A New Biosurfactant/Bioemulsifier from Gordonia alkanivorans Strain 1B: Production and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism and Culture Conditions
2.3. Emulsification Assays
2.4. Purification Process
2.4.1. BS/BE Extraction/Concentration
2.4.2. Chemical and Biochemical Composition
2.4.3. Dialysis of the Extract
2.5. Critical Micelle Concentration (CMC) Determination
2.6. Oil Spreading Test
2.7. Effect of Temperature
3. Results and Discussion
3.1. Preliminary Screening
3.2. Alternative Low-Cost Carbon Sources
3.3. Cellular Emulsifying Activity
3.4. Partial Purification and BS/BE Characterization
3.4.1. Chemical and Biochemical Composition
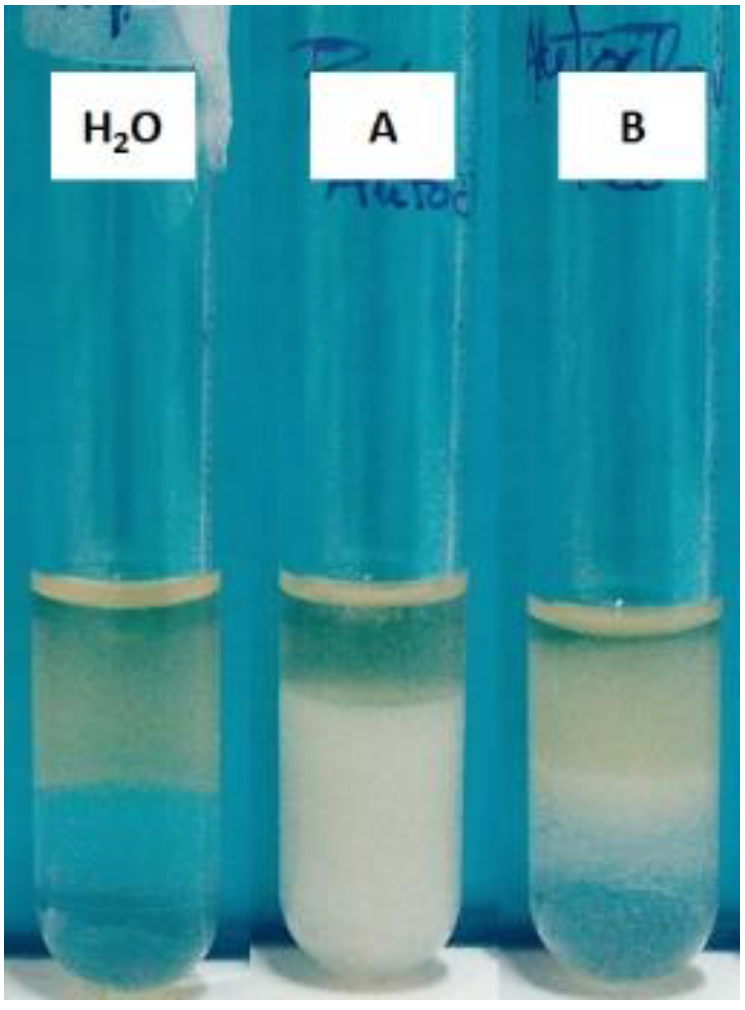
3.4.2. CMC Determination
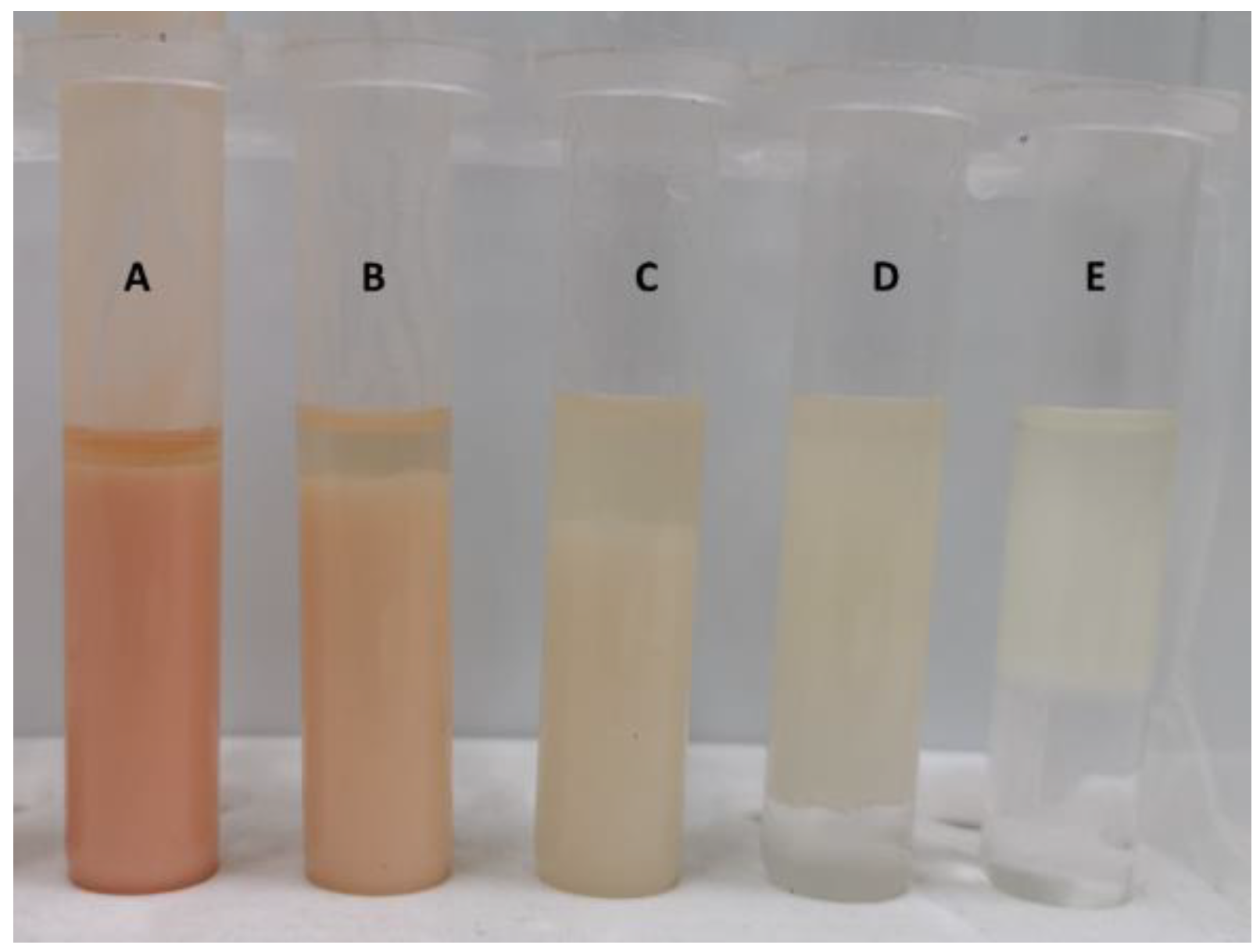
3.4.3. Dialysis and Further Characterization
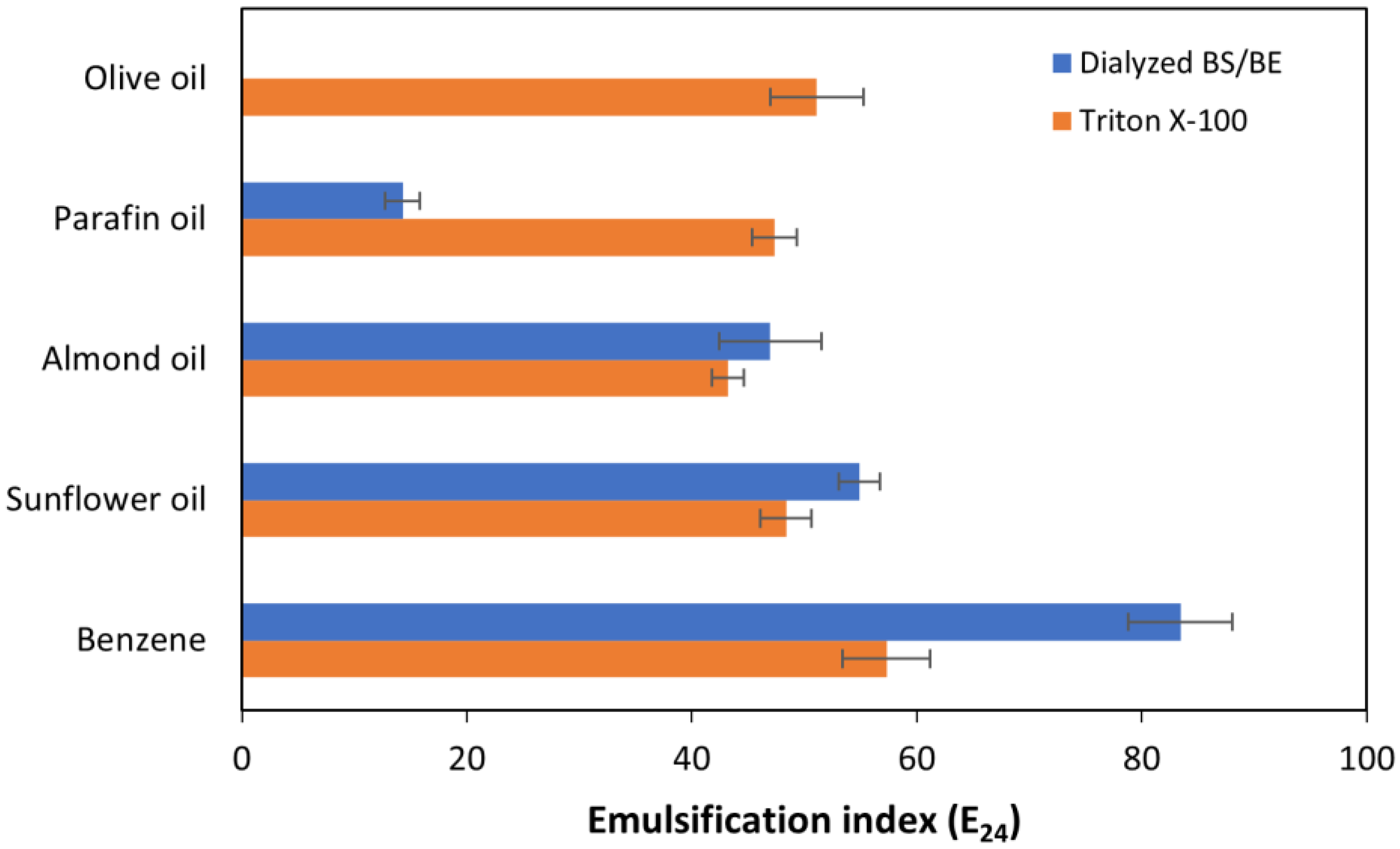
3.5. Effect of Temperature on BS/BE Properties
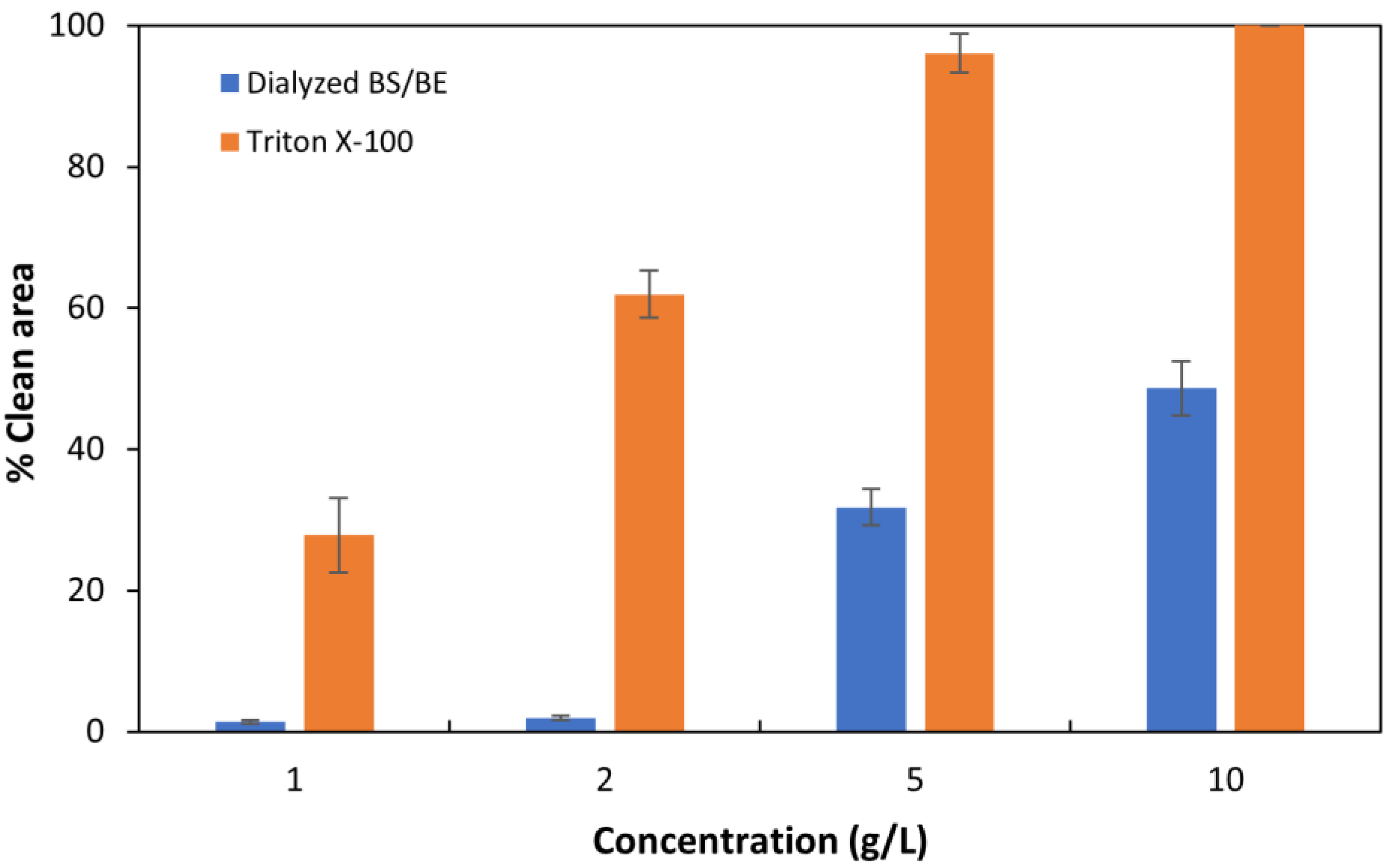
3.6. Oil Spreading Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Research and Markets Global Laundry Detergents Industry (2020 to 2027)—Key Market Trends and Drivers. Available online: https://www.prnewswire.com/news-releases/global-laundry-detergents-industry-2020-to-2027---key-market-trends-and-drivers-301289066.html (accessed on 3 November 2021).
- Fei, D.; Zhou, G.-W.; Yu, Z.-Q.; Gang, H.-Z.; Liu, J.-F.; Yang, S.-Z.; Ye, R.-Q.; Mu, B.-Z. Low-Toxic and Nonirritant Biosurfactant Surfactin and Its Performances in Detergent Formulations. J. Surfactants Deterg. 2020, 23, 109–118. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, Natural Alternatives to Synthetic Surfactants: Physicochemical Properties and Applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Ngueagni, P.T. A Review on New Aspects of Lipopeptide Biosurfactant: Types, Production, Properties and Its Application in the Bioremediation Process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Sajayan, A.; Priyadharshini, G.B.; Selvin, J.; Kiran, G.S. Revealing the Efficacy of Thermostable Biosurfactant in Heavy Metal Bioremediation and Surface Treatment in Vegetables. Front. Microbiol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Biosurfactant Production by Native Marine Bacteria (Acinetobacter Calcoaceticus P1-1A) Using Waste Carbon Sources: Impact of Process Conditions. Can. J. Chem. Eng. 2021, 99, 2386–2397. [Google Scholar] [CrossRef]
- Franzetti, A.; Caredda, P.; la Colla, P.; Pintus, M.; Tamburini, E.; Papacchini, M.; Bestetti, G. Cultural Factors Affecting Biosurfactant Production by Gordonia Sp. BS29. Int. Biodeterior. Biodegrad. 2009, 63, 943–947. [Google Scholar] [CrossRef]
- Nazareth, T.C.; Zanutto, C.P.; Maass, D.; Ulson De Souza, A.A.; Ulson De Souza, S.M.D.A.G. Impact of Oxygen Supply on Surfactin Biosynthesis Using Brewery Waste as Substrate. J. Environ. Chem. Eng. 2021, 9, 105372. [Google Scholar] [CrossRef]
- Mouafo, T.H.; Mbawala, A.; Ndjouenkeu, R. Effect of Different Carbon Sources on Biosurfactants’ Production by Three Strains of Lactobacillus spp. BioMed Res. Int. 2018, 2018, 5034783. [Google Scholar] [CrossRef]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. Harnessing the Catabolic Versatility of Gordonia Species for Detoxifying Pollutants. Biotechnol. Adv. 2019, 37, 382–402. [Google Scholar] [CrossRef]
- Jackisch-Matsuura, A.B.; Santos, L.S.; Eberlin, M.N.; de Faria, A.F.; Matsuura, T.; Grossman, M.J.; Durrant, L.R. Production and Characterization of Surface-Active Compounds from Gordonia Amicalis. Braz. Arch. Biol. Technol. 2014, 57, 138–144. [Google Scholar] [CrossRef]
- Arenskötter, M.; Bröker, D.; Steinbüchel, A. Biology of the Metabolically Diverse Genus Gordonia. Appl. Environ. Microbiol. 2004, 70, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, A.; Bestetti, G.; Caredda, P.; la Colla, P.; Tamburini, E. Surface-Active Compounds and Their Role in the Access to Hydrocarbons in Gordonia Strains. FEMS Microbiol. Ecol. 2008, 63, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Fusconi, R.; Maria Nascimento Assunção, R.; de Moura Guimarães, R.; Rodrigues Filho, G.; Eduardo da Hora Machado, A. Exopolysaccharide Produced by Gordonia Polyisoprenivorans CCT 7137 in GYM Commercial Medium and Sugarcane Molasses Alternative Medium: FT-IR Study and Emulsifying Activity. Carbohydr. Polym. 2010, 79, 403–408. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, G.; Sun, D.; Wu, T.; Li, Y. Enhanced Separation of Emulsified Oil from Alkaline-Surfactant-Polymer Flooding Produced Water by Gordonia Sp. TD-4: From Interactions to Mitigation Strategies and Bio-Demulsifying Mechanisms. J. Clean. Prod. 2022, 337, 130538. [Google Scholar] [CrossRef]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. Uptake and Detoxification of Diesel Oil by a Tropical Soil Actinomycete Gordonia Amicalis HS-11: Cellular Responses and Degradation Perspectives. Environ. Pollut. 2020, 263, 114538. [Google Scholar] [CrossRef]
- Qi, P.; Sun, D.; Gao, J.; Liu, S.; Wu, T.; Li, Y. Demulsification and Bio-Souring Control of Alkaline-Surfactant-Polymer Flooding Produced Water by Gordonia Sp. TD-4. Sep. Purif. Technol. 2021, 263, 118359. [Google Scholar] [CrossRef]
- Saeki, H.; Sasaki, M.; Komatsu, K.; Miura, A.; Matsuda, H. Oil Spill Remediation by Using the Remediation Agent JE1058BS That Contains a Biosurfactant Produced by Gordonia Sp. Strain JE-1058. Bioresour. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef]
- Laorrattanasak, S.; Rongsayamanont, W.; Khondee, N.; Paorach, N.; Soonglerdsongpha, S.; Pinyakong, O.; Luepromchai, E. Production and Application of Gordonia Westfalica GY40 Biosurfactant for Remediation of Fuel Oil Spill. Water Air Soil Pollut. 2016, 227, 325. [Google Scholar] [CrossRef]
- Alves, L.; Salgueiro, R.; Rodrigues, C.; Mesquita, E.; Matos, J.; Gírio, F.M. Desulfurization of Dibenzothiophene, Benzothiophene, and Other Thiophene Analogs by a Newly Isolated Bacterium, Gordonia Alkanivorans Strain 1B. Appl. Biochem. Biotechnol.-Part A Enzym. Eng. Biotechnol. 2005, 120, 199–208. [Google Scholar] [CrossRef]
- Alves, L.; Melo, M.; Mendonça, D.; Simões, F.; Matos, J.; Tenreiro, R.; Gírio, F.M. Sequencing, Cloning and Expression of the Dsz Genes Required for Dibenzothiophene Sulfone Desulfurization from Gordonia Alkanivorans Strain 1B. Enzym. Microb. Technol. 2007, 40, 1598–1603. [Google Scholar] [CrossRef]
- Alves, L.; Paixão, S.M. Toxicity Evaluation of 2-Hydroxybiphenyl and Other Compounds Involved in Studies of Fossil Fuels Biodesulphurisation. Bioresour. Technol. 2011, 102, 9162–9166. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Paixão, S.M. Fructophilic Behaviour of Gordonia Alkanivorans Strain 1B during Dibenzothiophene Desulfurization Process. New Biotechnol. 2014, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Paixão, S.M.; Pacheco, R.; Ferreira, A.F.; Silva, C.M. Biodesulphurization of Fossil Fuels: Energy, Emissions and Cost Analysis. RSC Adv. 2015, 5, 34047–34057. [Google Scholar] [CrossRef]
- Alves, L.; Paixão, S.M. Enhancement of Dibenzothiophene Desulfurization by Gordonia Alkanivorans Strain 1B Using Sugar Beet Molasses as Alternative Carbon Source. Appl. Biochem. Biotechnol. 2014, 172, 3297–3305. [Google Scholar] [CrossRef]
- Paixão, S.M.; Silva, T.P.; Arez, B.F.; Alves, L. Advances in the Reduction of the Costs Inherent to Fossil Fuels Biodesulfurization Towards Its Potential Industrial Application. In Nanocomposites for the Desulfurization of Fuels; Saleh, T., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 235–283. [Google Scholar]
- Silva, T.P.; Paixão, S.M.; Teixeira, A.V.; Roseiro, J.C.; Alves, L. Optimization of Low Sulfur Carob Pulp Liquor as Carbon Source for Fossil Fuels Biodesulfurization. J. Chem. Technol. Biotechnol. 2013, 88, 919–923. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Roseiro, J.C.; Alves, L. Jerusalem Artichoke as Low-Cost Fructose-Rich Feedstock for Fossil Fuels Desulphurization by a Fructophilic Bacterium. J. Appl. Microbiol. 2015, 118, 609–618. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. Ability of Gordonia Alkanivorans Strain 1B for High Added Value Carotenoids Production. RSC Adv. 2016, 6, 58055–58063. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Paixão, S.M.; Silva, T.P.; Roseiro, J.C.; Alves, L. Influence of Culture Conditions towards Optimal Carotenoid Production by Gordonia Alkanivorans Strain 1B. Bioprocess Biosyst. Eng. 2018, 41, 143–155. [Google Scholar] [CrossRef]
- Silva, T.P.; Alves, L.; Paixão, S.M. Effect of Dibenzothiophene and Its Alkylated Derivatives on Coupled Desulfurization and Carotenoid Production by Gordonia Alkanivorans Strain 1B. J. Environ. Manag. 2020, 270, 110825. [Google Scholar] [CrossRef]
- Dubey, K.V.; Charde, P.N.; Meshram, S.U.; Shendre, L.P.; Dubey, V.S.; Juwarkar, A.A. Surface-Active Potential of Biosurfactants Produced in Curd Whey by Pseudomonas Aeruginosa Strain-PP2 and Kocuria Turfanesis Strain-J at Extreme Environmental Conditions. Bioresour. Technol. 2012, 126, 368–374. [Google Scholar] [CrossRef]
- Squillaci, G.; Finamore, R.; Diana, P.; Restaino, O.F.; Schiraldi, C.; Arbucci, S.; Ionata, E.; la Cara, F.; Morana, A. Production and Properties of an Exopolysaccharide Synthesized by the Extreme Halophilic Archaeon Haloterrigena Turkmenica. Appl. Microbiol. Biotechnol. 2016, 100, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.S.; Coutinho, J.O.P.A.; Júnior, A.C.; Rosa, C.A.; Siqueira, E.P.; Santos, V.L. Characterization of New Biosurfactant Produced by Trichosporon Montevideense CLOA 72 Isolated from Dairy Industry Effluents. J. Basic Microbiol. 2009, 49, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, C.; Portier, E.; Corre, M.H.; Schlusselhuber, M.; Depayras, S.; Berjeaud, J.M.; Verdon, J. Highlighting the Potency of Biosurfactants Produced by Pseudomonas Strains as Anti-Legionella Agents. BioMed Res. Int. 2018, 2018, 8194368. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein. J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- Lewis, T.; Nichols, P.D.; McMeekin, T.A. Evaluation of Extraction Methods for Recovery of Fatty Acids from Lipid-Producing Microheterotrophs. J. Microbiol. Methods 2000, 43, 107–116. [Google Scholar] [CrossRef]
- Willumsen, P.A.; Karlson, U. Screening of Bacteria, Isolated from PAH-Contaminated Soils, for Production of Biosurfactants and Bioemulsifiers. Biodegradation 1996, 7, 415–423. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.; Kaytash, A.; Rasekh, B. Stabilization of Water/Gas Oil Emulsions by Desulfurizing Cells of Gordonia Alkanivorans RIPI90A. Microbiology 2007, 153, 1573–1581. [Google Scholar] [CrossRef][Green Version]
- Ivanova, A.E.; Borzenkov, I.A.; Sokolova, D.S. Catabolic Potential and Surfactant Activity of Halotolerant Hydrocarbon-Oxidizing Bacteria. Microbiology 2021, 90, 405–415. [Google Scholar] [CrossRef]
- Putri, M.; Hertadi, R. Effect of Glycerol as Carbon Source for Biosurfactant Production by Halophilic Bacteria Pseudomonas Stutzeri BK-AB12. Procedia Chem. 2015, 16, 321–327. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Gomes, U.V.R.; Silva, R.O.; Sarubbo, L.A.; Ribeiro, E. The Potential Application of Biosurfactant Produced by Pseudomonas Aeruginosa TGC01 Using Crude Glycerol on the Enzymatic Hydrolysis of Lignocellulosic Material. Biodegradation 2019, 30, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Liu, Y.; Wu, X. Biological Process of Alkane Degradation by Gordonia Sihwaniensis. ACS Omega 2022, 7, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Saranya, P.; Bhavani, P.; Swarnalatha, S.; Sekaran, G. Biosequestration of Chromium(III) in an Aqueous Solution Using Cationic and Anionic Biosurfactants Produced from Two Different Bacillus Sp.—A Comparative Study. RSC Adv. 2015, 5, 80596–80611. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; López-Prieto, A.; Lopez-Álvarez, M.; Pérez-Davila, S.; Serra, J.; González, P.; Cruz, J.M.; Moldes, A.B. Characterization and Cytotoxic Effect of Biosurfactants Obtained from Different Sources. ACS Omega 2020, 5, 31381–31390. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, A.; Caredda, P.; Ruggeri, C.; la Colla, P.; Tamburini, E.; Papacchini, M.; Bestetti, G. Potential Applications of Surface Active Compounds by Gordonia Sp. Strain BS29 in Soil Remediation Technologies. Chemosphere 2009, 75, 801–807. [Google Scholar] [CrossRef]
- Delegan, Y.; Sargsyan, A.; Hovhannisyan, N.; Babayan, B.; Petrikov, K.; Vainstein, M. Analysis of Genome Sequence and Trehalose Lipid Production Peculiarities of the Thermotolerant Gordonia Strain. J. Basic Microbiol. 2020, 60, 14–21. [Google Scholar] [CrossRef]
- Iwahori, K.; Tokutomi, T.; Miyata, N.; Fujita, M. Formation of Stable Foam by the Cells and Culture Supernatant of Gordonia (Nocardia) Amarae. J. Biosci. Bioeng. 2001, 92, 77–79. [Google Scholar] [CrossRef]
- Alvarez, V.M.; Guimarães, C.R.; Jurelevicius, D.; de Castilho, L.V.A.; de Sousa, J.S.; da Mota, F.F.; Freire, D.M.G.; Seldin, L. Microbial Enhanced Oil Recovery Potential of Surfactin-Producing Bacillus Subtilis AB2.0. Fuel 2020, 272, 117730. [Google Scholar] [CrossRef]
- Youssef, N.H.; Duncan, K.E.; Nagle, D.P.; Savage, K.N.; Knapp, R.M.; McInerney, M.J. Comparison of Methods to Detect Biosurfactant Production by Diverse Microorganisms. J. Microbiol. Methods 2004, 56, 339–347. [Google Scholar] [CrossRef]




| Carbon-Source | OD600 | Biomass (g/L) | pH | BS/BE Concentration in CE 2 (g/L) | E24 (S) 3 (%) | E24 (CEs) (%) |
|---|---|---|---|---|---|---|
| Olive oil | 10.2 | 7.15 | 5.05 | 8.66 | 26.9 | 57.1 |
| n-Hexadecane | 5.3 | 5.67 | 4.47 | 2.24 | 25.9 | 0 |
| Ethanol | 3.1 | 1.08 | 4.23 | 1.41 | 0 | 0 |
| Ammonium ferric citrate 1 | 0.8 | 0.86 | 8.26 | 3.39 | 0 | 65.4 |
| Mannose | 11.5 | 4.71 | 4.87 | 7.78 | 0 | 40.0 |
| Sucrose | 9.8 | 3.46 | 5.52 | 1.72 | 40.4 | 74.1 |
| YMB | 9.8 | 4.20 | 6.4 | 11.52 | 33.3 | 76.9 |
| Hydrophobic Layer | Emulsification Index (E24, %) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| n-Heptane | 70.0 | 64.0 | 60.9 | 0 | 0 |
| Olive oil | 52.8 | 43.8 | 45.0 | 40.0 | 38.6 |
| Frying oil | 62.5 | 66.7 | 56.3 | 36.4 | 9.4 |
| Gear oil | 54.8 | 59.4 | 13.8 | 43.5 | 56.1 |
| Ethyl acetate | 53.8 | 55.6 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.P.; Paixão, S.M.; Tavares, J.; Gil, C.V.; Torres, C.A.V.; Freitas, F.; Alves, L. A New Biosurfactant/Bioemulsifier from Gordonia alkanivorans Strain 1B: Production and Characterization. Processes 2022, 10, 845. https://doi.org/10.3390/pr10050845
Silva TP, Paixão SM, Tavares J, Gil CV, Torres CAV, Freitas F, Alves L. A New Biosurfactant/Bioemulsifier from Gordonia alkanivorans Strain 1B: Production and Characterization. Processes. 2022; 10(5):845. https://doi.org/10.3390/pr10050845
Chicago/Turabian StyleSilva, Tiago P., Susana M. Paixão, João Tavares, Cátia V. Gil, Cristiana A. V. Torres, Filomena Freitas, and Luís Alves. 2022. "A New Biosurfactant/Bioemulsifier from Gordonia alkanivorans Strain 1B: Production and Characterization" Processes 10, no. 5: 845. https://doi.org/10.3390/pr10050845
APA StyleSilva, T. P., Paixão, S. M., Tavares, J., Gil, C. V., Torres, C. A. V., Freitas, F., & Alves, L. (2022). A New Biosurfactant/Bioemulsifier from Gordonia alkanivorans Strain 1B: Production and Characterization. Processes, 10(5), 845. https://doi.org/10.3390/pr10050845










