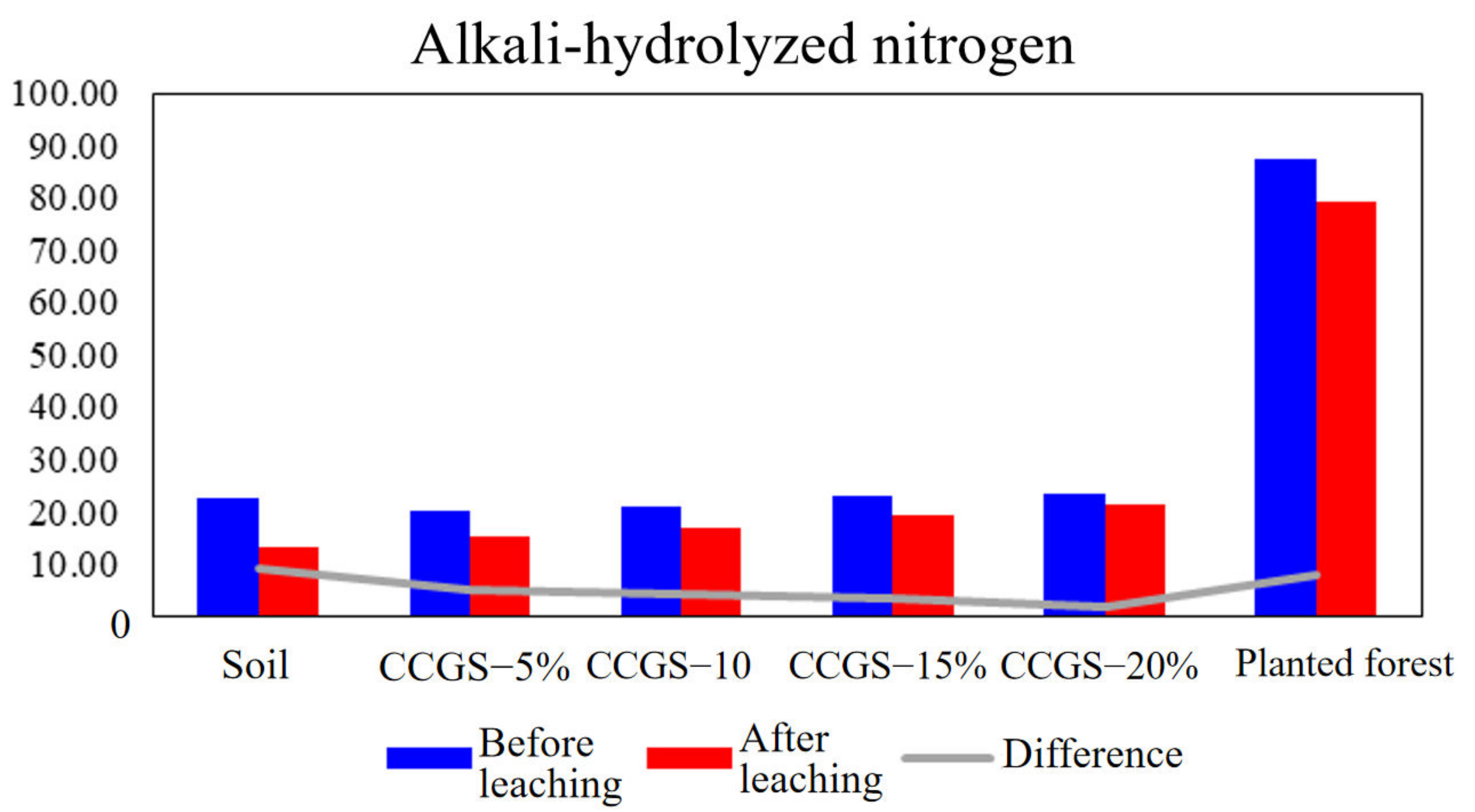
5. Feasibility Study of Gasification Slag Soil Improvement
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ministry of Natural Resources. China Mineral Resources Report; Geological Publishing House: Beijing, China, 2021. [Google Scholar]
- Shuai, H. Intrinsic Characteristics of CGS and Phase Evolution during CRN Process; Xi’an University of Architecture and Technology: Xi’an, China, 2015. [Google Scholar]
- Zhu, J.F.; Li, J.; Yan, L.; Shang, Y.; Wang, Y.; Li, Q.; Wang, J. Research progress and application prospect of coal gasification slag resource utilization. Clean Coal Technol. 2021, 27, 11–21. [Google Scholar]
- Yuan, H. Fundamental Research on Intrinsic Characteristics and Application of Coal Gasification Slag; Xi’an University of Architecture and Technology: Xi’an, China, 2020. [Google Scholar]
- Yang, S.; Shi, L.J. Component analysis and comprehensive utilization of coal gasification fine slag. Coal Chem. Ind. 2013, 4, 34–36, 43. [Google Scholar]
- Zhao, Y.B.; Wu, H.J.; Zhang, X.B.; Liu, H.G.; Jing, Y.H.; Yuan, Y. Preparation of Porous Ceramics from Coal Gasification Residue. Clean Coal Technol. 2016, 22, 7–11. [Google Scholar] [CrossRef]
- Yin, H.F.; Tang, Y.; Ren, Y. Basic characteristics and application of Texaco gasifier slag. Coal Convers. 2009, 32, 30–33. [Google Scholar]
- Li, G.Y.; Zhang, T.; Huo, L. Basic Performance and Application of LurgiCGS “C”. In Proceedings of the First Symposium on Solid Waste Treatment and Eco-Environmental Materials for the Establishment of Solid Waste Branch of China Silicate Society, Beijing, China, August 2015; China Silicate Society: Beijing, China, 2015. [Google Scholar]
- Acosta, A.; Aineto, M.; Iglesias, I.; Romero, M.; Rincón, J. Physico-chemical characterization of slag waste coming from GICC thermal power plant. Mater. Lett. 2001, 50, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Gong, M.; Lester, E.; Wang, F.; Zhou, Z.; Yu, Z. Characterisation of residual carbon from entrained-bed coal water slurry gasifiers. Fuel 2007, 86, 972–982. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhou, Z.J.; Gao, X.X.; Yu, G.; Gong, X. The gasification reactivity of unburned carbon present in gasification slag from entrained-flow gasifier. Fuel Process. Technol. 2009, 90, 1062–1070. [Google Scholar] [CrossRef]
- Hu, W.H. Basic Research on Activation Separation and Resource Utilization of Aluminum-Silicon Components in Coal Gasification Slag; University of Chinese Academy of Sciences: Hangzhou, China, 2019. [Google Scholar]
- Shang, Z.F.; Ma, J.L.; Zhang, J.; Xu, D.Y.; Zhang, L.Y.; Zhou, J.Q.; Duan, X.Y.; Zhang, X.M. Research status and utilization technology prospect of CGS. Solid Waste Pollut. Control. Technol. 2017, 7, 712–717. [Google Scholar]







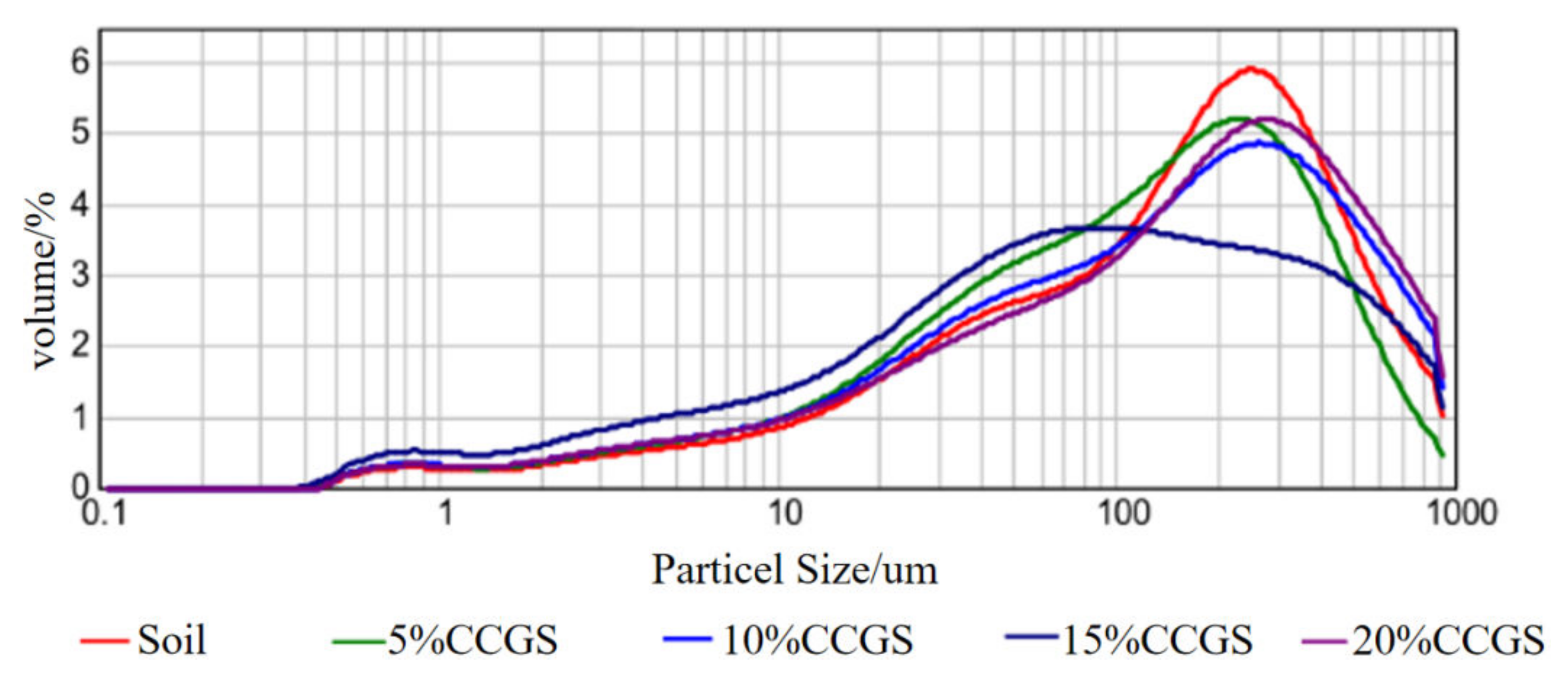




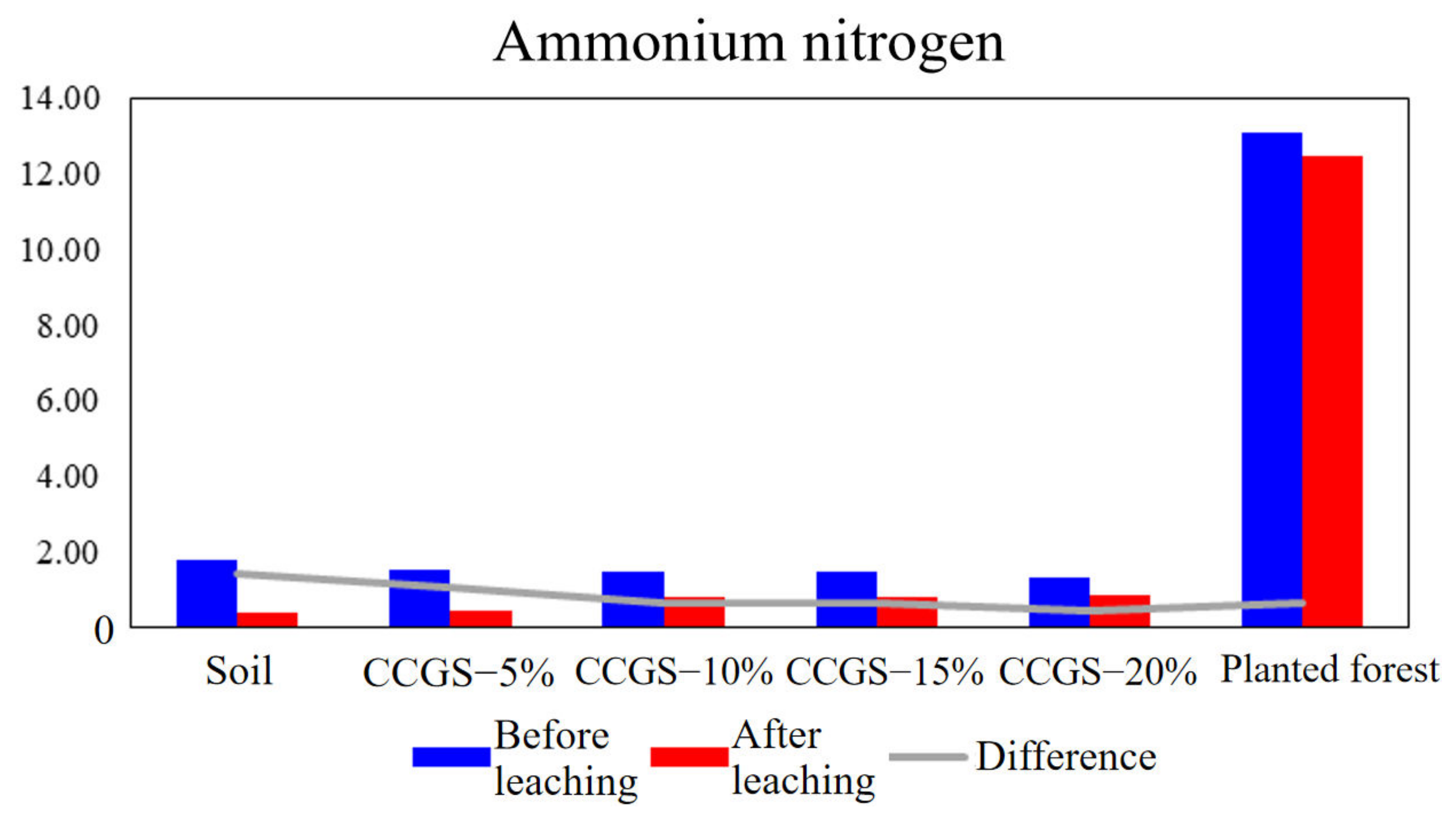
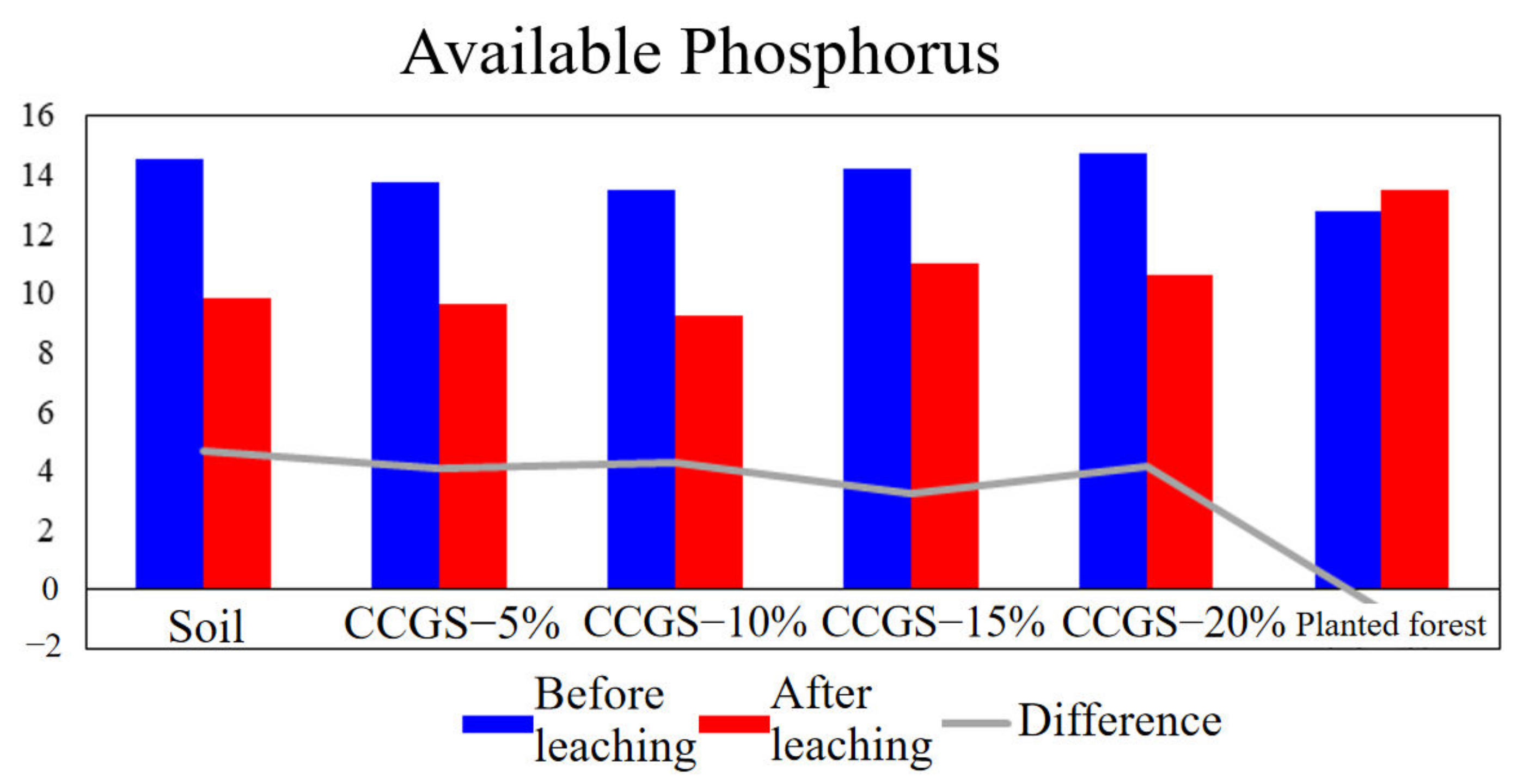


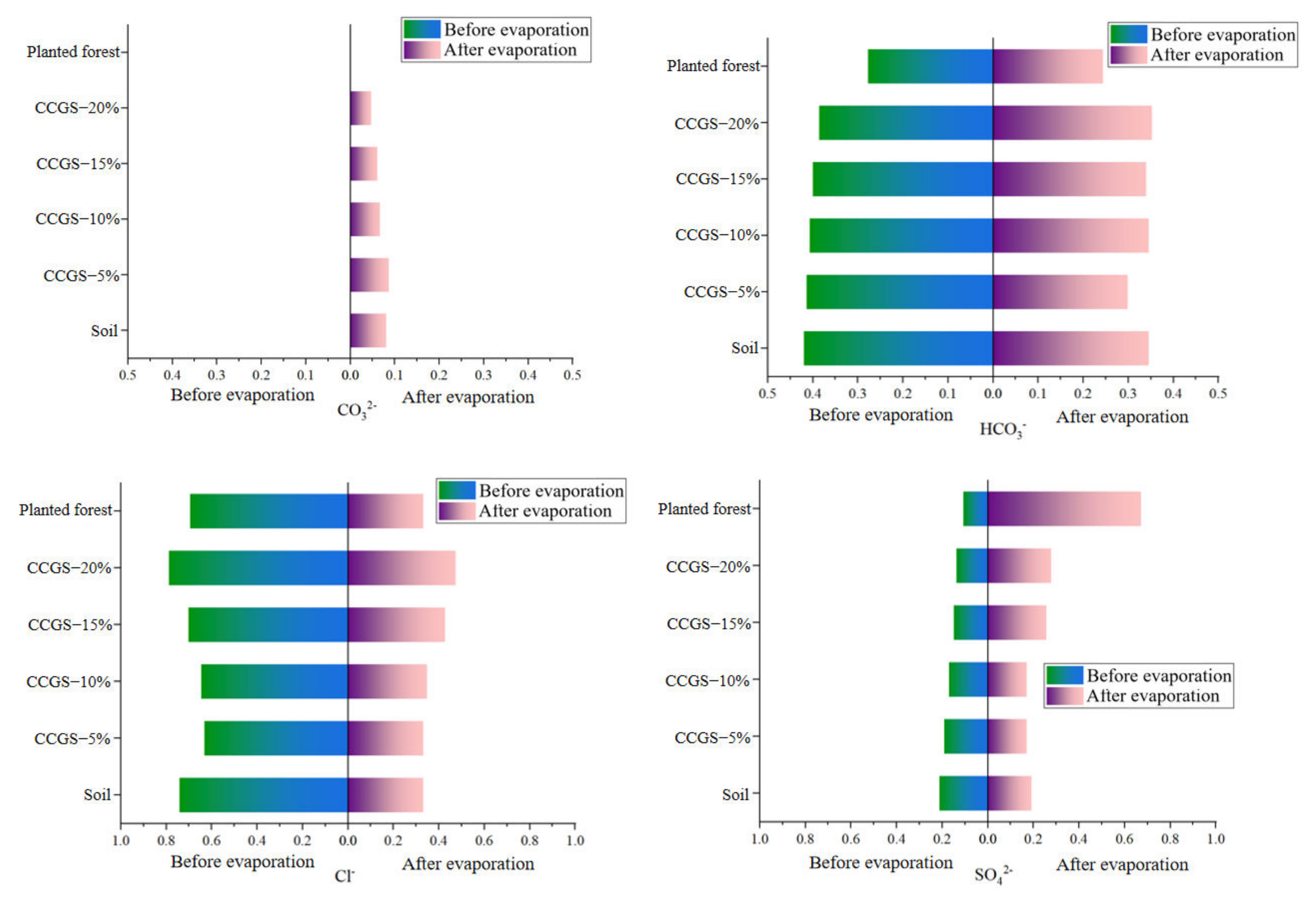
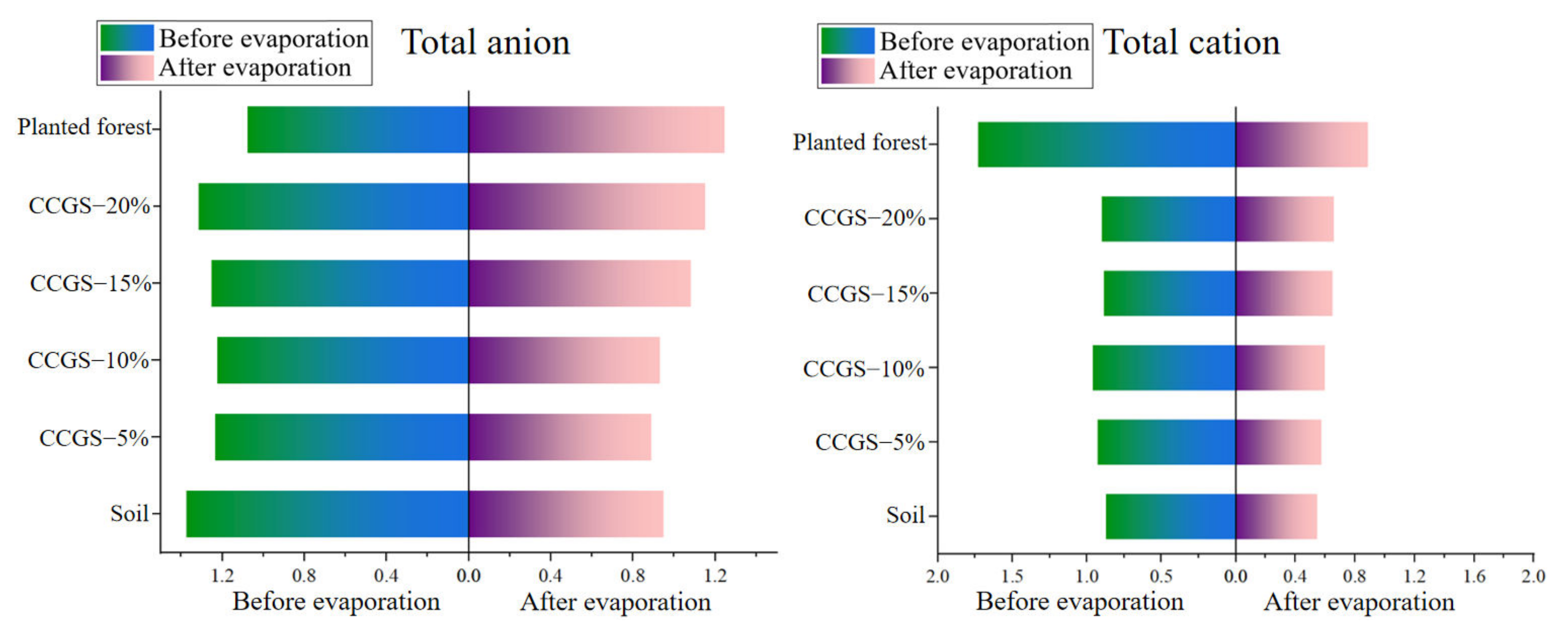

| Treatment | Clayey Particles (<0.002 mm, %) | Silty Particles (0.05~0.002 mm,%) | Sandy Particles (2~0.05 mm,%) | |
|---|---|---|---|---|
| Soil | 100% | 2.2 | 22.2 | 75.6 |
| CCGS | 100% | 0 | 5.32 | 94.68 |
| 5% | 1.31 | 22.31 | 76.39 | |
| 10% | 1.2 | 20.27 | 78.53 | |
| 15% | 1.1 | 18.54 | 80.35 | |
| 20% | 0.86 | 17.02 | 82.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Li, X.; Zhang, K.; Wang, P.; Xue, P.; Zhang, H. Research on the Application of Coal Gasification Slag in Soil Improvement. Processes 2022, 10, 2690. https://doi.org/10.3390/pr10122690
Zhang R, Li X, Zhang K, Wang P, Xue P, Zhang H. Research on the Application of Coal Gasification Slag in Soil Improvement. Processes. 2022; 10(12):2690. https://doi.org/10.3390/pr10122690
Chicago/Turabian StyleZhang, Ruimei, Xiaonan Li, Kai Zhang, Pengfei Wang, Peifeng Xue, and Hailong Zhang. 2022. "Research on the Application of Coal Gasification Slag in Soil Improvement" Processes 10, no. 12: 2690. https://doi.org/10.3390/pr10122690





