Recovery of Phenolic Compounds and Antioxidants from Coffee Pulp (Coffea canephora) Waste Using Ultrasound and Microwave-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Pulp
2.2. Chemicals of Experiments
2.3. Experimental Design
2.3.1. Ultrasound-Assisted Extraction (UAE)
2.3.2. Microwave-Assisted Extraction (MAE)
2.4. Determination of Bioactive Compounds
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.4.3. Determination of Caffeine and Chlorogenic Acid
2.5. Determination of Antioxidant Capacity
2.5.1. ABTS Assay
2.5.2. DPPH Assay
2.5.3. FRAP Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Impact of UAE Extraction Parameters on Recovery Yields of Bioactives and Antioxidant Properties from Coffee Pulp
3.1.1. Effect of Ultrasonic Time
3.1.2. Effect of Temperature
3.1.3. Effect of Ultrasonic Power
3.2. Impact of MAE Extraction Parameters on Recovery Yields of Bioactives and Antioxidant Properties from Coffee Pulp
3.2.1. Effect of Radiation Time
3.2.2. Effect of Radiation Power
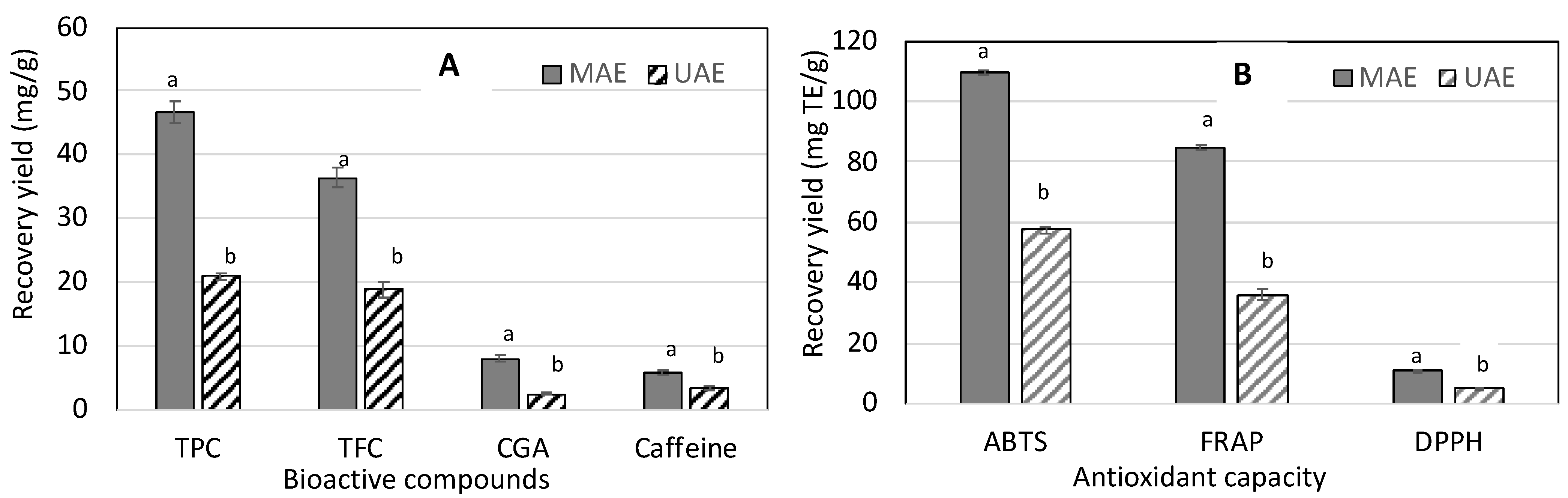
3.3. Comparison of UAE and MAE on the Recovery of Bioactive Compounds and Antioxidant Capacity from Coffee Pulp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopra, V.; Peter, K. Handbook of Industrial Crops; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Ramalakshmi, K.; Kubra, I.; Rao, L. Physicochemical Characteristics of Green Coffee: Comparison of Graded and Defective Beans. J. Food Sci. 2007, 72, S333–S337. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Padmapriya, R.; Tharian, J.A.; Thirunalasundari, T. Coffee waste management-An overview. Int. J. Curr. Sci. 2013, 9, 83–91. [Google Scholar]
- Wintgens, J.N. The Coffee Plant. Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wiley-Vch: Weinheim, Germany, 2009; pp. 1–24. [Google Scholar]
- Lashermes, P.; Andrade, A.C.; Etienne, H. Genomics of Coffee One of the World’s Largest Traded Commodities, in Genomics of Tropical Crop Plants; Moore, P.H., Ming, R., Eds.; Springer: New York, NY, USA, 2008; pp. 203–226. [Google Scholar]
- Tran, T.M.K.; Akanbi, T.; Kirkman, T.; Nguyen, M.H.; Van Vuong, Q. Optimal Aqueous Extraction Conditions as A Green Technique for Recovery of Phenolic Antioxidants from Robusta Dried Coffee Pulp. Eur. J. Eng. Res. Sci. 2020, 5, 1069–1074. [Google Scholar] [CrossRef]
- Tran, T.M.K.; Kirkman, T.; Nguyen, M.; Van Vuong, Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6, e04498. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Rojas, J.U.; Verreth, J.; Amato, S.; Huisman, E. Biological treatments affect the chemical composition of coffee pulp. Bioresour. Technol. 2003, 89, 267–274. [Google Scholar] [CrossRef]
- Murthy, P.S.; Manjunatha, M.R.; Sulochannama, G.; Naidu, M.M. Extraction, characterization and bioactivity of coffee anthocyanins. Eur. J. Biol. Sci. 2012, 4, 13–19. [Google Scholar]
- Ramirez-Coronel, M.A.; Marnet, N.; Kolli, V.K.; Roussos, S.; Guyot, S.; Augur, C. Characterization and Estimation of Proanthocyanidins and Other Phenolics in Coffee Pulp (Coffea arabica) by Thiolysis−High-Performance Liquid Chromatography. J. Agric. Food Chem. 2004, 52, 1344–1349. [Google Scholar] [CrossRef]
- Belay, A.; Gholap, A. Characterization and determination of chlorogenic acids (CGA) in coffee beans by UV-Vis spectroscopy. Afr. J. Pure Appl. Chem. 2009, 3, 34–240. [Google Scholar]
- Tran, T.M.K.; Akanbi, T.; Kirkman, T.; Nguyen, M.H.; Van Vuong, Q. Maximising Recovery of Bioactive Compounds from Coffee Pulp Waste Using Microwave-assisted Extraction. Eur. J. Eng. Technol. Res. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, N.; Qu, B. An Improved Ultrasonic-Assisted Extraction Method by Optimizing the Ultrasonic Frequency for Enhancing the Extraction Efficiency of Lycopene from Tomatoes. Food Anal. Methods 2016, 9, 2288–2298. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Vuong, Q.; Hirun, S.; Roach, P.; Bowyer, M.C.; Phillips, P.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J. Food Process. Preserv. 2016, 41, e13148. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Rostagno, M.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Carcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Mou, H.; Kong, Q. Ultrasound-assisted extraction and characterization of anthocyanins from purple corn bran. J. Food Process. Preserv. 2017, 42, e13377. [Google Scholar] [CrossRef] [Green Version]
- Dailey, A.; Vuong, Q.V. Optimisation of Ultrasonic Conditions as an Advanced Extraction Technique for Recovery of Phenolic Compounds and Antioxidant Activity from Macadamia (Macadamia tetraphylla) Skin Waste. Technologies 2015, 3, 302–320. [Google Scholar] [CrossRef] [Green Version]
- Dailey, A.; Vuong, Q.V. Optimum Conditions for Microwave Assisted Extraction for Recovery of Phenolic Compounds and Antioxidant Capacity from Macadamia (Macadamia tetraphylla) Skin Waste Using Water. Processes 2015, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Saifullah, M.; McCullum, R.; Vuong, Q. Maximising extraction yields of gallic acid and hesperetin from lemon myrtle (Backhousia citriodora) leaf using microwave assisted extraction. Results Chem. 2020, 2, 100080. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Mechanochemical extraction of antioxidant phenolic compounds from Mediterranean and medicinal Laurus nobilis: A comparative study with other traditional and green novel techniques. Ind. Crop. Prod. 2019, 141, 111805. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Chen, J.-C.; Liu, D.-H.; Ye, X.-Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochemistry 2009, 16, 57–62. [Google Scholar] [CrossRef]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crop. Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
| Time (min) | TPC (mg GAE/g DW) | TFC (mg CE/g DW) | CGA (mg/g DW) | Caffeine (mg/g DW) | ABTS (mg TE/g DW) | DPPH (mg TE/g DW) | FRAP (mg TE/g DW) |
|---|---|---|---|---|---|---|---|
| 5 | 6.26 ± 0.35 c | 4.53 ± 0.25 c | 1.31 ± 0.10 b | 1.87 ± 0.09 a | 13.35 ± 0.76 a | 1.33 ± 0.08 c | 9.50 ±0.90 a |
| 15 | 8.70 ± 0.34 b | 6.47 ± 0.16 b | 1.70 ± 0.15 ab | 2.25 ± 0.035 b | 14.89 ± 0.41 b | 1.74 ± 0.06 b | 9.69 ± 0.99 a |
| 25 | 9.14 ± 0.82 ab | 6.54 ± 0.93 b | 1.72 ± 0.49 ab | 2.31 ± 0.10 b | 15.19 ± 0.49 b | 1.78 ± 0.21 ab | 9.73 ± 0.36 a |
| 35 | 9.72 ± 0.15 ab | 6.91 ± 0.20 ab | 1.67 ± 0.11 ab | 2.47 ± 0.21 b | 15.21 ± 0.28 b | 1.87 ± 0.07 ab | 10.28 ± 0.89 a |
| 45 | 9.74 ± 0.19 ab | 7.25 ± 0.36 ab | 1.77 ± 0.33 a | 2.53 ± 0.18 b | 15.64 ± 0.02 b | 1.84 ± 0.04 ab | 10.33 ± 0.52 a |
| 55 | 9.88 ± 0.15 a | 8.09 ± 0.76 a | 1.78 ± 0.12 a | 2.54 ± 0.11 b | 15.69 ± 0.09 b | 2.04 ± 0.12 a | 10.17 ± 0.25 a |
| 65 | 9.44 ± 0.38 ab | 7.59 ± 0.40 ab | 1.73 ± 0.06 ab | 2.42 ± 0.08 b | 15.48 ± 0.40 b | 1.9 ± 0.07 ab | 9.75 ± 0.21 a |
| Temp (°C) | TPC (mg GAE/g DW) | TFC (mg CE/g DW) | CGA (mg/g DW) | Caffeine (mg/g DW) | ABTS (mg TE/g DW) | DPPH (mg TE/g DW) | FRAP (mg TE/g DW) |
|---|---|---|---|---|---|---|---|
| 30 | 8.71 ± 0.93 a | 7.75 ± 0.25 a | 2.15 ± 0.32 a | 2.94 ± 0.18 a | 28.04 ± 0.85 a | 2.78 ± 0.02 a | 15.18 ± 0.95 a |
| 40 | 10.48 ± 0.94 a | 9.35 ± 0.51 a | 2.24 ± 0.52 a | 2.99 ± 0.07 a | 31.09 ± 1.88 a | 3.24 ± 0.03 a | 18.72 ± 0.33 b |
| 50 | 14.58 ± 1.32 b | 11.29 ± 1.03 b | 2.54 ± 0.36 a | 3.20 ± 0.25 a | 40.57 ± 3.19 b | 4.33 ± 0.34 b | 20.76 ± 1.31 bc |
| 60 | 15.14 ± 0.23 b | 13.15 ± 0.51 c | 2.65 ± 0.06 a | 2.89 ± 0.19 a | 45.78 ± 1.36 b | 4.68 ± 0.26 b | 22.32 ± 0.63 c |
| Power of Machine (W) | TPC (mg GAE/g DW) | TFC (mg CE/g DW) | CGA (mg/g DW) | Caffeine (mg/g DW) | ABTS (mg TE/g DW) | DPPH (mg TE/g DW) | FRAP (mg TE/g DW) |
|---|---|---|---|---|---|---|---|
| 150 | 14.81 ± 0.19 a | 11.60 ± 0.41 a | 2.64 ± 0.41 a | 2.72 ± 0.23 a | 41.92 ± 0.68 a | 4.10 ± 0.07 a | 24.12 ± 0.61 a |
| 200 | 15.68 ± 0.85 a | 12.32 ± 0.35 a | 2.39 ± 0.29 a | 2.73 ± 0.17 a | 45.84 ± 0.64 b | 4.88 ± 0.10 b | 25.97 ± 0.47 a |
| 250 | 20.86 ± 0.58 b | 18.77 ± 1.07 b | 2.31 ± 0.13 a | 3.32 ± 0.26 b | 57.65 ± 1.16 c | 5.20 ± 0.14 c | 35.85 ± 1.80 b |
| Time (min) | TPC (mg GAE/g DW) | TFC (mg CE/g DW) | CGA (mg/g DW) | Caffeine (mg/g DW) | ABTS (mg TE/g DW) | DPPH (mg TE/g DW) | FRAP (mg TE/g DW) |
|---|---|---|---|---|---|---|---|
| 10 | 25.48 ± 0.99 d | 19.11 ± 0.83 d | 5.66 ± 0.08 d | 4.52 ± 0.07 a | 59.53 ± 2.49 g | 6.16 ± 0.50 b | 36.96 ± 1.85 d |
| 20 | 27.53 ± 1.26 cd | 20.84 ± 1.69 cd | 5.96 ± 0.18 cd | 3.18 ± 2.75 a | 63.18 ± 2.97 f | 6.40 ± 0.54 b | 39.58 ± 1.05 cd |
| 30 | 29.03 ± 0.68 c | 22.07 ± 0.43 c | 5.49 ± 0.26 d | 4.71 ± 0.49 a | 65.17 ± 2.38 e | 6.85 ± 0.28 b | 43.49 ± 0.18 bc |
| 40 | 30.17 ± 0.46 c | 23.17 ± 0.61 c | 6.59 ± 0.40 bc | 4.84 ± 0.42 a | 68.47 ± 1.7 d | 7.01 ± 0.36 b | 46.65 ± 0.20 b |
| 50 | 38.73 ± 0.59 b | 32.81 ± 0.75 ab | 7.33 ± 0.23 ab | 5.70 ± 0.33 a | 78.11 ± 0.47 c | 9.44 ± 0.29 a | 63.44 ± 0.85 a |
| 60 | 39.13 ± 0.16 b | 34.30 ± 0.39 a | 7.57 ± 0.32 a | 5.77 ± 0.33 a | 79.07 ± 0.52 b | 9.83 ± 0.23 a | 65.12 ± 0.33 a |
| 70 | 43.38 ± 0.85 a | 33.86 ± 0.83 ab | 7.33 ± 0.27 ab | 5.45 ± 0.22 a | 82.34 ± 1.1 a | 10.56 ± 0.21 a | 65.55 ± 0.69 a |
| 80 | 40.90 ± 1.67 ab | 31.52 ± 1.42 b | 6.92 ± 0.57 ab | 5.38 ± 0.13 a | 80.43 ± 0.36 ab | 9.70 ± 0.73 a | 60.91 ± 1.15 a |
| Power of Machine (W) | TPC (mg GAE/g DW) | TFC (mg CE/g DW | CGA (mg/g DW) | Caffeine (mg/g DW) | ABTS (mg TE/g DW) | FRAP (mg TE/g DW) | DPPH (mg TE/g DW) |
|---|---|---|---|---|---|---|---|
| 300 | 44.15 ± 0.06 ab | 34.50 ± 1.25 a | 7.62 ± 0.59 a | 5.76 ± 0.19 a | 105.48 ± 1.54 b | 83.61 ± 0.83 a | 10.37 ± 0.16 ab |
| 400 | 45.49 ± 1.35 ab | 35.82 ± 1.96 a | 7.58 ± 0.88 a | 5.36 ± 0.59 a | 116.08 ± 2.32 a | 84.65 ± 1.73 a | 10.54 ± 0.29 ab |
| 500 | 45.02 ± 0.82 ab | 35.49 ± 0.97 a | 7.33 ± 0.27 a | 5.45 ± 0.22 a | 107.56 ± 1.50 b | 84.13 ± 2.46 a | 10.65 ± 0.29 ab |
| 600 | 45.61 ± 1.67 ab | 36.79 ± 1.42 a | 8.12 ± 0.49 a | 5.88 ± 0.12 a | 109.27 ± 2.68 ab | 84.63 ± 1.05 a | 11.02 ± 0.46 a |
| 700 | 46.72 ± 1.86 a | 36.44 ± 1.54 a | 7.87 ± 0.54 a | 5.74 ± 0.29 a | 109.55 ± 0.64 ab | 84.73 ± 0.88 a | 11.02 ± 0.21 a |
| 800 | 43.03 ± 1.14 b | 36.00 ± 1.02 a | 8.09 ± 0.35 a | 5.39 ± 0.69 a | 86.73 ± 4.22 c | 72.37 ± 0.33 b | 9.81 ± 0.09 b |
| 900 | 42.79 ± 1.27 b | 34.42 ± 1.85 a | 7.29 ± 1.40 a | 5.29 ± 1.44 a | 84.81 ± 2.57 c | 71.26 ± 3.10 b | 10.00 ± 0.52 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.M.K.; Akanbi, T.O.; Kirkman, T.; Nguyen, M.H.; Vuong, Q.V. Recovery of Phenolic Compounds and Antioxidants from Coffee Pulp (Coffea canephora) Waste Using Ultrasound and Microwave-Assisted Extraction. Processes 2022, 10, 1011. https://doi.org/10.3390/pr10051011
Tran TMK, Akanbi TO, Kirkman T, Nguyen MH, Vuong QV. Recovery of Phenolic Compounds and Antioxidants from Coffee Pulp (Coffea canephora) Waste Using Ultrasound and Microwave-Assisted Extraction. Processes. 2022; 10(5):1011. https://doi.org/10.3390/pr10051011
Chicago/Turabian StyleTran, Thy Minh Kieu, Taiwo O. Akanbi, Timothy Kirkman, Minh H. Nguyen, and Quan Van Vuong. 2022. "Recovery of Phenolic Compounds and Antioxidants from Coffee Pulp (Coffea canephora) Waste Using Ultrasound and Microwave-Assisted Extraction" Processes 10, no. 5: 1011. https://doi.org/10.3390/pr10051011
APA StyleTran, T. M. K., Akanbi, T. O., Kirkman, T., Nguyen, M. H., & Vuong, Q. V. (2022). Recovery of Phenolic Compounds and Antioxidants from Coffee Pulp (Coffea canephora) Waste Using Ultrasound and Microwave-Assisted Extraction. Processes, 10(5), 1011. https://doi.org/10.3390/pr10051011








