A Review of Numerical Research on the Pressure Swing Adsorption Process
Abstract
:1. Introduction
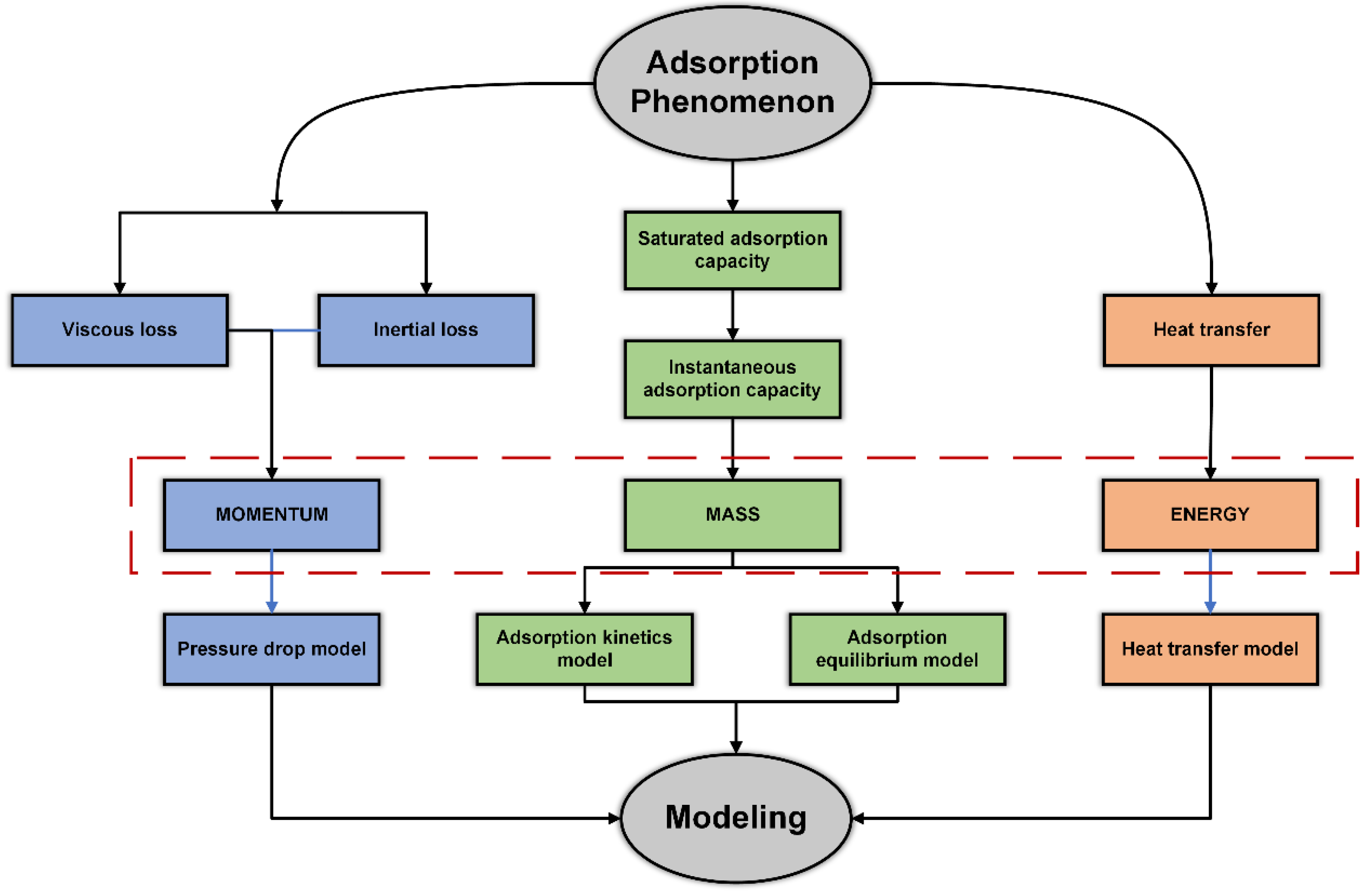
2. PSA Modeling
2.1. Adsorption-Kinetics Model
2.2. Pressure-Drop Model
2.3. Fluid-Flow Model
2.4. Special Treatments for Energy Balance
2.4.1. Heat of Adsorbed Phases and the Heat of Adsorption
2.4.2. Non-Isothermal Conditions and Thermal Equilibrium
2.4.3. Adiabatic, Thin-Wall, and Rigorous Models of Heat Transfer to Environment
2.5. One-Dimensional Process Model
3. PSA Numerical Optimization and Control
3.1. Optimization Strategies
3.1.1. CSS Definition
3.1.2. Detailed Model
3.1.3. Surrogate Model
3.2. Control Strategy
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrotra, A.; Ebner, A.D.; Ritter, J.A. Arithmetic approach for complex PSA cycle scheduling. Adsorption 2010, 16, 113–126. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, Y.; Yu, X.; Shen, Y.; He, D.; Tang, Z.; Li, W.; Zhang, D. Simulation and analysis of dual-reflux pressure swing adsorption using silica gel for blue coal gas initial separation. Int. J. Hydrogen Energy 2021, 46, 683–696. [Google Scholar] [CrossRef]
- Sees, M.D.; Kirkes, T.; Chen, C.-C. A simple and practical process modeling methodology for pressure swing adsorption. Comput. Chem. Eng. 2021, 147, 107235. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Shen, Y.; Fu, Q.; Zhang, D.; Fu, B. Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR). Int. J. Hydrogen Energy 2018, 43, 19057–19074. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Fu, Q.; Zhang, D. CO enrichment from low-concentration syngas by a layered-bed VPSA process. Ind. Eng. Chem. Res. 2017, 56, 6741–6754. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, Y.; Li, D.; Fu, Q.; Zhang, D.; Na, P. Dual-reflux pressure swing adsorption process for carbon dioxide capture from dry flue gas. Int. J. Greenh. Gas Control 2017, 65, 55–64. [Google Scholar] [CrossRef]
- Feng, L.; Shen, Y.; Wu, T.; Liu, B.; Zhang, D.; Tang, Z. Adsorption equilibrium isotherms and thermodynamic analysis of CH4, CO2, CO, N2 and H2 on NaY Zeolite. Adsorption 2020, 26, 1101–1111. [Google Scholar] [CrossRef]
- Rosner, F.; Chen, Q.; Rao, A.; Samuelsen, S.; Jayaraman, A.; Alptekin, G. Thermo-economic analyses of IGCC power plants employing warm gas CO2 separation technology. Energy 2019, 185, 541–553. [Google Scholar] [CrossRef]
- Marcoberardino, G.D.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green hydrogen production from raw biogas: A techno-economic investigation of conventional processes using pressure swing adsorption unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Grande, C.A. Advances in pressure swing adsorption for gas separation. Int. Sch. Res. Not. 2012, 2012, 982934. [Google Scholar] [CrossRef] [Green Version]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Li, S.; Deng, S.; Zhao, L.; Zhao, R.; Lin, M.; Du, Y.; Lian, Y. Mathematical modeling and numerical investigation of carbon capture by adsorption: Literature review and case study. Appl. Energy 2018, 221, 437–449. [Google Scholar] [CrossRef]
- Xiao, Y.; Qiu, S.; Zhao, Q.; Zhu, Y.; Godiya, C.B.; He, G. Numerical simulation of low-concentration CO2 adsorption on fixed bed using finite element analysis. Chin. J. Chem. Eng. 2020, 36, 47–56. [Google Scholar] [CrossRef]
- Golubyatnikov, O.; Akulinin, E.; Dvoretsky, S.; Dvoretsky, D. To the problem of forming the equation system for pressure swing adsorption mathematical model. Chem. Prod. Process Model. 2021. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Y.; Shen, Y.; Sun, W.; Fu, Q.; Yan, H.; Zhang, D. Experiment and simulation for separating CO2/N2 by dual-reflux pressure swing adsorption process. Chem. Eng. J. 2016, 297, 315–324. [Google Scholar] [CrossRef]
- Xing, R.; Shi, W.; Shen, Y.; Liu, B.; Zhang, D. Vacuum pressure swing adsorption system for N2/CO2 separation in consideration of unstable feed concentration. Adsorption 2019, 25, 1147–1158. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, H.; Shen, Y.; Qin, Y.; Zhang, D.; Zhou, Y. Optimal design and control of pressure swing adsorption process for N2/CH4 separation. J. Clean. Prod. 2018, 170, 704–714. [Google Scholar] [CrossRef]
- Xu, M.; Wu, H.-C.; Lin, Y.S.; Deng, S. Simulation and optimization of pressure swing adsorption process for high-temperature air separation by perovskite sorbents. Chem. Eng. J. 2018, 354, 62–74. [Google Scholar] [CrossRef]
- Chen, Q.; Rosner, F.; Rao, A.; Samuelsen, S.; Jayaraman, A.; Alptekin, G. Simulation of elevated temperature solid sorbent CO2 capture for pre-combustion applications using computational fluid dynamics. Appl. Energy 2019, 237, 314–325. [Google Scholar] [CrossRef]
- Haghpanah, R.; Majumder, A.; Nilam, R.; Rajendran, A.; Farooq, S.; Karimi, I.A.; Amanullah, M. Multiobjective optimization of a four-step adsorption process for postcombustion CO2 capture via finite volume simulation. Ind. Eng. Chem. Res. 2013, 52, 4249–4265. [Google Scholar] [CrossRef]
- Anna, H.R.S.; Barreto, A.G., Jr.; Tavares, F.W.; de Souza, M.B., Jr. Machine learning model and optimization of a PSA unit for methane-nitrogen separation. Comput. Chem. Eng. 2017, 104, 377–391. [Google Scholar] [CrossRef]
- Rashki, M.; Azarkish, H.; Rostamian, M.; Bahrpeyma, A. Classification correction of polynomial response surface methods for accurate reliability estimation. Struct. Saf. 2019, 81, 101869. [Google Scholar] [CrossRef]
- Gaspar, B.; Teixeira, A.P.; Soares, C.G. Assessment of the efficiency of Kriging surrogate models for structural reliability analysis. Probabilistic Eng. Mech. 2014, 37, 24–34. [Google Scholar] [CrossRef]
- Zheng, X.G.; Yao, H.; Huang, Y. Orthogonal numerical simulation on multi-factor design for rapid pressure swing adsorption. Adsorption 2017, 23, 685–697. [Google Scholar] [CrossRef]
- Beck, J.; Friedrich, D.; Brandani, S.; Fraga, E.S. Multi-objective optimisation using surrogate models for the design of VPSA systems. Comput. Chem. Eng. 2015, 82, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Towne, A.; Schmidt, O.T.; Colonius, T. Spectral proper orthogonal decomposition and its relationship to dynamic mode decomposition and resolvent analysis. J. Fluid Mech. 2018, 847, 821–867. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lin, J.; Peng, Q.; Wang, D.; Yang, T.; Sorooshian, S.; Liu, X.; Zhuang, J. Modeling and simulating of reservoir operation using the artificial neural network, support vector regression, deep learning algorithm. J. Hydrol. 2018, 565, 720–736. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Ma, S.; Tong, L.; Xiao, J.; Bénard, P.; Chahine, R. Artificial neural network based optimization for hydrogen purification performance of pressure swing adsorption. Int. J. Hydrogen Energy 2019, 44, 5334–5344. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y.; Guan, Z.; Zhang, D.; Tang, Z.; Li, W. Multi-objective optimization of ANN-based PSA model for hydrogen purification from steam-methane reforming gas. Int. J. Hydrogen Energy 2021, 46, 11740–11755. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Fang, L.; Böwer, P.; Wark, M.; Bénard, P.; Chahine, R. Machine learning—Based optimization for hydrogen purification performance of layered bed pressure swing adsorption. Int. J. Energy Res. 2020, 44, 4475–4492. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, A.; Tao, W.; Ma, S.; Bénard, P.; Chahine, R. Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons. Energies 2021, 14, 2450. [Google Scholar] [CrossRef]
- Akulinin, E.; Golubyatnikov, O.; Dvoretsky, D.; Dvoretsky, S. Optimization and analysis of pressure swing adsorption process for oxygen production from air under uncertainty. Chem. Ind. Chem. Eng. Q. 2020, 26, 89–104. [Google Scholar] [CrossRef]
- Nogueira, I.B.R.; Martins, M.A.F.; Regufe, M.J.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M. Big data-based optimization of a pressure swing adsorption unit for syngas purification: On mapping uncertainties from a metaheuristic technique. Ind. Eng. Chem. Res. 2020, 59, 14037–14047. [Google Scholar] [CrossRef]
- Hao, Z.; Caspari, A.; Schweidtmann, A.M.; Vaupel, Y.; Lapkin, A.A.; Mhamdi, A. Efficient hybrid multiobjective optimization of pressure swing adsorption. Chem. Eng. J. 2021, 423, 130248. [Google Scholar] [CrossRef]
- Martins, M.A.F.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B.R. Artificial Intelligence-oriented economic non-linear model predictive control applied to a pressure swing adsorption unit: Syngas purification as a case study. Sep. Purif. Technol. 2021, 276, 119333. [Google Scholar] [CrossRef]
- Zhou, L.; Qu, Z.G.; Chen, L.; Tao, W.Q. Lattice Boltzmann simulation of gas-solid adsorption processes at pore scale level. J. Comput. Phys. 2015, 300, 800–813. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Fyrillas, M.M.; Park, J. Variable diffusivity homogeneous surface diffusion model and analysis of merits and fallacies of simplified adsorption kinetics equations. J. Hazard. Mater. 2019, 367, 224–245. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. An exponential kinetic model for adsorption at solid/solution interface. Chem. Eng. J. 2013, 215, 65–71. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, H.; Tsai, C.-J.; Yang, X.; Xing, Y.; Zhang, C.; Xiao, P.; Webley, P.A. A numerical modelling study of SO2 adsorption on activated carbons with new rate equations. Chem. Eng. J. 2018, 353, 858–866. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z.; Liu, H. Multiple-relaxation-time lattice Boltzmann simulation for flow, mass transfer, and adsorption in porous media. Phys. Rev. E 2017, 96, 13313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, A.; Talu, O. Role of pressure drop on rapid pressure swing adsorption performance. Ind. Eng. Chem. Res. 2017, 56, 5715–5723. [Google Scholar] [CrossRef]
- Baghapour, B.; Rouhani, M.; Sharafian, A.; Kalhori, S.B.; Bahrami, M. A pressure drop study for packed bed adsorption thermal energy storage. Appl. Therm. Eng. 2018, 138, 731–739. [Google Scholar] [CrossRef]
- Myers, T.G.; Font, F.; Hennessy, M.G. Mathematical modelling of carbon capture in a packed column by adsorption. Appl. Energy 2020, 278, 115565. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Ali Abd, A.; Roslee Othman, M.; Helwani, Z. Evaluation of thermal effects on carbon dioxide breakthrough curve for biogas upgrading using pressure swing adsorption. Energy Convers. Manag. 2021, 247, 114752. [Google Scholar] [CrossRef]
- Ebner, A.D.; Mehrotra, A.; Ritter, J.A. Graphical approach for complex PSA cycle scheduling. Adsorption 2009, 15, 406–421. [Google Scholar] [CrossRef]
- Ebner, A.D.; Mehrotra, A.; Ritter, J.A. Graphical unit block approach for complex PSA cycle scheduling of parallel interacting trains of columns and tanks. Adsorption 2015, 21, 229–241. [Google Scholar] [CrossRef]
- Mehrotra, A.; Ebner, A.D.; Ritter, J.A. Simplified graphical approach for complex PSA cycle scheduling. Adsorption 2011, 17, 337–345. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ho, J.G.S.; Ritter, J.A. Graphical approach for formulating pressure swing adsorption cycle schedules with unlimited equalization steps. Adsorption 2018, 24, 221–232. [Google Scholar] [CrossRef]
- Park, Y.; Kang, J.H.; Moon, D.K.; Jo, Y.S.; Lee, C.H. Parallel and series multi-bed pressure swing adsorption processes for H2 recovery from a lean hydrogen mixture. Chem. Eng. J. 2021, 408, 127299. [Google Scholar] [CrossRef]
- Liu, B.; Yu, X.; Shi, W.; Shen, Y.; Zhang, D.; Tang, Z. Two-stage VSA/PSA for capturing carbon dioxide (CO2) and producing hydrogen (H2) from steam-methane reforming gas. Int. J. Hydrogen Energy 2020, 45, 24870–24882. [Google Scholar] [CrossRef]
- Lu, B.; Shen, Y.; Tang, Z.; Zhang, D.; Chen, G. Vacuum pressure swing adsorption process for coalbed methane enrichment. Chin. J. Chem. Eng. 2021, 32, 264–280. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Manović, V. Production of negative-emission biomethane by twin double-bed pressure swing adsorption with tail gas sequestration. Chem. Eng. J. 2021, 408, 127312. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lin, P.-W.; Chen, W.-H.; Yen, F.-Y.; Yang, H.-S.; Chou, C.-T. Biogas Upgrading by Pressure Swing Adsorption with Design of Experiments. Processes 2021, 9, 1325. [Google Scholar] [CrossRef]
- Van Chinh, P.; Hieu, N.T.; Tien, V.D.; Nguyen, T.-Y.; Nguyen, H.N.; Anh, N.T.; Thom, D. Van Simulation and Experimental Study of a Single Fixed-Bed Model of Nitrogen Gas Generator Working by Pressure Swing Adsorption. Processes 2019, 7, 654. [Google Scholar] [CrossRef] [Green Version]
- Durán, I.; Rubiera, F.; Pevida, C. Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem. Eng. J. 2022, 428, 132564. [Google Scholar] [CrossRef]
- Subraveti, S.G.; Li, Z.; Prasad, V.; Rajendran, A. Machine learning-based multiobjective optimization of pressure swing adsorption. Ind. Eng. Chem. Res. 2019, 58, 20412–20422. [Google Scholar] [CrossRef]
- Rebello, C.M.; Martins, M.A.F.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B.R. A novel standpoint of Pressure Swing Adsorption processes multi-objective optimization: An approach based on feasible operation region mapping. Chem. Eng. Res. Des. 2022, 178, 590–601. [Google Scholar] [CrossRef]
- Capra, F.; Gazzani, M.; Joss, L.; Mazzotti, M.; Martelli, E. MO-MCS, a derivative-free algorithm for the multiobjective optimization of adsorption processes. Ind. Eng. Chem. Res. 2018, 57, 9977–9993. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, A.; Shao, J.; Chi, T. A knowledge-informed and pareto-based artificial bee colony optimization algorithm for multi-objective land-use allocation. ISPRS Int. J. Geo-Inf. 2018, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Khajuria, H.; Pistikopoulos, E.N. Optimization and control of pressure swing adsorption processes under uncertainty. AIChE J. 2013, 59, 120–131. [Google Scholar] [CrossRef]
- Nilchan, S.; Pantelides, C.C. On the optimisation of periodic adsorption processes. Adsorption 1998, 4, 113–147. [Google Scholar] [CrossRef]
- Tsay, C.; Pattison, R.C.; Baldea, M. A pseudo-transient optimization framework for periodic processes: Pressure swing adsorption and simulated moving bed chromatography. AIChE J. 2018, 64, 2982–2996. [Google Scholar] [CrossRef]
- Sun, W.; Shen, Y.; Zhang, D.; Yang, H.; Ma, H. A systematic simulation and proposed optimization of the pressure swing adsorption process for N2/CH4 separation under external disturbances. Ind. Eng. Chem. Res. 2015, 54, 7489–7501. [Google Scholar] [CrossRef]
- Ding, Z.; Han, Z.; Fu, Q.; Shen, Y.; Tian, C.; Zhang, D. Optimization and analysis of the VPSA process for industrial-scale oxygen production. Adsorption 2018, 24, 499–516. [Google Scholar] [CrossRef]
- Leperi, K.T.; Yancy-Caballero, D.; Snurr, R.Q.; You, F. 110th Anniversary: Surrogate Models Based on Artificial Neural Networks To Simulate and Optimize Pressure Swing Adsorption Cycles for CO2 Capture. Ind. Eng. Chem. Res. 2019, 58, 18241–18252. [Google Scholar] [CrossRef]
- Moustapha, M.; Bourinet, J.-M.; Guillaume, B.; Sudret, B. Comparative study of Kriging and support vector regression for structural engineering applications. ASCE-ASME J. Risk Uncertain. Eng. Syst. Part A Civ. Eng. 2018, 4, 4018005. [Google Scholar] [CrossRef] [Green Version]
- Cozad, A.; Sahinidis, N.V.; Miller, D.C. Learning surrogate models for simulation-based optimization. AIChE J. 2014, 60, 2211–2227. [Google Scholar] [CrossRef]
- Zhang, N.; Bénard, P.; Chahine, R.; Yang, T.; Xiao, J. Optimization of pressure swing adsorption for hydrogen purification based on Box-Behnken design method. Int. J. Hydrogen Energy 2021, 46, 5403–5417. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, W.; Zhang, D.; Na, P.; Fu, B. The removal and capture of CO2 from biogas by vacuum pressure swing process using silica gel. J. CO2 Util. 2018, 27, 259–271. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Ismagilova, E.; Aarts, G.; Coombs, C.; Crick, T.; Duan, Y.; Dwivedi, R.; Edwards, J.; Eirug, A.; et al. Artificial Intelligence (AI): Multidisciplinary perspectives on emerging challenges, opportunities, and agenda for research, practice and policy. Int. J. Inf. Manag. 2021, 57, 101994. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Xu, X.; Klotz, E.; Newman, S.T. Intelligent manufacturing in the context of industry 4.0: A review. Engineering 2017, 3, 616–630. [Google Scholar] [CrossRef]
- Gholami, A.; Bonakdari, H.; Ebtehaj, I.; Mohammadian, M.; Gharabaghi, B.; Khodashenas, S.R. Uncertainty analysis of intelligent model of hybrid genetic algorithm and particle swarm optimization with ANFIS to predict threshold bank profile shape based on digital laser approach sensing. Measurement 2018, 121, 294–303. [Google Scholar] [CrossRef]
- Luan, J.; Yao, Z.; Zhao, F.; Song, X. A novel method to solve supplier selection problem: Hybrid algorithm of genetic algorithm and ant colony optimization. Math. Comput. Simul. 2019, 156, 294–309. [Google Scholar] [CrossRef]
- Urich, M.D.; Vemula, R.R.; Kothare, M. V Multivariable model predictive control of a novel rapid pressure swing adsorption system. AIChE J. 2018, 64, 1234–1245. [Google Scholar] [CrossRef]
- Hui, P.; Ping, W.; Weihua, L. Model Based Fractional Order PID Controller Design and Simulation of Pressure Swing Adsorption. In Proceedings of the 2019 Chinese Control Conference (CCC), Guangzhou, China, 27–30 July 2019; pp. 2880–2883. [Google Scholar]
- Akulinin, E.I.; Ishin, A.A.; Skvortsov, S.A.; Dvoretsky, D.S.; Dvoretsky, S.I. Optimization of adsorption processes with cyclic variable pressure in gas mixture separation. Adv. Mater. Technol. 2017, 3, 51–60. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Xing, R.; Zhang, D.-H.; Shen, Y.-H.; Fu, Q.; Ding, Z.-Y.; Tian, C.-X. Vacuum pressure swing adsorption system for N2/CH4 separation under uncertainty. Chem. Eng. Res. Des. 2019, 142, 245–256. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; López López, G.; Alvarado Martínez, V.M.; de J. Sorcia Vázquez, F.; Brizuela Mendoza, J.A.; Martínez García, M. Parametric study and control of a pressure swing adsorption process to separate the water-ethanol mixture under disturbances. Sep. Purif. Technol. 2020, 236, 116214. [Google Scholar] [CrossRef]





| Step | Z = 0 | Z = L |
|---|---|---|
| Adsorption | ||
 | ||
| Equalization and co-current depressurization | ||
 | ||
| Blowdown | ||
 | ||
| Purge | ||
 | ||
| Equalization pressurizationand Pressurization | ||
 |
| Category | Equation |
|---|---|
| Component mass balance | |
| Total mass balance | |
| Gas and Solid phase | |
| Bed wall | |
| Momentum balance | |
| Mass transfer | |
| Langmuir isotherm | |
| Ideal gas equation |
| Category | Equation |
|---|---|
| Vacuum pump and compressor | |
| Buffer tank | |
| Valve | Unidirection: if inlet. P > outlet. P, then F = Cv(inlet. P − outlet. P); if inlet. P ≤ outlet. P, then F = 0 Bidirection: F = Cv(inlet. P > outlet. P) |
| Purity (%) | |
| Recovery (%) | |
| Energy consumption (MJ/kg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Shen, Y.; Tang, Z.; Li, W.; Zhang, D. A Review of Numerical Research on the Pressure Swing Adsorption Process. Processes 2022, 10, 812. https://doi.org/10.3390/pr10050812
Zhang R, Shen Y, Tang Z, Li W, Zhang D. A Review of Numerical Research on the Pressure Swing Adsorption Process. Processes. 2022; 10(5):812. https://doi.org/10.3390/pr10050812
Chicago/Turabian StyleZhang, Runye, Yuanhui Shen, Zhongli Tang, Wenbin Li, and Donghui Zhang. 2022. "A Review of Numerical Research on the Pressure Swing Adsorption Process" Processes 10, no. 5: 812. https://doi.org/10.3390/pr10050812
APA StyleZhang, R., Shen, Y., Tang, Z., Li, W., & Zhang, D. (2022). A Review of Numerical Research on the Pressure Swing Adsorption Process. Processes, 10(5), 812. https://doi.org/10.3390/pr10050812






