Research on Adsorption and Desorption Performance of Gas-Phase Naphthalene on Hydrophobic Modified FDU-15
Abstract
:1. Introduction
2. Experimental Part
2.1. Adsorbents and Characterization
2.2. Adsorption Experiment
2.2.1. Adsorption Experiment Device
2.2.2. Calculation of Adsorption Capacity
2.3. Desorption Experiment
2.3.1. Desorption Experimental Method
2.3.2. Determination of Activation Energy
2.3.3. Determining the Probable Mechanism Function
3. Experimental Results and Discussion
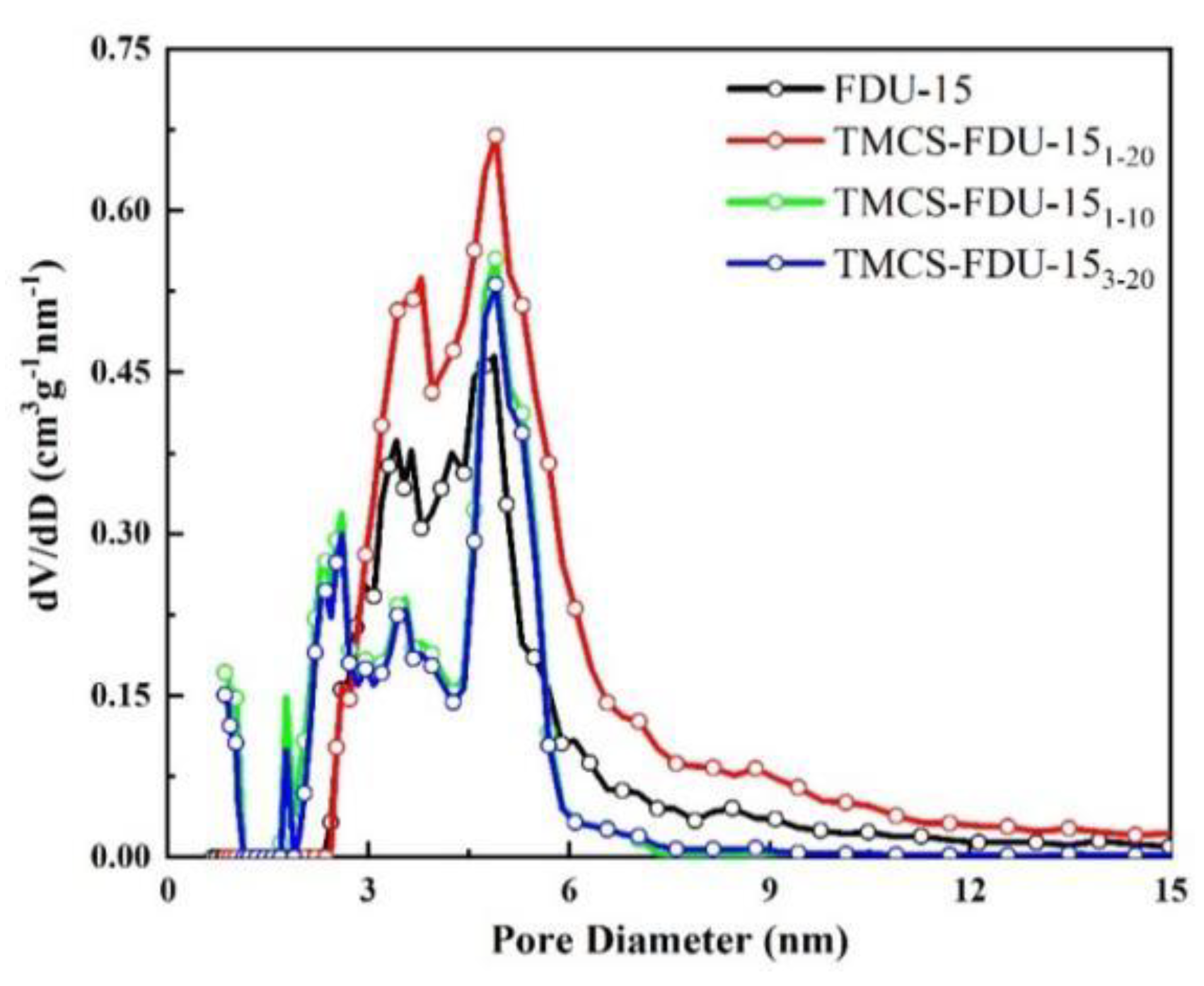
3.1. Characterization of Adsorbents
3.1.1. Analysis of Channel Characteristics
3.1.2. FTIR Analysis
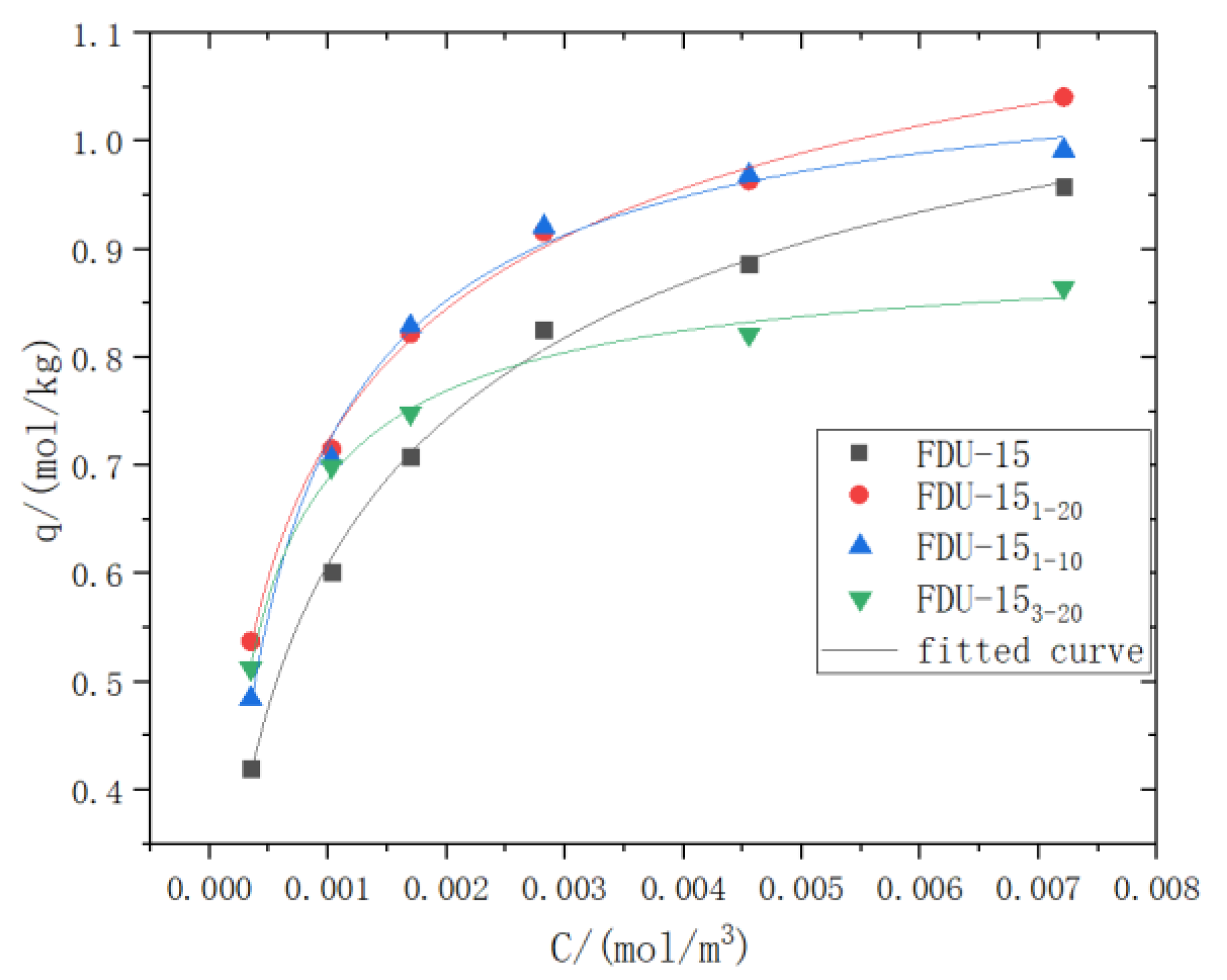
3.2. Adsorption Performance Analysis
3.3. Desorption Performance Analysis
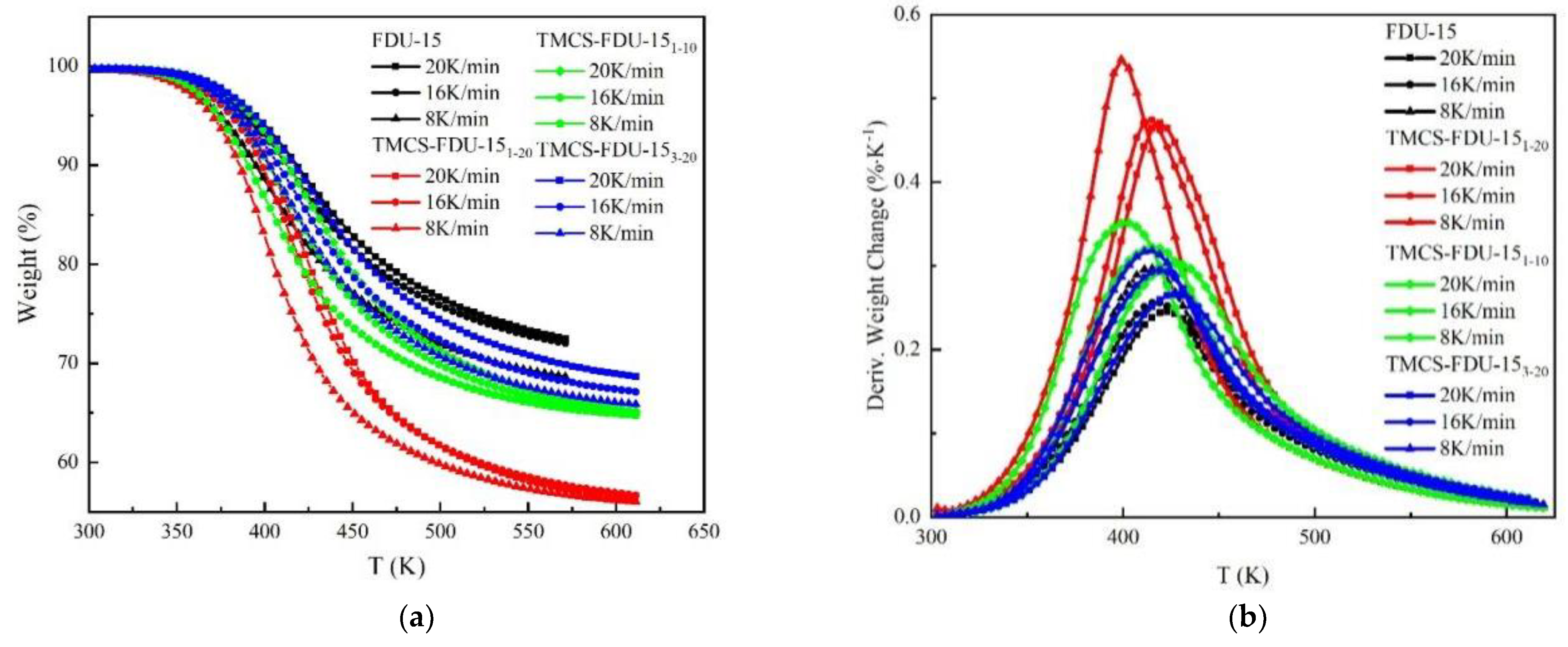
3.3.1. Desorption Curve Analysis
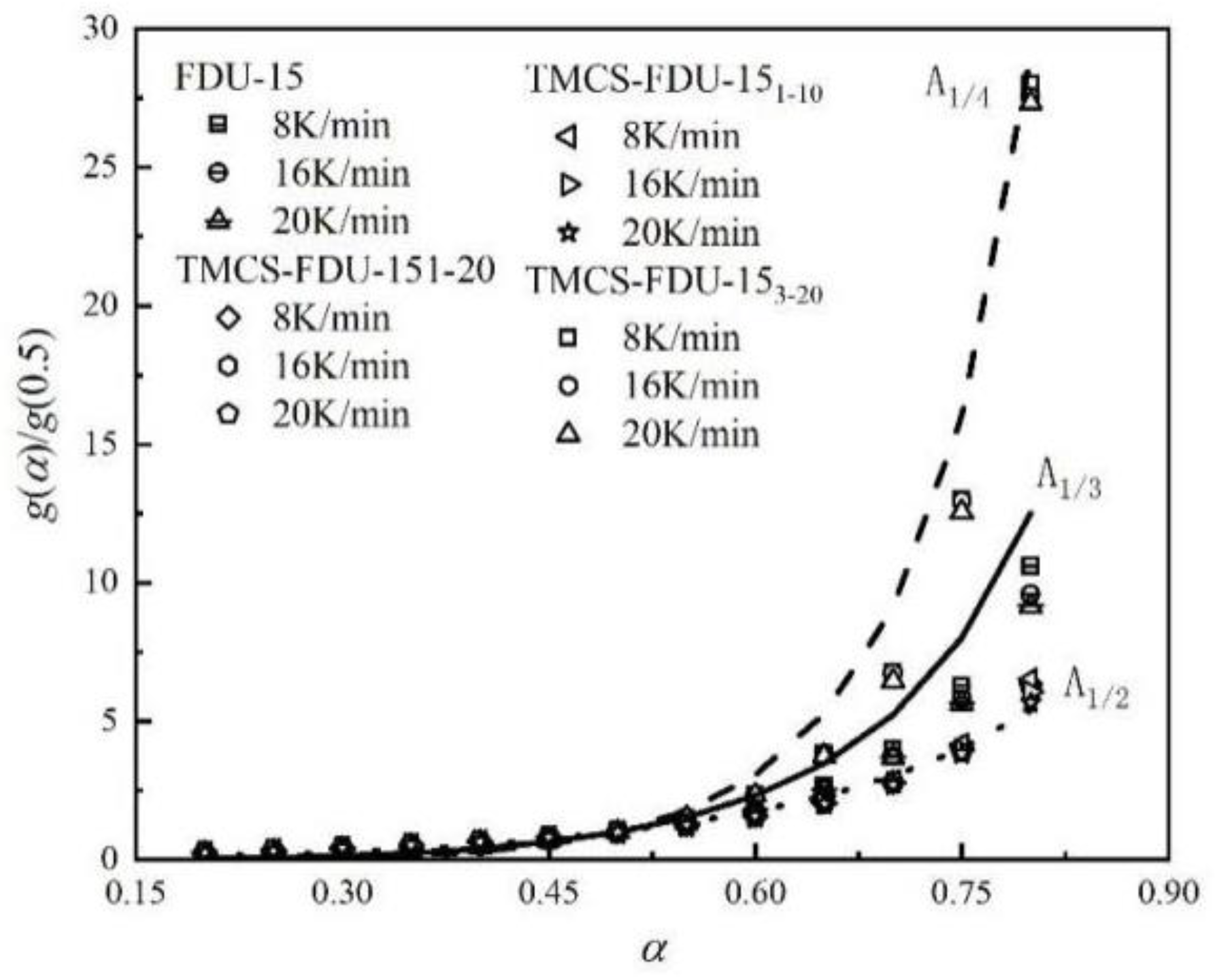
3.3.2. Desorption Kinetics Parameter Calculation
4. Conclusions
- (1)
- Hydrophobic modification of FDU-15 adsorbent with trimethylchlorosilane (TMCS) can increase the content of micropores and specific surface area of FDU-15. In this study, the specific surface area of modification using FDU-15 followed TMCS-FDU-151-20 > TMCS-FDU-151-10 > TMCS-FDU-153-20 > FDU-15.
- (2)
- Hydrophobic modification of FDU-15 with an appropriate amount of TMCS was beneficial to improve the adsorption performance of the adsorbent for naphthalene at low concentrations. Under the experimental conditions studied in this paper, TMCS-FDU-151-20 and TMCS-FDU-151-10 modified with a small amount of TMCS can significantly improve the naphthalene adsorption capacity at low concentrations.
- (3)
- When the mass ratio of TMCS and FDU-15 was 1:10, the adsorbent’s pore structure and surface hydrophobicity can reach an optimal balance. Under this condition, the desorption activation energy of the adsorbent for naphthalene decreased from 60.98 kJ/mol of the raw FDU-15 and reduced to a minimum of 50.28 kJ/mol.
- (4)
- Based on experiment conditions, the desorption mechanism functions of naphthalene on FDU-15 and modified FDU-15 were in accordance with the JMA equation, which is characterized by the formation of nuclei and the rate of growth.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, X.; Li, Z.; Liu, Y.; Xing, Y.; Wei, J.; Yang, B.; Tsai, C.J. Research progress of gaseous polycyclic aromatic hydrocarbons purification by adsorption. Aerosol Air Qual. Res. 2019, 19, 911. [Google Scholar] [CrossRef] [Green Version]
- Mukwevho, N.; Gusain, R.; Fosso-Kankeu, E.; Kumar, N.; Waanders, F.; Ray, S.S. Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite. J. Ind. Eng. Chem. 2020, 81, 393. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, P.; Wu, Z.; Cravotto, G. In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions. Processes 2021, 9, 391. [Google Scholar] [CrossRef]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene-an environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Jiang, L.; Li, Z.; Liu, Y.; Wang, H.; Yang, R.T. Molecular simulation of naphthalene, phenanthrene, and pyrene adsorption on MCM-41. Int. J. Mol. Sci. 2019, 20, 665. [Google Scholar] [CrossRef] [Green Version]
- Dowaidar, A.M.; El-Shahawi, M.S.; Ashour, I. Adsorption of polycyclic aromatic hydrocarbons onto activated carbon from non-aqueous media: The Influence of the Organic Solvent Polarity. Sep. Sci. Technol. 2007, 42, 3609. [Google Scholar] [CrossRef]
- Mastral, A.M.; García, T.; Callén, M.S.; Navarro, M.V.; Galban, J. Assessement of phenanthrene removal from hot gas by porous carbons. Energy Fuels 2001, 15, 1. [Google Scholar] [CrossRef]
- Li, F.; Chen, J.; Hu, X.; He, F.; Bean, E.; Tsang, D.C.; Gao, B. Applications of carbonaceous adsorbents in the remediation of polycyclic aromatic hydrocarbon-contaminated sediments: A review. J. Clean. Prod. 2020, 255, 120263. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Yang, X.; Xing, Y.; Tsai, C.; Yang, Q.; Yang, R.T. Performance of mesoporous silicas (MCM-41 and SBA-15) and carbon (CMK-3) in the removal of gas-phase naphthalene: Adsorption capacity, rate and regenerability. Rsc Adv. 2016, 6, 21193–21203. [Google Scholar] [CrossRef]
- Kosuge, K.; Kubo, S.; Kikukawa, N.; Takemori, M. Effect of pore structure in mesoporous silicas on VOC dynamic adsorption/desorption performance. Langmuir 2007, 23, 3095. [Google Scholar] [CrossRef]
- Loussala, H.M.; Han, S.; Feng, J.; Sun, M.; Feng, J.; Fan, J.; Pei, M. Mesoporous silica hybridized by ordered mesoporous carbon for in-tube solid-phase microextraction. J. Sep. Sci. 2020, 43, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Lu, G.Q.; Whittaker, A.K.; Millar, G.J.; Zhu, H.Y. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Yang, X.; Xing, Y.; Tsai, C.J.; Meng, M.; Yang, R.T. Performance of mesoporous silicas and carbon in adsorptive removal of phenanthrene as a typical gaseous polycyclic aromatic hydrocarbon. Microporous Mesoporous Mater. 2017, 239, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Li, Z.; Meng, M.; Yang, X.; Bian, W.; Yang, R.T. Adsorption and desorption of gaseous naphthalene on carbonaceous sorbents: Insights into advantageous pore sizes and morphologies. J. Clean. Prod. 2021, 314, 127905. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, Y.S.; Yang, X.; Meng, M.M.; Zhang, C.Z. Study of naphthalene desorption properties on typical mesoporous. J. Eng. Thermophys. 2017, 38, 1478. [Google Scholar]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Yang, H.; Li, Z.; Zhao, D. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef]
- Ndamanisha, J.C.; Guo, L. Ordered mesoporous carbon for electrochemical sensing: A review. Anal. Chim. Acta 2012, 747, 19–28. [Google Scholar] [CrossRef]
- Wu, Z.; Webley, P.A.; Zhao, D. Comprehensive study of pore evolution, mesostructural stability, and simultaneous surface functionalization of ordered mesoporous carbon (FDU-15) by wet oxidation as a promising adsorbent. Langmuir 2010, 26, 10277–10286. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, G.; Liu, L.; Yu, Y.; Chen, A. Preparation of mesoporous carbon from biomass for heavy metal ion adsorption. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 102–108. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Gan, G.; Wang, L.; Fan, S.; Wang, P.; Liu, S. Functionalized Activated Carbon for Competing Adsorption of Volatile Organic Compounds and Water. ACS Appl. Mater. Interfaces 2021, 13, 56510–56518. [Google Scholar] [CrossRef]
- Kim, K.-D.; Park, E.J.; Seo, H.O.; Jeong, M.-G.; Kim, Y.D.; Lim, D.C. Effect of Thin Hydrophobic Films for Toluene Adsorption and Desorption Behavior on Activated Carbon Fiber Under Dry and Humid Conditions. Chem. Eng. J. 2012, 200–202, 133–139. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; He, Z.; Wang, P.; Yan, Y.; Ran, J. Hydrophobic Modified Activated Carbon Using PDMS for the Adsorption of VOCs in Humid Condition. Sep. Purif. Technol. 2020, 239, 116517–116527. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Y.; Chen, T.; Tang, F.; Cai, J.; Ma, J. Trimethylchlorosilane Modified Activated Carbon for the Adsorption of VOCs at High Humidity. Sep. Purif. Technol. 2021, 272, 118659. [Google Scholar] [CrossRef]
- Li, H.Y.; Guo, J.F. Study on mesoporous SBA-15 modified by organochlorosilane and its hydrophobicity. Chem. World 2011, 52, 389. [Google Scholar]
- Wang, H.; Wang, T.; Han, L.; Tang, M.; Zhong, J.; Huang, W.; Chen, R. VOC adsorption and desorption behavior of hydrophobic, functionalized SBA-15. J. Mater. Res. 2016, 31, 516. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, Y.S.; Yang, X.; Xing, Y.; Yang, Q.; Yang, R.T. Adsorption thermodynamics and desorption properties of gaseous polycyclic aromatic hydrocarbons on mesoporous adsorbents. Adsorption 2017, 23, 361. [Google Scholar] [CrossRef]
- Delle Site, A. The vapor pressure of environmentally significant organic chemicals: A review of methods and data at ambient temperature. J. Phys. Chem. Ref. Data 1997, 26, 157. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, Y.; Yang, X.; Xing, Y.; Tsai, C.; Wang, Z.; Yang, R.T. Desorption of polycyclic aromatic hydrocarbons on mesoporous sorbents: Thermogravimetric experiments and kinetics study. Ind. Eng. Chem. Res. 2016, 55, 1183. [Google Scholar] [CrossRef]
- Janković, B.; Adnađević, B.; Jovanović, J. Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: Thermogravimetric analysis. Thermochim. Acta. 2007, 452, 106. [Google Scholar] [CrossRef]
- Wanjun, T.; Yuwen, L.; Xi, Y.; Cunxin, W. Kinetic studies of the calcination of ammonium metavanadate by thermal methods. Ind. Eng. Chem. Res. 2004, 43, 2054. [Google Scholar] [CrossRef]
- Salonen, J.; Lehto, V.P.; Laine, E. Thermal oxidation of free-standing porous silicon films. Appl. Phys. Lett. 1997, 70, 637. [Google Scholar] [CrossRef]
- Stevens, R.W., Jr.; Siriwardane, R.V.; Logan, J. In situ fourier transform infrared (FTIR) investigation of CO2 adsorption onto zeolite materials. Energy Fuels 2008, 22, 3070. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina. Chem. Eng. Sci. 2001, 56, 3781. [Google Scholar] [CrossRef]
- Yang, X.; Epiepang, F.E.; Li, J.; Wei, Y.; Liu, Y.; Yang, R.T. Sr-LSX zeolite for air separation. Chem. Eng. J. 2019, 362, 482. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Yang, X.; Wang, Z.; Yang, Q.; Yang, R.T. Desorption kinetics of naphthalene and acenaphthene over two activated carbons via thermogravimetric analysis. Energy Fuels 2015, 29, 5303. [Google Scholar] [CrossRef]
- Drewien, C.A.; Tallant, D.R.; Eatough, M.O. Thermal stability and decomposition kinetics of Li2Al4CO3(OH)12·3H2O. J. Mater. Sci. 1996, 31, 4321. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, Y.S.; Jiang, L.J. Research progress on adsorption purification technology of gaseous polycyclic aromatic hydrocarbons. Chin. J. Eng. 2018, 40, 127. [Google Scholar]
- Wang, K.L.; Huang, B.C.; Liu, D.M.; Ye, D. Ordered mesoporous carbons with various pore sizes: Preparation and naphthalene adsorption performance. J. Appl. Polym. Sci. 2012, 125, 3368. [Google Scholar] [CrossRef]


| Symbol | Model | Differential Form | Integral Form |
|---|---|---|---|
| Diffusion Model | |||
| D1 | one-dimensional diffusion | (1/2)α−1 | α2 |
| D2 | two-dimensional diffusion | [−ln(1 − α)]−1 | α + (1 − α) ln(1 − α) |
| D3 | 3D Diffusion (ZLT Equation) | (3/2)(1 − α)4/3 [(1 − α)−1/3 − 1]−1 | [(1 − α) − 1/3 − 1]2 |
| Nucleation model | |||
| Am | Random Nucleation and Nucleation Growth (JMA(Johnson-Mehl-Arvami) equation; parameters m = 4, 3, 2, 3/2, 4/3, 1, 2/3, 1/2, 1/3, 1/4) | m(1 − α)[−ln(1 − α)]1−1/m | [−ln(1 − α)]1/m |
| geometric shrinkage model | |||
| R2 | Phase boundary reaction (cylindrical symmetry) | 2(1 − α)1/2 | 1 − (1 − α)1/2 |
| R3 | Phase boundary reaction (spherical symmetry) | 3(1 − α)2/3 | 1 − (1 − α)1/3 |
| Reaction series model | |||
| F1(A1) | First-order | 1 − α | −ln(1 − α) |
| F2 | Second order | (1 − α)2 | (1 − α)−1 − 1 |
| F3 | Third order | (1 − α)3 | 0.5 [(1 − α)−2 − 1] |
| Sample | SBET (m2/g) | Vp (cm3·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) | dp (nm) |
|---|---|---|---|---|---|
| FDU-15 | 1149 | 1.39 | 0.00 | 1.39 | 4.9 |
| TMCS-FDU-15 1-20 | 1733 | 1.25 | 0.00 | 1.25 | 4.9 |
| TMCS-FDU-15 1-10 | 1382 | 1.14 | 0.09 | 1.05 | 4.9 |
| TMCS-FDU-15 3-20 | 1237 | 1.06 | 0.06 | 1.00 | 4.9 |
| Adsorbent | qm | b | n | R2/% |
|---|---|---|---|---|
| FDU-15 | 1.283 | 59.865 | 0.607 | 99.5 |
| TMCS-FDU-151-20 | 1.354 | 46.373 | 0.536 | 99.5 |
| TMCS-FDU-151-10 | 1.102 | 704.934 | 0.857 | 99.2 |
| TMCS-FDU-153-20 | 0.910 | 895.218 | 0.821 | 99.2 |
| Desorption Kinetics Three Factors | Adsorbent | |||
|---|---|---|---|---|
| FDU-15 | TMCS-FDU-151-20 | TMCS-FDU-151-10 | TMCS-FDU-153-20 | |
| Model | A1/3 | A1/2 | A1/2 | A1/4 |
| Ea (kJ/mol) | 60.98 | 60.59 | 50.28 | 95.3 |
| ln A (min−1) | 11.00 | 15.81 | 12.48 | 20.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Liu, Y.; Meng, M.; Li, Z.; Wang, H.; Liu, W.; Yang, X. Research on Adsorption and Desorption Performance of Gas-Phase Naphthalene on Hydrophobic Modified FDU-15. Processes 2022, 10, 574. https://doi.org/10.3390/pr10030574
Zhao C, Liu Y, Meng M, Li Z, Wang H, Liu W, Yang X. Research on Adsorption and Desorption Performance of Gas-Phase Naphthalene on Hydrophobic Modified FDU-15. Processes. 2022; 10(3):574. https://doi.org/10.3390/pr10030574
Chicago/Turabian StyleZhao, Chunyu, Yingshu Liu, Miaomiao Meng, Ziyi Li, Haihong Wang, Wenhai Liu, and Xiong Yang. 2022. "Research on Adsorption and Desorption Performance of Gas-Phase Naphthalene on Hydrophobic Modified FDU-15" Processes 10, no. 3: 574. https://doi.org/10.3390/pr10030574
APA StyleZhao, C., Liu, Y., Meng, M., Li, Z., Wang, H., Liu, W., & Yang, X. (2022). Research on Adsorption and Desorption Performance of Gas-Phase Naphthalene on Hydrophobic Modified FDU-15. Processes, 10(3), 574. https://doi.org/10.3390/pr10030574







