ZIF for CO2 Capture: Structure, Mechanism, Optimization, and Modeling
Abstract
:1. Introduction
1.1. CO2 Capture Technologies
1.2. Aim of Present Review
- To summarize recent developments in understanding of structure and characterization of ZIF, mechanisms of CO2 capture using ZIF, and current challenges.
- To highlight opportunities for the researchers by summarizing innovative optimisation methods reported in the literature for ZIF application for CO2 capture.
- We have not cited any article in open literature that provides a comprehensive review of modelling of ZIF for CO2 capture.
1.3. Methodology of Present Review
2. ZIFs for CO2 Capture
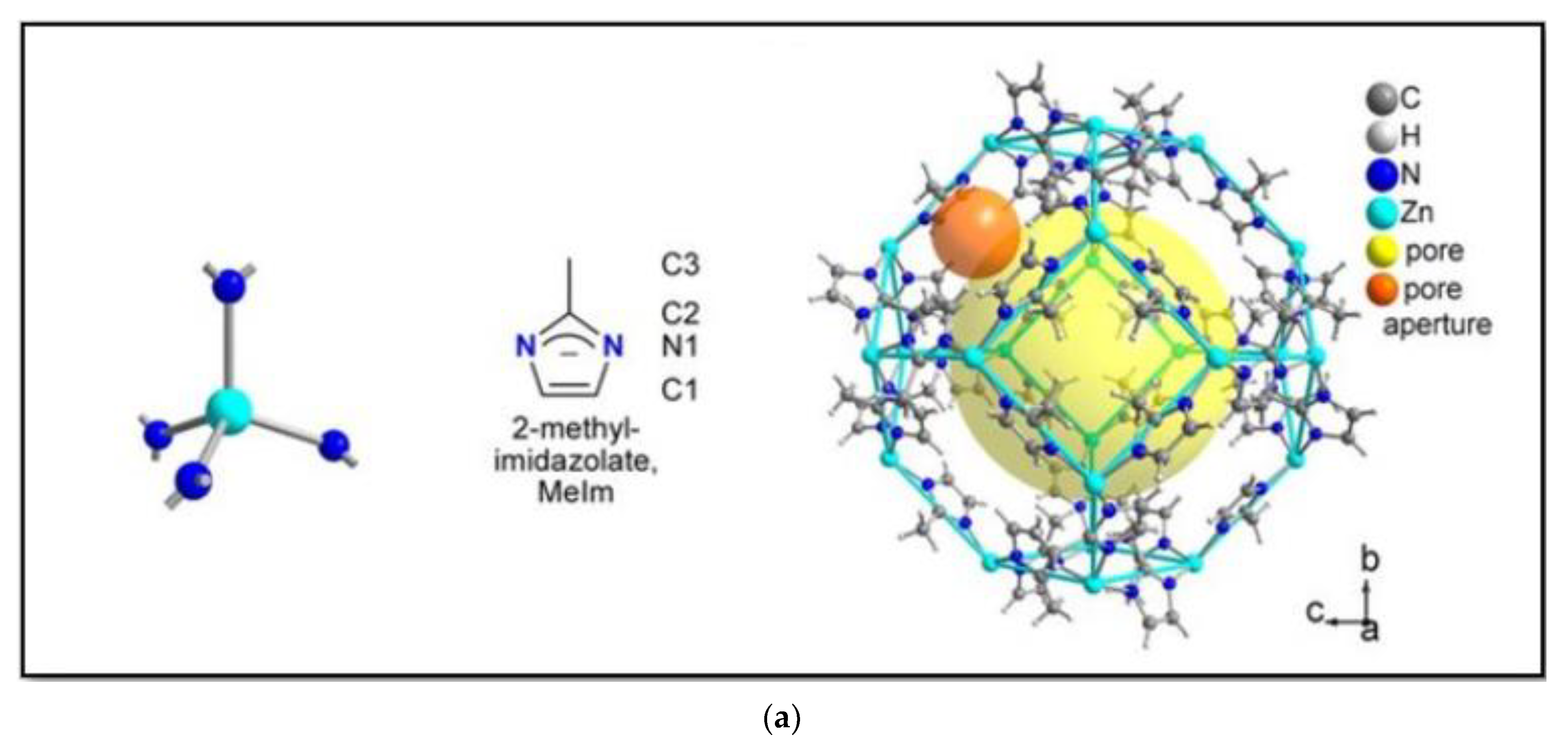
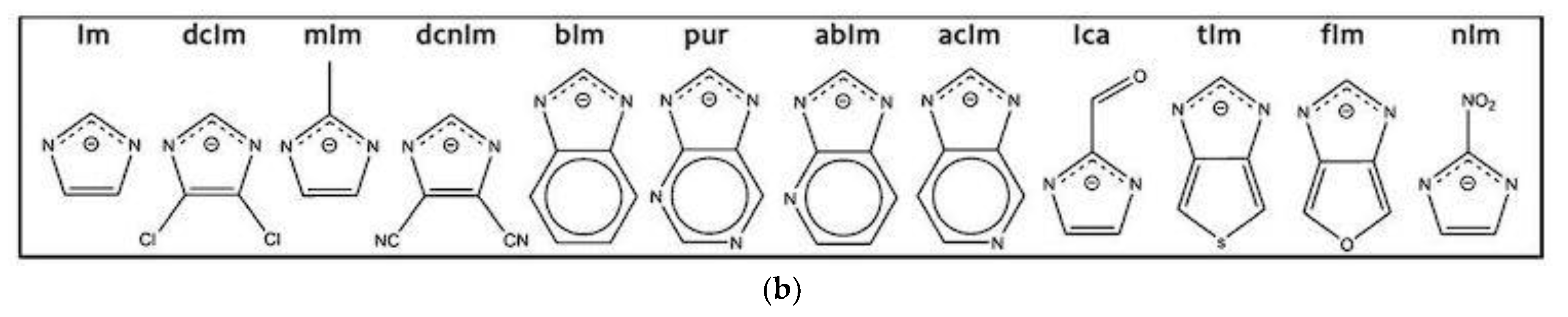
2.1. ZIF Structure
2.2. Synthesis Techniques for ZIFs
2.3. Mechanism of CO2 Capture
2.4. ZIF Characterization
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Isotherms
2.4.3. X-ray Diffraction (XRD) Analysis
2.4.4. Thermogravimetric Analysis (TGA)
3. Optimization Techniques for Improving CO2 Capture by ZIF
4. Modelling ZIF for CO2 Capture
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. Our World Data. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 4 December 2022).
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Pettinari, C.; Tombesi, A. Metal–organic frameworks for carbon dioxide capture. MRS Energy Sustain. 2020, 7, 35. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.-X.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Saha, S.; Chandra, S.; Garai, B.; Banerjee, R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Piscopo, C.G.; Loebbecke, S. Strategies to Enhance Carbon Dioxide Capture in Metal-Organic Frameworks. Chempluschem 2020, 85, 538–547. [Google Scholar] [CrossRef]
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical structure, network topology, and porosity effects on the mechanical properties of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2010, 107, 9938–9943. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Accounts Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, D.; Zhang, Z.; Li, H.; Chen, G.; Liu, B. A pilot-scale experimental study on CO2 capture using Zeolitic imidazolate framework-8 slurry under normal pressure. Appl. Energy 2019, 248, 104–114. [Google Scholar] [CrossRef]
- Song, F.; Cao, Y.; Zhao, Y.; Jiang, R.; Xu, Q.; Yan, J.; Zhong, Q. Ion-Exchanged ZIF-67 Synthesized by One-Step Method for Enhancement of CO2 Adsorption. J. Nanomater. 2020, 2020, e1508574. [Google Scholar] [CrossRef]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A Combined Experimental–Computational Investigation of Carbon Dioxide Capture in a Series of Isoreticular Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic Imidazolate Frameworks: Synthesis, Functionalization, and Catalytic/Adsorption Applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Kukkar, P.; Kim, K.-H.; Kukkar, D.; Singh, P. Recent advances in the synthesis techniques for zeolitic imidazolate frameworks and their sensing applications. Coord. Chem. Rev. 2021, 446, 214109. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chien, H.-T.; Lo, Y.; Hao Che, C.; Wang, T.; Kang, D.-Y. Synthesis of Zeolitic Imidazolate Framework Core–Shell Nanosheets Using Zinc-Imidazole Pseudopolymorphs. ACS Appl. Mater. Interfaces 2015, 7, 18353–18361. [Google Scholar] [CrossRef]
- Qian, X.; Ren, Q.; Wu, X.; Sun, J.; Wu, H.; Lei, J. Enhanced Water Stability in Zn-Doped Zeolitic Imidazolate Framework-67 (ZIF-67) for CO2 Capture Applications. ChemistrySelect 2018, 3, 657–661. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, W.; Guo, W.; An, L.; Xia, D.; Zou, R. Functional Zeolitic-Imidazolate-Framework-Templated Porous Carbon Materials for CO2 Capture and Enhanced Capacitors. Chem. Asian J. 2013, 8, 1879–1885. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; De France, K.; Zhang, P.; Zhao, X.; Zhang, Q. Porous carbon frameworks with high CO2 capture capacity derived from hierarchical polyimide/zeolitic imidazolate frameworks composite aerogels. Chem. Eng. J. 2020, 395, 124927. [Google Scholar] [CrossRef]
- Abdelhamid, H. A Review on Removal of Carbon Dioxide (CO2) using Zeolitic Imidazolate Frameworks: Adsorption and Conversion via Catalysis. Catalysis 2022. [Google Scholar] [CrossRef]
- Sr, V.; Ma, C. Highly Permeable Zeolite Imidazolate Framework-8 Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Railey, P.; Song, Y.; Liu, T.; Li, Y. Metal organic frameworks with immobilized nanoparticles: Synthesis and applications in photocatalytic hydrogen generation and energy storage. Mater. Res. Bull. 2017, 96, 385–394. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Chapter 2—Adsorbent. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 71–210. [Google Scholar]
- Fazari, F. Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study; Massachusetts Institute of Technology: Cambridge, MA, USA, 2010. [Google Scholar]
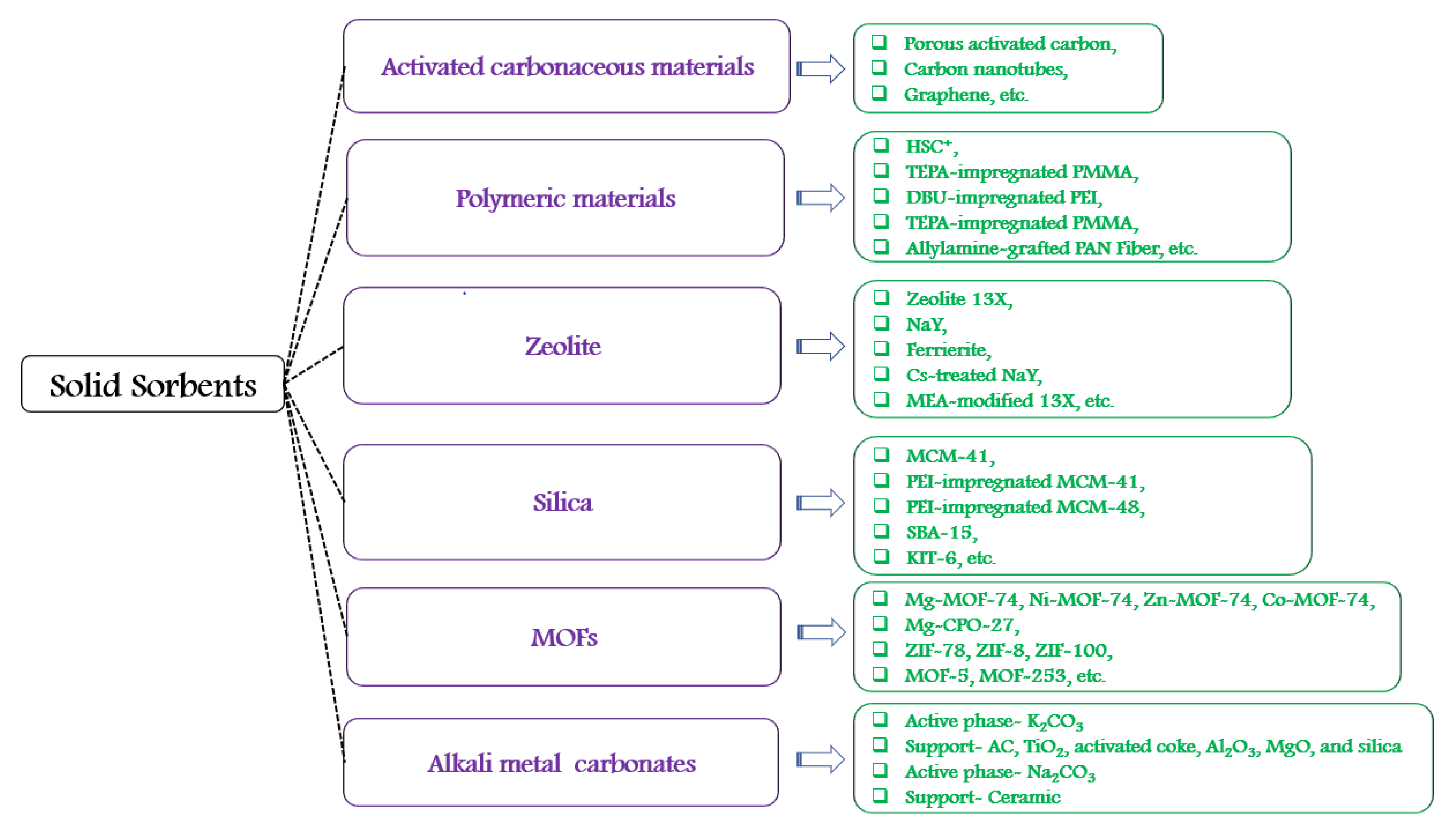
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; He, Y. CO2 Capture Using Solid Sorbents. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2349–2404. [Google Scholar]
- Ünveren, E.E.; Monkul, B.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Dunstan, M.; Donat, F.; Bork, A.H.; Grey, C.; Müller, C. CO2 capture using solid sorbents: Fundamental aspects, mechanistic insights and recent advances. Chem. Rev. 2021, 121, 12681–12745. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2011, 51, 1438–1463. [Google Scholar] [CrossRef]
- Dao, D.S.; Yamada, H.; Yogo, K. Enhancement of CO2 Adsorption/Desorption Properties of Solid Sorbents Using Tetraethylenepentamine/Diethanolamine Blends. ACS Omega 2020, 5, 23533–23541. [Google Scholar] [CrossRef]
- Dinda, S.; Murge, P.C.; Paruchuri, B.C. A study on zeolite-based adsorbents for $$\hbox CO2 capture. Bull. Mater. Sci. 2019, 42, 240. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Zhao, N.; Li, J.; Xiao, F.; Sun, Y. Carbon Dioxide Capture by MgO-modified MCM-41 Materials. Adsorpt. Sci. Technol. 2009, 27, 593–601. [Google Scholar] [CrossRef]
- Yamada, H.; Dao, D.; Chowdhury, F.; Fujiki, J.; Goto, K.; Yogo, K. Development of Amine-impregnated Solid Sorbents for CO2 capture. Energy Procedia 2014, 63, 2346–2350. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hou, L.; Yu, S.; Xi, B.; Zhao, Y.; Xia, X. MCM-41 impregnated with A zeolite precursor: Synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chem. Eng. J. 2013, 223, 678–687. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Ghoshal, A.K. Novel Pore-Expanded MCM-41 for CO2 Capture: Synthesis and Characterization. Langmuir ACS J. Surf. Colloids 2013, 29, 3491–3499. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, B.; Zhao, Y.; Yang, T. Fabrication of the magnetic mesoporous silica Fe-MCM-41-A as efficient adsorbent: Performance, kinetics and mechanism. Sci. Rep. 2021, 11, 26. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.; Miller, B.; Scaroni, A. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2001, 16, 1463–1469. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Cao, Y.; Lineberry, Q.; Pan, W.-P. Evaluation of CO2 adsorption capacity of solid sorbents. J. Therm. Anal. Calorim. 2011, 106, 199–205. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of Activated Carbon and MCM-41 from Bagasse and Rice Husk and their Carbon Dioxide Adsorption Capacity. 2. J. Sustain. Energy Environ. 2010, 2, 77–81. [Google Scholar]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Sodiq, A.; Feron, P.H.M. 29—Commercial liquid absorbent-based PCC processes. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 757–778. [Google Scholar]
- Chi, W.S.; Hwang, S.; Lee, S.-J.; Park, S.; Bae, Y.-S.; Ryu, D.Y.; Kim, J.H.; Kim, J. Mixed matrix membranes consisting of SEBS block copolymers and size-controlled ZIF-8 nanoparticles for CO2 capture. J. Membr. Sci. 2015, 495, 479–488. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. A 2017, 6, 1887–1899. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, B.-C. Efficient elimination of caffeine from water using Oxone activated by a magnetic and recyclable cobalt/carbon nanocomposite derived from ZIF-67. Dalton Trans. 2016, 45, 3541–3551. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, E.; Khan, E.A.; Lai, Z. Synthesis and characterization of ZIF-69 membranes and separation for CO2/CO mixture. J. Membr. Sci. 2010, 353, 36–40. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Huang, K.-W.; Ko, B.-T.; Lin, K.-Y.A. Bifunctional ZIF-78 heterogeneous catalyst with dual Lewis acidic and basic sites for carbon dioxide fixation via cyclic carbonate synthesis. J. CO2 Util. 2017, 22, 178–183. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, Y.; Pan, X.; Gao, L.; Xiao, G. Pyridyl Ionic Liquid Functionalized ZIF-90 for Catalytic Conversion of CO2 into Cyclic Carbonates. Catal. Lett. 2020, 150, 3561–3571. [Google Scholar] [CrossRef]
- Ilicak, I.; Boroglu, M.S.; Durmus, A.; Boz, I. Influence of ZIF-95 on structure and gas separation properties of polyimide-based mixed matrix membranes. J. Nat. Gas Sci. Eng. 2021, 91, 103941. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Qiao, Z.; Diestel, L.; Zhou, J.; Huang, A.; Caro, J. Polydopamine-based synthesis of a zeolite imidazolate framework ZIF-100 membrane with high H2/CO2 selectivity. J. Mater. Chem. A 2015, 3, 4722–4728. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Wang, Y.; Kuang, T. ZIF-8-Based Membranes for Carbon Dioxide Capture and Separation. ACS Sustain. Chem. Eng. 2017, 5, 11204–11214. [Google Scholar] [CrossRef]
- Van der Perre, S.; Van Assche, T.; Bozbiyik, B.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorptive Characterization of the ZIF-68 Metal-Organic Framework: A Complex Structure with Amphiphilic Properties. Langmuir 2014, 30, 8416–8424. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Bian, Z.; Hu, J.; Liu, H. Dynamic hydrophobic hindrance effect of zeolite@zeolitic imidazolate framework composites for CO2 capture in the presence of water. J. Mater. Chem. A 2015, 3, 8091–8097. [Google Scholar] [CrossRef]
- Sławek, A.; Roztocki, K.; Majda, D.; Jaskaniec, S.; Vlugt, T.J.H.; Makowski, W. Adsorption of n-alkanes in ZIF-8: Influence of crystal size and framework dynamics. Microporous Mesoporous Mater. 2021, 312, 110730. [Google Scholar] [CrossRef]
- Aniruddha, R.; Sreedhar, I. Process optimization for enhanced carbon capture and cyclic stability using adsorbents derived from coal fly ash. Environ. Sci. Pollut. Res. 2021, 268. [Google Scholar] [CrossRef]
- Payra, S.; Challagulla, S.; Indukuru, R.R.; Chakraborty, C.; Tarafder, K.; Ghosh, B.; Roy, S. The structural and surface modification of zeolitic imidazolate frameworks towards reduction of encapsulated CO2. N. J. Chem. 2018, 42, 19205–19213. [Google Scholar] [CrossRef]
- Song, X.; Yu, J.; Wei, M.; Li, R.; Pan, X.; Yang, G.; Tang, H. Ionic Liquids-Functionalized Zeolitic Imidazolate Framework for Carbon Dioxide Adsorption. Materials 2019, 12, 2361. [Google Scholar] [CrossRef]
- Ban, Y.; Li, Y.; Liu, X.; Peng, Y.; Yang, W. Solvothermal synthesis of mixed-ligand metal–organic framework ZIF-78 with controllable size and morphology. Microporous Mesoporous Mater. 2013, 173, 29–36. [Google Scholar] [CrossRef]
- Ghahramaninezhad, M.; Mohajer, F.; Niknam Shahrak, M. Improved CO2 capture performances of ZIF-90 through sequential reduction and lithiation reactions to form a hard/hard structure. Front. Chem. Sci. Eng. 2020, 14, 425–435. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Lo, T.N.H.; Kim, J.; Nguyen, H.T.D.; Le, T.B.; Cordova, K.E.; Furukawa, H. Mixed-Metal Zeolitic Imidazolate Frameworks and their Selective Capture of Wet Carbon Dioxide over Methane. Inorg. Chem. 2016, 55, 6201–6207. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Biswal, B.P.; Banerjee, R.; Kurungot, S. Zeolitic Imidazolate Framework (ZIF)-Derived, Hollow-Core, Nitrogen-Doped Carbon Nanostructures for Oxygen-Reduction Reactions in PEFCs. Chem. A Eur. J. 2013, 19, 9335–9342. [Google Scholar] [CrossRef]
- Adamu, A.; Russo-Abegão, F.R.; Boodhoo, K. Process intensification technologies for CO2 capture and conversion—A review. BMC Chem. Eng. 2020, 2, 2. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Baliban, R.C.; Elia, J.A.; Floudas, C.A. Modeling, Simulation, and Optimization of Postcombustion CO2 Capture for Variable Feed Concentration and Flow Rate. 2. Pressure Swing Adsorption and Vacuum Swing Adsorption Processes. Ind. Eng. Chem. Res. 2012, 51, 15665–15682. [Google Scholar] [CrossRef]
- Li, S.; Zhiwei, H. Application of intelligent optimization techniques in CO2 geological storage and utilization (CGUS). IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012147. [Google Scholar] [CrossRef]
- Valencia-Marquez, D.; Flores-Tlacuahuac, A.; Vasquez-Medrano, R. An optimization approach for CO2 capture using ionic liquids. J. Clean. Prod. 2017, 168, 1652–1667. [Google Scholar] [CrossRef]
- Lie, J.A.; Vassbotn, T.; Hägg, M.-B.; Grainger, D.; Kim, T.-J.; Mejdell, T. Optimization of a membrane process for CO2 capture in the steelmaking industry. Int. J. Greenh. Gas Control 2007, 1, 309–317. [Google Scholar] [CrossRef]
- He, X. A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain. Soc. 2018, 8, 34. [Google Scholar] [CrossRef]
- Abraha, Y.W.; Tsai, C.-W.; Niemantsverdriet, J.W.H.; Langner, E.H.G. Optimized CO2 Capture of the Zeolitic Imidazolate Framework ZIF-8 Modified by Solvent-Assisted Ligand Exchange. ACS Omega 2021, 6, 21850–21860. [Google Scholar] [CrossRef]
- Sze, L.; Yeong, Y.F.; Lau, K.K.; Mohd Shariff, D. Synthesis of zeolitic imidazolate frameworks (ZIF)-8 membrane and its process optimization study in separation of CO2 from natural gas. J. Chem. Technol. Biotechnol. 2016, 92, 420–431. [Google Scholar] [CrossRef]
- Song, Y.; He, M.; Zhao, J.; Jin, W. Structural manipulation of ZIF-8-based membranes for high-efficiency molecular separation. Sep. Purif. Technol. 2021, 270, 118722. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Zhang, S.; Xie, X.; Xia, W. Enhancement thermal stability and CO2 adsorption property of ZIF-8 by pre-modification with polyaniline. Mater. Res. Express 2020, 7, 025304. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Li, H.; Chowdhary, J.; Huang, L.; He, X.; MacKerell, A.D.; Roux, B. Drude Polarizable Force Field for Molecular Dynamics Simulations of Saturated and Unsaturated Zwitterionic Lipids. J. Chem. Theory Comput. 2017, 13, 4535–4552. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, A.; Milchevskiy, Y.; Lyubartsev, A. AMBER-ii: New Combining Rules and Force Field for Perfluoroalkanes. J. Phys. Chem. B 2015, 119, 14563–14573. [Google Scholar] [CrossRef]
- Ye, W.; Ji, D.; Wang, W.; Luo, R.; Chen, H.-F. Test and Evaluation of ff99IDPs Force Field for Intrinsically Disordered Proteins. J. Chem. Inf. Model. 2015, 55, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.; MacCallum, J.L.; Brini, E.; Simmerling, C.; Dill, K.A. Grid-Based Backbone Correction to the ff12SB Protein Force Field for Implicit-Solvent Simulations. J. Chem. Theory Comput. 2015, 11, 4770–4779. [Google Scholar] [CrossRef]
- Matczak, P. A Test of Various Partial Atomic Charge Models for Computations on Diheteroaryl Ketones and Thioketones. Computation 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, M.; Murthy, V.; Mackinnon, I.D.R. Evaluation of DFT methods to calculate structure and partial atomic charges for zeolite N. Comput. Mater. Sci. 2020, 171, 109225. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Stone, A.J. Distributed Multipole Analysis: Stability for Large Basis Sets. J. Chem. Theory Comput. 2005, 1, 1128–1132. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef]
- Besler, B.H.; Merz, K.M., Jr.; Kollman, P.A. Atomic charges derived from semiempirical methods. J. Comput. Chem. 1990, 11, 431–439. [Google Scholar] [CrossRef]
- Chirlian, L.E.; Francl, M.M. Atomic charges derived from electrostatic potentials: A detailed study. J. Comput. Chem. 1987, 8, 894–905. [Google Scholar] [CrossRef]
- Hu, H.; Lu, Z.; Yang, W. Fitting Molecular Electrostatic Potentials from Quantum Mechanical Calculations. J. Chem. Theory Comput. 2007, 3, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, C.; Liu, B.; Liu, D.; Yang, Q.; Zhong, C. Large-scale computational screening of metal-organic frameworks for CH4/H2 separation. AIChE J. 2012, 58, 2078–2084. [Google Scholar] [CrossRef]
- Atci, E.; Keskin, S. Understanding the Potential of Zeolite Imidazolate Framework Membranes in Gas Separations Using Atomically Detailed Calculations. J. Phys. Chem. C 2012, 116, 15525–15537. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ozcan, A.; Keskin, S. Computational screening of ZIFs for CO2 separations. Mol. Simul. 2015, 41, 713–726. [Google Scholar] [CrossRef]
- Yilmaz Kanargi, G.; Keskin, S. Predicting the Performance of Zeolite Imidazolate Framework/Polymer Mixed Matrix Membranes for CO2, CH4, and H2 Separations Using Molecular Simulations. Ind. Eng. Chem. Res. 2012, 51, 14218–14228. [Google Scholar] [CrossRef]
- Battisti, A.; Taioli, S.; Garberoglio, G. Zeolitic imidazolate frameworks for separation of binary mixtures of CO2, CH4, N2 and H2: A computer simulation investigation. Microporous Mesoporous Mater. 2011, 143, 46–53. [Google Scholar] [CrossRef]
- Keskin, S. Atomistic Simulations for Adsorption, Diffusion, and Separation of Gas Mixtures in Zeolite Imidazolate Frameworks. J. Phys. Chem. C 2010, 115, 800–807. [Google Scholar] [CrossRef]
- Guo, H.; Shi, F.; Ma, Z.; Liu, X. Molecular Simulation for Adsorption and Separation of CH4/H2 in Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2010, 114, 21891. [Google Scholar] [CrossRef]
- Yang, Y.; Shin, Y.K.; Li, S.; Bennett, T.; Van Duin, A.; Mauro, J. Enabling Computational Design of ZIFs Using ReaxFF. J. Phys. Chem. B 2018, 122, 9616–9624. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Liu, D.; Liu, B.; Chen, G.; Zhong, C. Effect of temperature on gas adsorption and separation in ZIF-8: A combined experimental and molecular simulation study. Chem. Eng. Sci. 2011, 66, 6297–6305. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Y.; Xia, Q.; Li, Z.; Xi, H. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 2012, 19, 25–37. [Google Scholar] [CrossRef]
- Wan, Z.; Zhou, G.; Dai, Z.; Li, L.; Hu, N.; Chen, X.; Yang, Z. Separation Selectivity of CH4/CO2 Gas Mixtures in the ZIF-8 Membrane Explored by Dynamic Monte Carlo Simulations. J. Chem. Inf. Model. 2020, 60, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.K.; Pazzona, F.G.; Suffritti, G.B.; Demontis, P.; Masia, M. Estimation of Partial Charges in Small Zeolite Imidazolate Frameworks from Density Functional Theory Calculations. J. Chem. Theory Comput. 2011, 7, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrouche, H.; Aguado, S.; Pérez-Pellitero, J.; Chizallet, C.; Siperstein, F.; Farrusseng, D.; Bats, N.; Nieto-Draghi, C. Experimental and Computational Study of Functionality Impact on Sodalite–Zeolitic Imidazolate Frameworks for CO2 Separation. J. Phys. Chem. C 2011, 115, 16425–16432. [Google Scholar] [CrossRef]
- Babarao, R.; Dai, S.; Jiang, D. Effect of Pore Topology and Accessibility on Gas Adsorption Capacity in Zeolitic–Imidazolate Frameworks: Bringing Molecular Simulation Close to Experiment. J. Phys. Chem. C 2011, 115, 8126–8135. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, C.; Yang, Q.; Zhong, C. Understanding the Adsorption and Diffusion of Carbon Dioxide in Zeolitic Imidazolate Frameworks: A Molecular Simulation Study. J. Phys. Chem. C 2009, 113, 5004–5009. [Google Scholar] [CrossRef]
- Liu, B.; Smit, B. Molecular Simulation Studies of Separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 2010, 114, 8515–8522. [Google Scholar] [CrossRef]
- Liu, J.; Keskin, S.; Sholl, D.; Johnson, K. Molecular Simulations and Theoretical Predictions for Adsorption and Diffusion of CH4/H2 and CO2/CH4 Mixtures in ZIFs. J. Phys. Chem. C 2011, 115, 12560–12566. [Google Scholar] [CrossRef]
- Rankin, R.; Liu, J.; Kulkarni, A.; Johnson, J.K. Adsorption and Diffusion of Light Gases in ZIF-68 and ZIF-70: A Simulation Study. J. Phys. Chem. C 2009, 113, 16906–16914. [Google Scholar] [CrossRef]



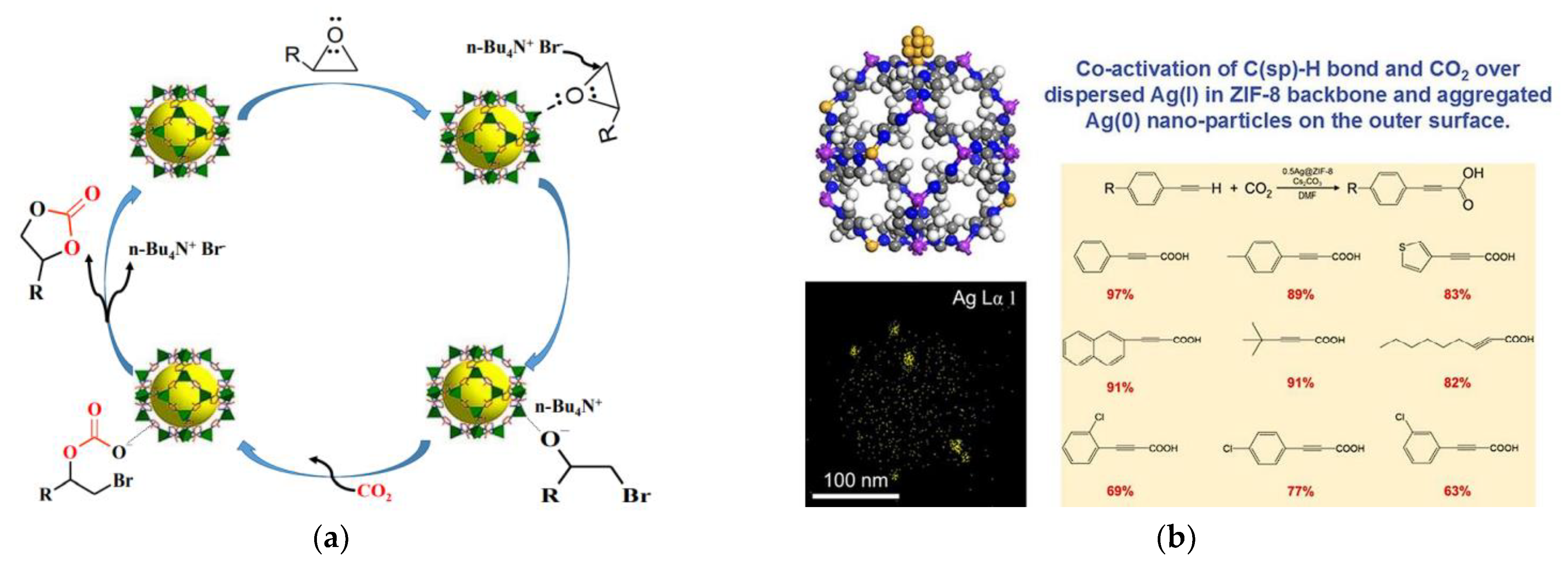
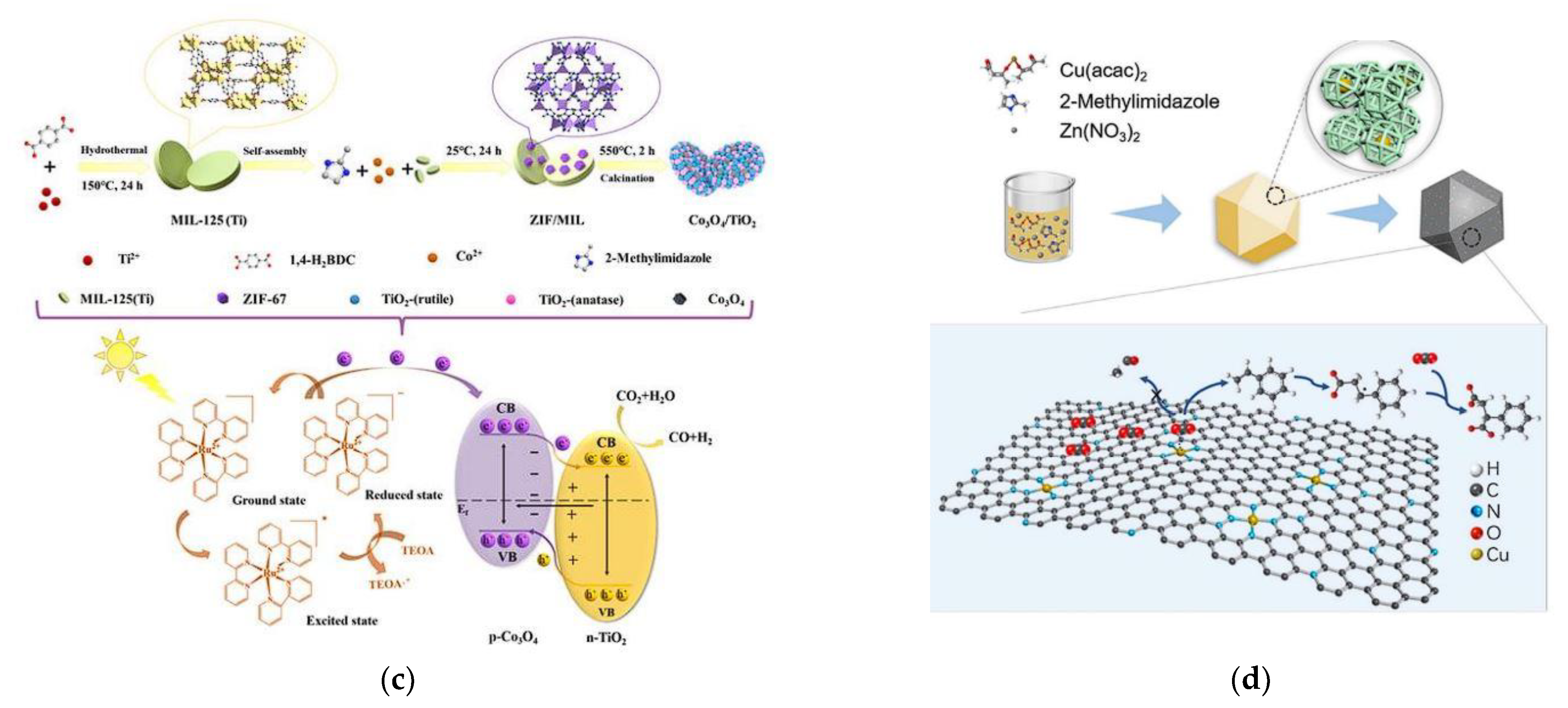
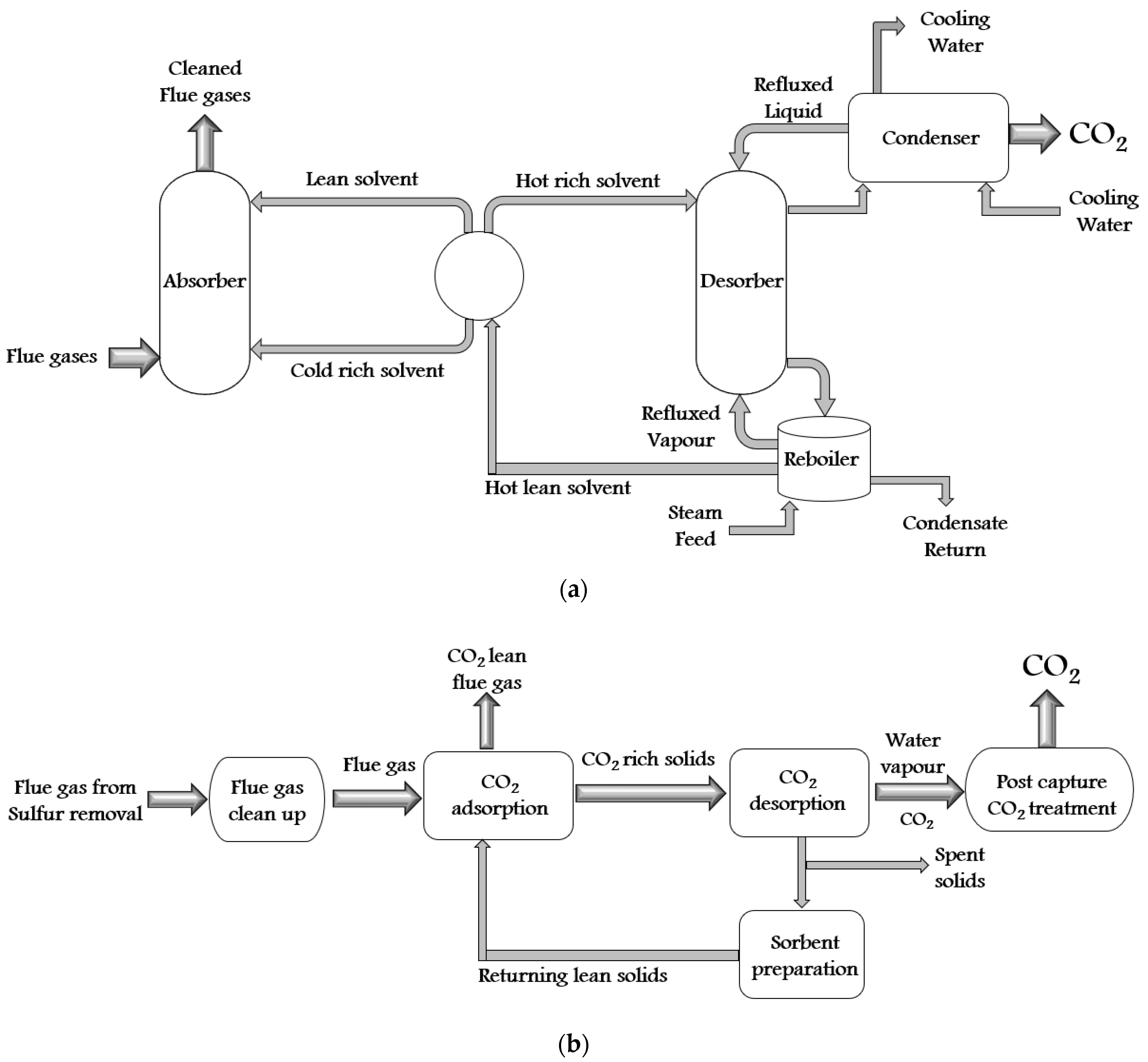
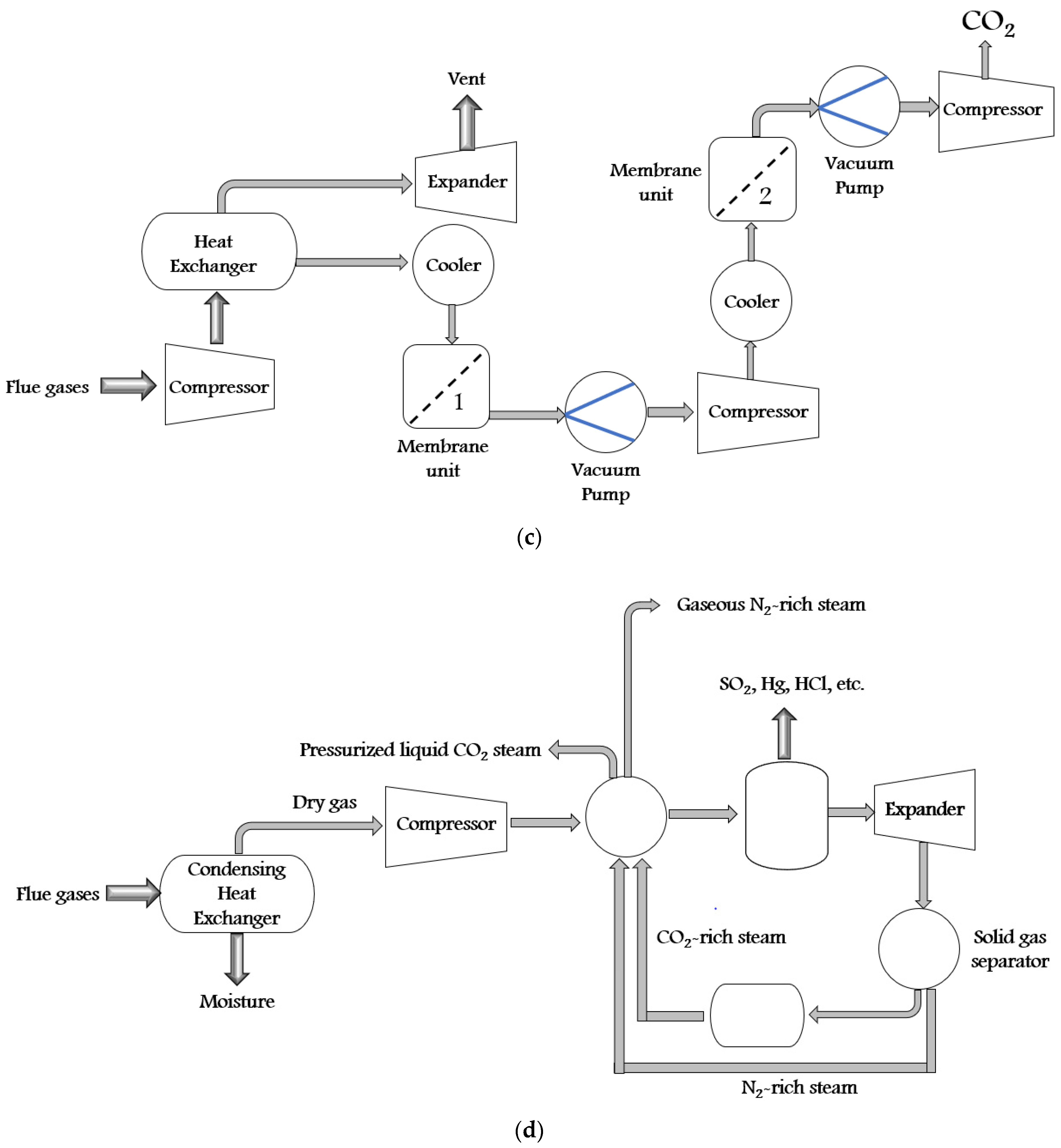

| S. No. | CO2 Capture Materials/Technologies | Advantages of Methods | Limitation of Methods |
|---|---|---|---|
| 1. | Amine scrubbing (Chemical sorbent) |
|
|
| 2. | Ionic liquids (Physical solvents) |
|
|
| 3. | Zeolites (Adsorption) |
|
|
| 4. | CO2 Membranes (Membranes) |
|
|
| 5. | Molecular sieves (Membranes) |
|
|
| 6. | Covalent organic framework (Adsorption) |
|
|
| 7. | Metal-organic frameworks (MOFs) (Adsorption) |
|
|
| S. No. | ZIFs | Crystal Structure | SEM | Topology | Ref. |
|---|---|---|---|---|---|
| 1. | ZIF-8 |  |  | sod | [43,51] |
| 2. | ZIF-68 |  |  | sod | [52] |
| 3. | ZIF-69 |  |  | gme | [46] |
| 4. | ZIF-78 |  |  | gme | [47] |
| 5. | ZIF-95 |  |  | poz | [49] |
| S. No. | ZIFs | Isotherms | Textural Property | Ref. | ||
|---|---|---|---|---|---|---|
| N2 | CO2 | SBET (m2/g) | Vpore (cm3/g) | |||
| 1. | ZIF-8, ZIF-L, ZIF-l@ZIF-8 |  |    | NA | 0.55, 0.06, 0.14 | [17] |
| 2. | ZIF-8 |  |  | 1699 | 0.52 | [54] |
| 3. | ZIF-67 |  |  | 2189.6 | NA | [18] |
| 4. | ZIF-8 ZIF67 |  |  | 1200 | NA | [20] |
| 5. | ZIF-67 and ZIF-67-IL |  |  | 1716 and 1707 | 0.72 and 0.65 | [57] |
| 6. | ZIF-68, ZIF-69, and ZIF-70 |  |  | 1220 1070 and 1970 | NA | [14] |
| 7. | ZIF-69 |  | NA | 874 | NA | [46] |
| 8. | ZIF-78 | NA |  | NA | NA | [58] |
| 9. | ZIF-95 |  | NA | 763.75 | 0.39 | [49] |
| 10. | ZIF-25, ZIF-71, ZIF-93, ZIF-96, and ZIF-97 | NA |  | 564, 652, 864, 960 and 1110 | NA | [13] |
| 11. | ZIF-204 | NA |  | 715 | NA | [60] |
| S. No. | ZIFs | XRD Pattern | Information | Ref. |
|---|---|---|---|---|
| 1. | ZIF-8 |  |
| [43] |
| 2. | ZIF-67/67-IL |  |
| [57] |
| 3. | ZIF-68 |  |
| [61] |
| 4. | ZIF-69 |  |
| [46] |
| 5. | ZIF-70 |  |
| [61] |
| 6. | ZIF-78 |  |
| [58] |
| 7. | ZIF-95 |  |
| [49] |
| 8. | ZIF-97/96/93/71/25 |  |
| [13] |
| S. No. | ZIFs | TGA Profile | Weight Loss between (Tem. K) | Ref. |
|---|---|---|---|---|
| 1. | ZIF-8 ZIF67 |  | 873–1173 673–823 | [20] |
| 2. | ZIF-67 |  | 623–773 | [12] |
| 3. | ZIF-67 |  | 373–403 | [18] |
| 4. | ZIF-69 |  | 643–693 | [46] |
| 5. | ZIF-95 |  | 623–1023 | [49] |
| S. No. | Non-Bonded Interactions | |
|---|---|---|
| 1. | Lennard Jones (LJ) potential |
|
| 2. | Buckingham potential |
|
| 3. | Electrostatic potential |
|
| Bonded interactions | ||
| 1. | Bond potential |
|
| 2. | Angle potential |
|
| 3. | Torsional (dihedral) angle potential |
|
| S. No. | FF | Highlights |
|---|---|---|
| 1. | Class 1 |
|
| 2. | Class 2 |
|
| 3. | Class 3 |
|
| Models | Theory | Highlights | Ref. |
|---|---|---|---|
| Mulliken | Partitioning the molecular wave function into atomic contribution |
| [78,79] |
| Natural population analysis (NPA) | Partitioning the molecular wave function into atomic contribution |
| [78,80] |
| Distributed multipole analysis of Gaussian (GDMA) | Partitioning the molecular wave function into atomic contribution |
| [78,81] |
| Atoms-in-molecules (AIM) | Partition the MED into atomic domains in the physical space |
| [78,82] |
| Hirshfeld | Partition the MED into atomic domains in the physical space |
| [78,83] |
| Charge model 5 (CM5) | Partition the MED into atomic domains in the physical space |
| [78,84] |
| Merz-Kollman-Singh (MKS) | Reproduction of the molecular electrostatic potential |
| [78,85] |
| Charge from electrostatic potentials (CHELP) | Reproduction of the molecular electrostatic potential |
| [78,86] |
| Charge from electrostatic potentials using a grid (CHELPG) | Reproduction of the molecular electrostatic potential |
| [72,78,87] |
| Hu-Lu-Yang (HLY) | Reproduction of the molecular electrostatic potential |
| [78,87] |
| S. No. | ZIF | Evaluation of APC | Interacting Potential | Interaction Techniques | Force Field | Simulation | Highlights | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | ZIF-1 | NA | LJ | Lorenz Berthelot | TraPPE and DREIDING | GCMC |
| [88] |
| 2. | ZIF-1 to ZIF-3 ZIF-6, ZIF-8,ZIF-10, ZIF-60, ZIF-65, ZIF-67, ZIF-68 ZIF-69, ZIF-78, ZIF-79, ZIF-81, ZIF-90, | CBAC | LJ | Lorenz Berthelot | UFF and DREIDING | GCMC |
| [89] |
| 3. | ZIF-1 to ZIF-3 ZIF-6, ZIF-8, ZIF-10 to ZIF-12, ZIF-60, ZIF-65, ZIF-67, ZIF-69, ZIF-78, ZIF-79, ZIF-81, ZIF-90, | DFT | LJ and electrostatic | Lorenz Berthelot | UFF | GCMC and EMD |
| [90] |
| 4. | ZIF-2, ZIF-3 ZIF-6, ZIF-8, ZIF-10 to ZIF-12, ZIF-60, ZIF-65, ZIF-67, ZIF-69, ZIF-78, ZIF-79, ZIF-81, ZIF-90 | DFT | LJ and electrostatic | Lorenz Berthelot | UFF | GCMC and EMD |
| [91] |
| 5. | ZF-2 to ZIF-10 | NA | LJ | - | UFF and DREIDING | GCMC |
| [92] |
| 6. | ZIF-3 and ZIF-10 | DFT | LJ | Lorenz Berthelot | UFF | GCMC and EMD |
| [93] |
| 7. | ZIF-3, ZIF-8, ZIF-10, ZIF-60, and ZIF-67 | NA | LJ | Lorenz Berthelot | UFF and TraPPE | GCMC |
| [94] |
| 8. | ZIF-4, ZIF-62, and ZIF-77 | NA | NA | NA | ReaxFF | NA |
| [95] |
| 9. | ZIF-8 | CBAC | LJ and Coulombic | Ewald Summation | TraPPE and DREIDING | GCMC |
| [96] |
| 10. | ZIF-8 | DFT | LJ and Coulombic | Lorenz Berthelot | UFF | GCMC |
| [97] |
| 11. | ZIF-8 | DFT | LJ | Lorenz Berthelot | AMBER | GCMC |
| [98] |
| 12. | ZIF-8 | DFT/REPEAT | NA | NA | UFF | GCMC |
| [99] |
| 13. | ZIF-8, ZIF-25, ZIF-90, ZIF-93, and ZIF-97 | DFT | LJ | Lorenz Berthelot and Ewald Summation | DREIDING | GCMC |
| [100] |
| 14. | ZIF-8 and ZIF-90 | DFT | Electrostatic surface | REPEAT | UFF | GCMC |
| [101] |
| 15. | ZIF-25, ZIF-71, ZIF-93, ZIF-96, and ZIF-97, | DFT | NA | Lorenz Berthelot | UFF | GCMC |
| [13] |
| 16. | ZIF-68 and ZIF-69 | DFT | LJ and Coulombic | Ewald Summation | UFF and DREIDING | GCMC |
| [102] |
| 17. | ZIF-68 and ZIF-69 | DFT | LJ | Lorenz Berthelot | UFF | GCMC |
| [103] |
| 18. | ZIF-68 and ZIF-69 | DFT | LJ and Coulombic | Lorenz Berthelot | TraPPE | GCMC |
| [104] |
| 19. | ZIF-68 and ZIF-70 | DFT | LJ | Lorenz Berthelot | UFF | GCMC and EMD |
| [105] |
| 20. | ZIF-68 and ZIF-70 | DFT | LJ | Lorenz Berthelot | UFF and DREIDING | GCMC and EMD |
| [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalauni, K.; Vedrtnam, A.; Wdowin, M.; Chaturvedi, S. ZIF for CO2 Capture: Structure, Mechanism, Optimization, and Modeling. Processes 2022, 10, 2689. https://doi.org/10.3390/pr10122689
Kalauni K, Vedrtnam A, Wdowin M, Chaturvedi S. ZIF for CO2 Capture: Structure, Mechanism, Optimization, and Modeling. Processes. 2022; 10(12):2689. https://doi.org/10.3390/pr10122689
Chicago/Turabian StyleKalauni, Kishor, Ajitanshu Vedrtnam, Magdalena Wdowin, and Shashikant Chaturvedi. 2022. "ZIF for CO2 Capture: Structure, Mechanism, Optimization, and Modeling" Processes 10, no. 12: 2689. https://doi.org/10.3390/pr10122689









