Thermal-Induced Autolysis Enzymes Inactivation, Protein Degradation and Physical Properties of Sea Cucumber, Cucumaria frondosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Pre-Treatment
2.2. Thermal Treatments
2.3. Cooking Yield
2.4. Water Content and Water Holding Capability (WHC)
2.5. Protein Characteristics
2.5.1. Crude Protein Content
2.5.2. Trichloroacetic Acid (TCA)-Soluble Nitrogen
2.5.3. Electrophoresis SDS-PAGE
2.6. Texture Profile Analysis (TPA)
2.7. Relative Enzymes Activity
2.8. Statistical Analysis and PCA
3. Results
3.1. Effects of Thermal Treatments on Cooking Yield
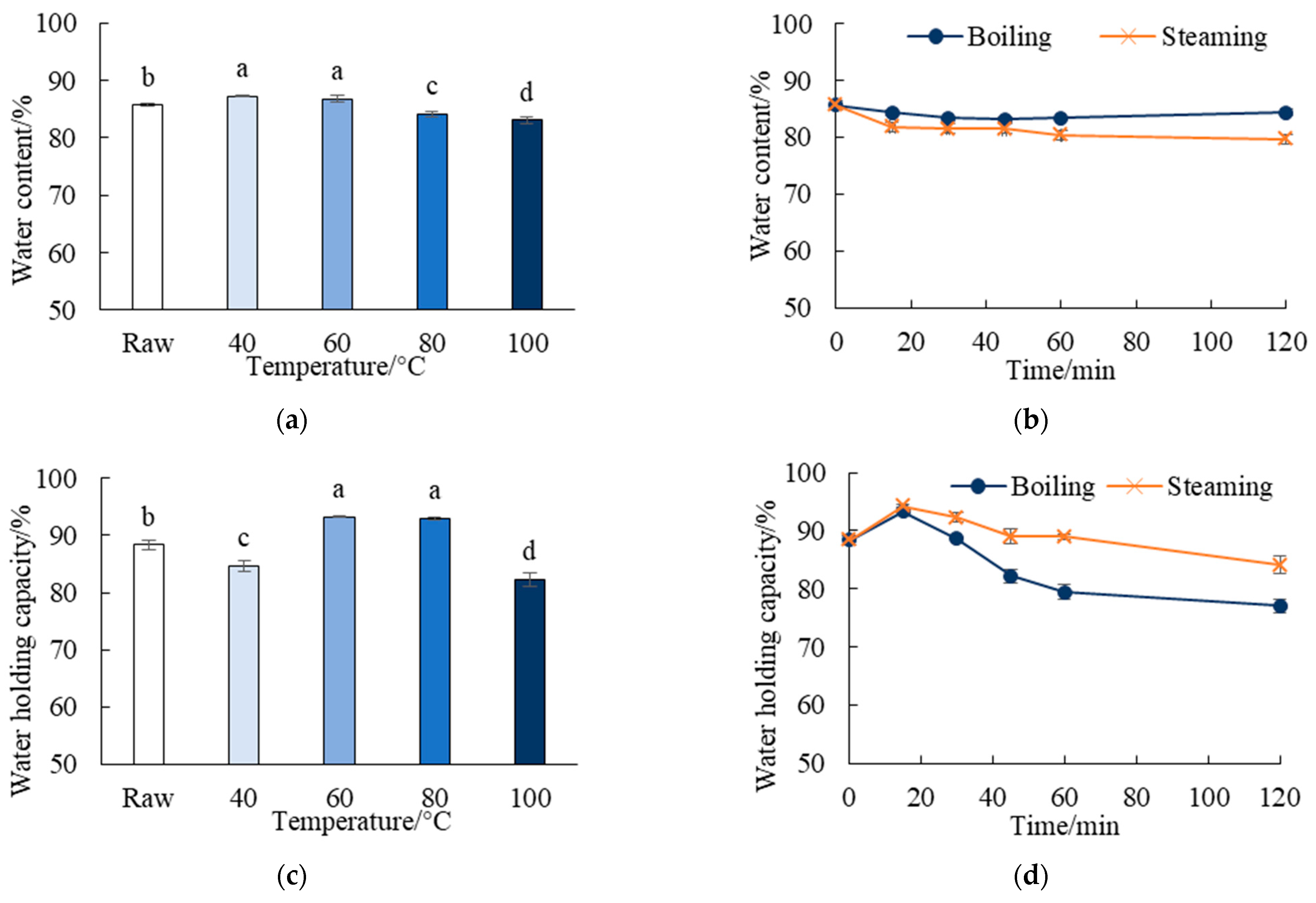
3.2. Effects of Thermal Treatments on the Water Content and WHC
3.3. Effects of Thermal Treatments on Protein Denaturation
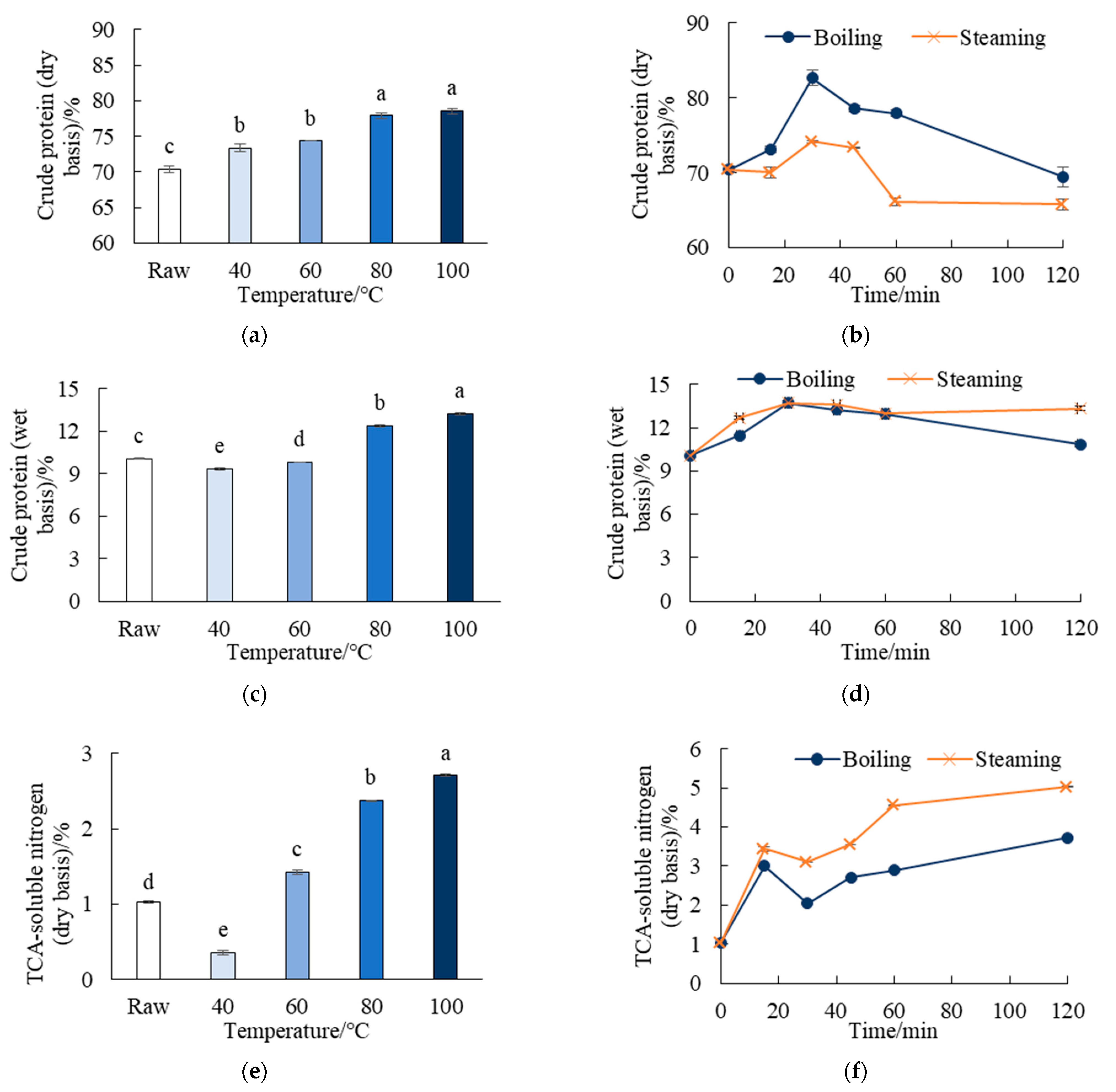
3.3.1. Crude Protein and TCA-Soluble Nitrogen
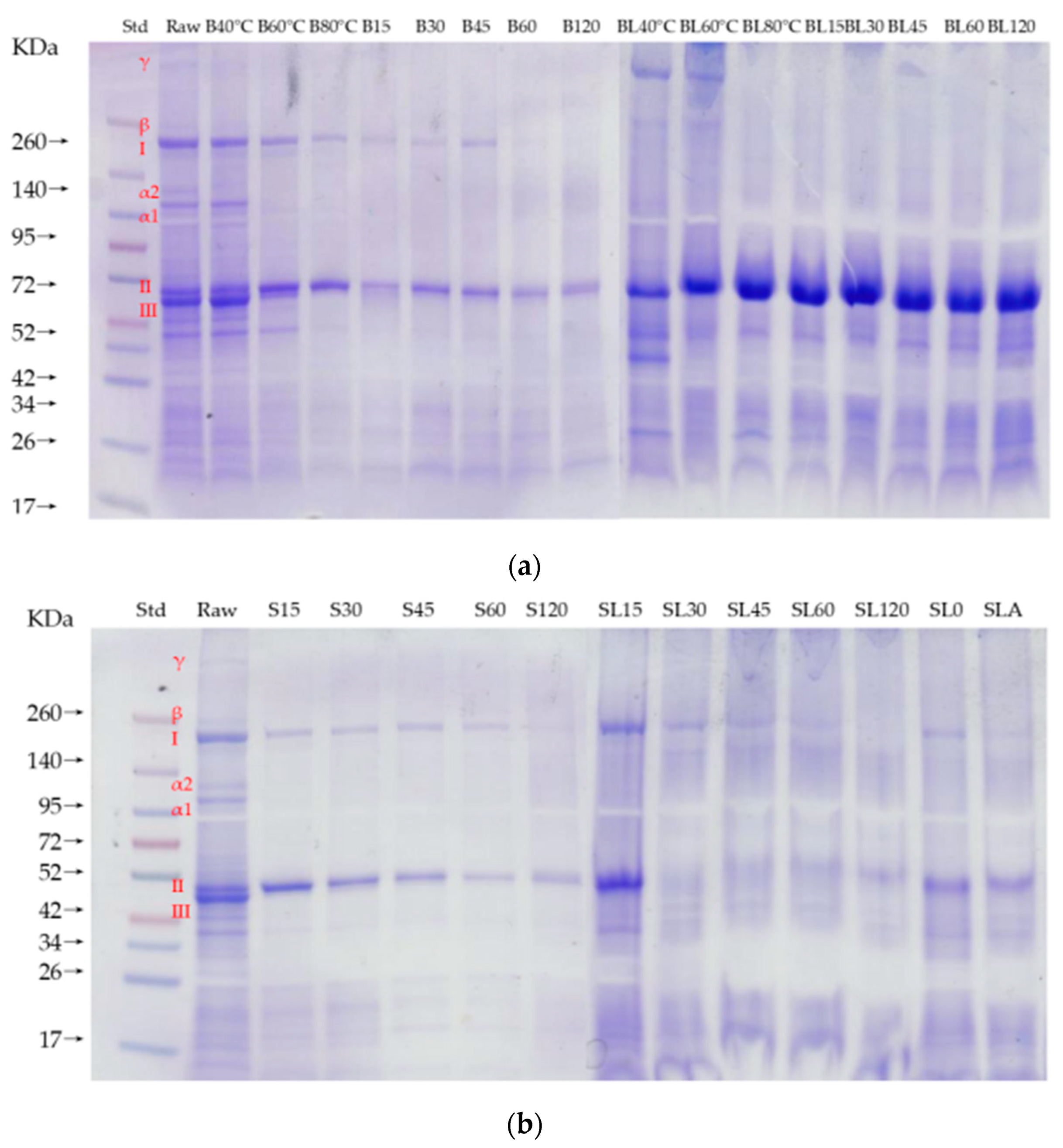
3.3.2. Protein Distribution Pattern
3.4. Effects of Thermal Treatments on Texture
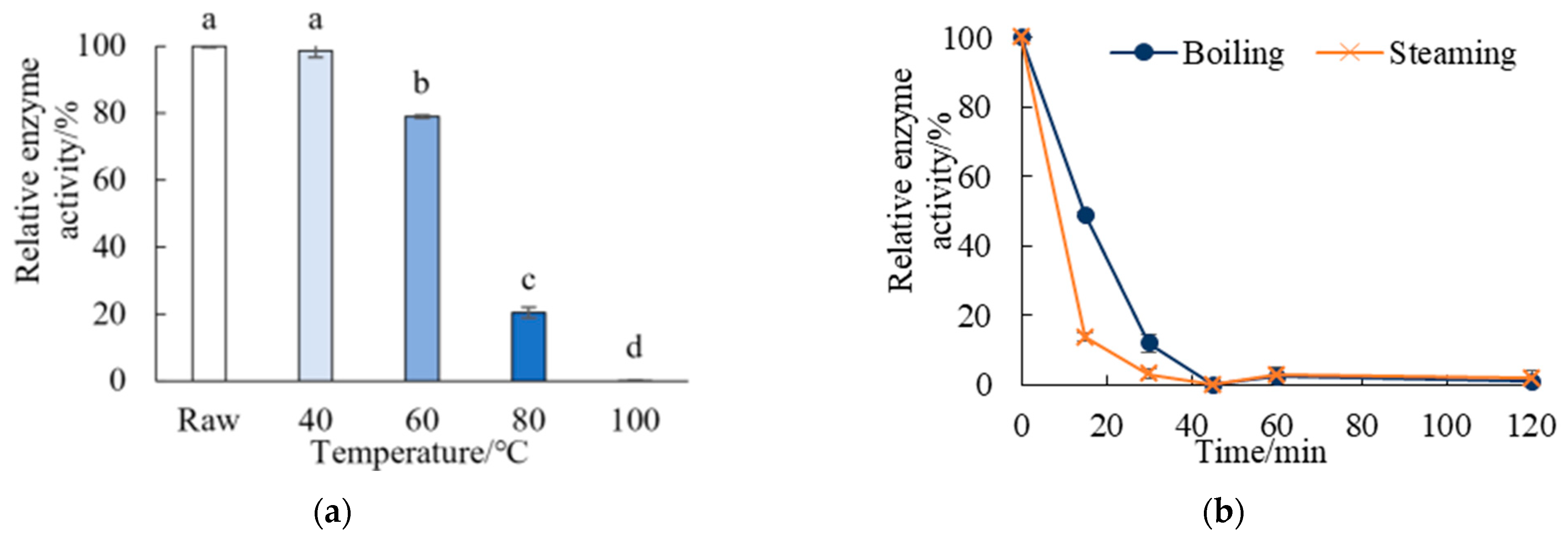
3.5. Thermal Inactivation Kinetics of Autolysis Enzymes
3.6. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Z.; Liu, L.; Liu, Q. Rheological changes of sea cucumber Stichipus japonicus during different heated times. Int. J. Fish. Aquac. 2011, 2, 258–262. [Google Scholar]
- Mizuta, S.; Koizumi, Y.; Yokoyama, Y.; Yoshinaka, R. Purification and immunochemical detection of a quantitatively major collagen in the dermis of sea cucumber Apostichopus japonicus. Chem. Biochem. 2022, 88, 173–180. [Google Scholar] [CrossRef]
- David, F.; Hubas, C.; Laguerre, H.; Badou, A.; Herault, G.; Bordelet, T.; Ameziane, N. Food sources, digestive efficiency and resource allocation in the sea cucumber Holothuria forskali (Echinodermata: Holothuroidea): Insights from pigments and fatty acids. Aquac. Nutr. 2019, 26, 1568–1583. [Google Scholar] [CrossRef]
- Montero, N.; Marras, B.; Nurchis, P.; Bettoschi, A.; Nurchis, P.; Coroneo, V.; Sanna, C.; Schintu, M. Trace metal levels in the edible tissues of sea cucumbers (Holothuria tubulosaand Holothuria polii) from Sardinia (Western Mediterranean). Ital. J. Food Saf. 2021, 10, 9576. [Google Scholar]
- Fisheries, B.O.; Agriculture, M.O. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 9, 7415–7420. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, E.M.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Song, Z.; Li, H.; Wen, J.; Zeng, Y.; Ye, X.; Zhao, W.; Xu, T.; Xu, N.; Zhang, D. Consumers’ attention on identification, nutritional compounds, and safety in heavy metals of Canadian sea cucumber in Chinese food market. Food Sci. Nutr. 2020, 8, 5962–5975. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Shi, D.; Wang, J.; Wang, Y.; Lou, Q.; Xue, C. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase-protein kinase B signal pathway. J. Biosci. Bioeng. 2014, 117, 457–463. [Google Scholar] [CrossRef]
- Nelson, E.J.; Macdonald, B.A.; Robinson, S.M.C. A Review of the Northern Sea Cucumber Cucumaria frondosa (Gunnerus, 1767) as a Potential Aquaculture Species. Rev. Fish. Sci. 2012, 20, 212–219. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, A.F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Dong, J. Study on Quality and Nutrient Composition of Sea Cucumber during Thermal Processing; Ocean University of China: Qingdao, China, 2015. [Google Scholar]
- Hernández-Sámano, A.C.; Guzmán-García, X.; García-Barrientos, R.; Ascencio-Valle, F.; Sierra-Beltrán, A.; Vallejo-Córdoba, B.; González-Córdova, A.F.; Torres-Llanez, M.J.; Guerrero-Legarreta, I. Extraction and characterization of Sea Cucumber Isostichopus fuscus Proteases, Collected at the Gulf of California, Mexico. Rev. Mex. Ing. Química. 2015, 14, 35–47. [Google Scholar]
- Zhu, B.; Han, B. Characterization and Purification of Holothurians Autoenzyme. Food Ferment. Ind. 2004, 30, 132–137. [Google Scholar]
- Zeng, S.; Zheng, M.; Chen, L.; Guo, Z.; Zheng, B. Inactivation Kinetics Model of Sea Cucunber Processed by High Pressure and the Storage Quality of Soft Canned Sea Cucumber. J. Chin. Inst. Food Sci. Technol. 2017, 17, 173–180. [Google Scholar]
- Chen, T.; Peng, Z.; Lu, J.; Li, B.; Hou, H. Self-degradation of Sea Cucumber body wall under 4C storage condition. J. Food Process. Preserv. 2015, 40, 715–723. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, Y.; Song, L.; Cheng, S.; Li, D.; Zhu, B.; Zhou, D.; Tan, M. Combination of NMR and MRI Techniques for Non-invasive Assessment of Sea Cucumber (Stichopus japonicas) Tenderization During Low-Temperature Heating Process. Food Anal. Methods 2017, 10, 2207–2216. [Google Scholar] [CrossRef]
- Stefaniak-Vidarsson, M.M.; Varsha AKaleb, M.G.; Marteinsdottir, G.; Fridjonsson, O.; Hreggvidsson, G.O.; Sigurjonsson, O.E.; Omarsdottir, S.; Kristbergsson, K. Bioactive effect of sulphated polysaccharides derived from orange-footed sea cucumber (Cucumaria frondosa) toward THP-1 macrophage. Bioact. Carbohydr. Diet. Fibre 2017, 12, 14–19. [Google Scholar] [CrossRef]
- Duan, X.; Wang, H.; Ren, G.; Zhu, K. Research progress of dry-cure technology of sea cucumber. Sci. Technol. Food Ind. 2012, 33, 427–431. [Google Scholar]
- Zhang, L.; Huang, X.; Miao, S.; Zeng, S.; Zhang, Y.; Zheng, B. Influence of ultrasound on the rehydration of dried sea cucumber (Stichopus japonicus). J. Food Eng. 2016, 178, 203–211. [Google Scholar] [CrossRef]
- Qi, H.; Fu, H.; Dong, X.; Feng, D.; Li, N.; Wen, C.; Nakamura, Y.; Zhu, B. Apoptosis induction is involved in UVA-induced autolysis in sea cucumber Stichopus japonicus. J. Photochem. Photobiol. B Biol. 2016, 158, 130–135. [Google Scholar] [CrossRef]
- Bi, J.; Li, Y.; Cheng, S.; Dong, X.; Kamal, T.; Zhou, D.; Li, D.; Jiang, P.; Zhu, B.-W.; Tan, M. Changes in Body Wall of Sea Cucumber during a two-step heating process assessed by Rheology, LF-NMR, and Texture Profile Analysis. Food Biophys. 2016, 11, 257–265. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Z.; Deng, J.; Liu, X.; Li, N.; Chen, S.; Li, Z. Effects of High Hydrostatic Pressure Treatment on Holothurians Autoenzyme Activity. Chin. J. High Press. Phys. 2009, 23, 377–383. [Google Scholar]
- Crapo, C.A.; Crawford, D.L. Influence of Polyphosphate Soak and Cooking Procedures on Yield and Quality of Dungeness Crab Meat. J. Food Sci. 1991, 56, 657–659. [Google Scholar] [CrossRef]
- Heerden, S.M.V.; Strydom, P.E. Nutrient retention values and cooking yield factors for three South African lamb and mutton cuts. J. Sci. Food Agric. 2017, 97, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.N.; Pinali, C.; Young, R.D.; Meek, K.M.; Quantock, A.J.; Knupp, C. Structural Interactions between Collagen and Proteoglycans Are Elucidated by Three-Dimensional Electron Tomography of Bovine Cornea. Structure 2010, 18, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D. Study on Physical Properties and Processing Technology of Sea Cucumber; Ocean University of China: Qingdao, China, 2006. [Google Scholar]
- Parkin, K.; Damodaran, S. Amino Acids, Peptides, and Proteins; CRC Press: New York, NY, USA, 2017; pp. 272–273. [Google Scholar]
- Zhao, Y.; Xue, Y.; Dong, J.; Li, Z.; Wang, Y.; Xue, C. Effect of Two Plant Extracts on the Stability of the Body Wall Collagen in Instant Sea Cucumber. Mod. Food Sci. Technol. 2015, 31, 113–119. [Google Scholar]
- Wu, F.; Xue, Y.; Liu, X.; Xue, C.; Wang, J.; Du, L.; Koretaro, T.; Wang, Y. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014, 64, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Junpeng, Z.; Wei, Z.; Hong, Y.; Lan, W. Effects of Extraction Methods on the Composition and Functional Properties of Fish Proteins. Mod. Agric. Equip. 2019, 40, 64–71. [Google Scholar]
- Hou, H.; Chen, T.; Peng, Z.; Zhang, Z.; Xue, C.; Li, B. Stability and degradation regulation of body wall gel of sea cucumber treated by high hydrostatic pressure. Trans. Chin. Soc. Agric. Eng. 2014, 30, 316–322. [Google Scholar]
- Cui, F.; Xue, C.; Li, Z.; Zhang, Y.; Dong, P.; Fu, X.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Saito, M.; Kunisaki, N.; Urano, N.; KIMURA, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Liu, Z.; Oliveira, A.C.M.; Su, Y. Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea Cucumber Stichopus monotuberculatus. J. Food Sci. 2015, 80, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.A.; Lyons-Levy, G.; Thurmond, F.A.; Koobt, T.J. Covalent composition of collagen fibrils from the dermis of the sea cucumber, Cucumaria frondosa, a tissue with mutable mechanical properties. Camp. Biochem. Physiol. 1995, 112A, 463–478. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Garrone, R.; Exposito, J.-Y. Sea urchin fibrillar collagen 2α chain participates in heterotrimeric molecules of (1α)22α stoichiometry. Matrix Biol. 2000, 19, 545–547. [Google Scholar] [CrossRef]
- Morales, J.; Montero, P.; Moral, A. Isolation and partial characterization of two types of muscle collagen in some cephalopods. J. Agric. Food Chem. 2000, 48, 2142–2148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zheng, J.; Tian, X.; Tang, Y.; Ding, G.; Yang, Z.; Jin, H. Pepsin-soluble collagen from the skin of Lophius litulo a preliminary study evaluating physicochemical, antioxidant, and wound healing properties. Mar. Drugs 2019, 17, 708. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (Apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272. [Google Scholar] [CrossRef]
- Chen, F.-C.; Hsieh, Y.-H.P. Porcine troponin I: A thermostable species marker protei. Meat Sci. 2002, 61, 55–60. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 106, 105779. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K-PKB pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, D.; Liu, Y.; Yu, M.; Liu, B.; Song, L.; Dong, X.; Qi, H.; Shahidi, F. Inhibitory effect of natural metal ion chelators on the autolysis of sea cucumber (Stichopus japonicus) and its mechanism. Food Res. Int. 2020, 133, 109205. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Q.; Yang, H. Seasonal biochemical changes in composition of body wall tissues of sea cucumber Apostichopus japonicus. Chin. J. Oceanol. Limnol. 2011, 29, 252–260. [Google Scholar] [CrossRef]
- Zhang, K.; Hou, H.; Bu, L.; Li, B.; Xue, C.; Peng, Z.; Su, S. Effects of heat treatment on the gel properties of the body wall of sea cucumber (Apostichopus japonicus). J. Food Sci. Technol. 2017, 54, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Almeida, G.M.D.; Mendonça, C.M.N.O.; Converti, A.; Oliveira, R.P.d.S. Kinetic and thermodynamic parameters of nisin thermoinactivation. J. Food Eng. 2020, 280, 109986. [Google Scholar]



| Group Abbreviation | Group Description | Hardness/g | Adhesiveness/g.s | Springiness | Cohesiveness | Chewiness/g | Resilience |
|---|---|---|---|---|---|---|---|
| Raw | Raw | 8895.19 ± 501.37 b | −331.97 ± 10.97 d | 0.73 ± 0.05 d | 0.33 ± 0.03 e | 1679.57 ± 295.47 g | 0.13 ± 0.02 g |
| B40 °C | Blanch 40 °C 45 min | 12,532.65 ± 288.70 a | −668.44 ± 37.01 e | 0.54 ± 0.05 e | 0.28 ± 0.04 f | 2020.45 ± 249.92 f g | 0.15 ± 0.03 g |
| B60 °C | Blanch 60 °C 45 min | 7416.39 ± 612.14 c | −143.01 ± 68.51 c | 0.80 ± 0.02 c | 0.67 ± 0.04 d | 4453.01 ± 576.48 a | 0.36 ± 0.01 f |
| B80 °C | Blanch 80 °C 45 min | 5955.04 ± 423.38 d | −30.88 ± 7.38 a | 0.86 ± 0.02 a, b | 0.78 ± 0.01 b c | 3995.48 ± 930.18 a, b | 0.44 ± 0.01 e |
| B15 | Boil 100 °C 15 min | 6504.57 ± 405.68 d | −41.72 ± 14.55 a, b | 0.84 ± 0.01 b c | 0.81 ± 0.01 a, b | 4176.96 ± 300.27 a | 0.47 ± 0.01 d |
| B30 | Boil 100 °C 30 min | 6394.92 ± 283.05 d | −27.89 ± 10.46 a | 0.89 ± 0.02 a, b | 0.82 ± 0.02 a | 4030.32 ± 665.58 a, b | 0.50 ± 0.02 b, c |
| B45 | Boil 100 °C 45 min | 4958.39 ± 627.12 e, f | −28.77 ± 12.12 a | 0.90 ± 0.03 a | 0.83 ± 0.01 a | 3301.81 ± 465.00 c | 0.51 ± 0.01 b |
| B60 | Boil 100 °C 60 min | 4542.24 ± 426.70 f | −37.07 ± 6.38 a | 0.89 ± 0.01 a, b | 0.82 ± 0.01 a | 3024.04 ± 608.18 c, d | 0.53 ± 0.01 a, b |
| B120 | Boil 100 °C 120 min | 3693.17 ± 558.31 g | −30.23 ± 8.47 a | 0.84 ± 0.06 b, c | 0.81 ± 0.03 a, b | 2788.71 ± 566.11 c, d, e | 0.55 ± 0.01 a |
| S15 | Steam 100 °C 15 min | 5162.88 ± 199.91 e | −72.37 ± 8.58 b | 0.86 ± 0.03 a, b | 0.77 ± 0.02 c | 3415.58 ± 242.77 b, c | 0.43 ± 0.01 e |
| S30 | Steam 100 °C 30 min | 4739.95 ± 450.27 e, f | −51.52 ± 12.91 a, b | 0.89 ± 0.03 a b | 0.81 ± 0.03 a b | 3398.32 ± 114.26 b, c | 0.48 ± 0.04 c, d |
| S45 | Steam 100 °C 45 min | 3459.53 ± 430.16 g, h | −44.11 ± 5.17 a b | 0.88 ± 0.06 a, b | 0.81 ± 0.03 a, b | 2654.81 ± 254.46 d, e, f | 0.47 ± 0.03 d |
| S60 | Steam 100 °C 60 min | 3443.25 ± 215.68 g, h | −46.29 ± 11.27 a b | 0.88 ± 0.04 a, b | 0.81 ± 0.01 a | 2514.34 ± 262.32 d, e, f | 0.48 ± 0.03 c d |
| S120 | Steam 100 °C 120 min | 3090.11 ± 256.76 h | −20.27 ± 3.43 a | 0.89 ± 0.02 a, b | 0.82 ± 0.01 a | 2260.60 ± 238.41 e, f, g | 0.52 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Liu, R.; Geirsdóttir, M.; Li, S.; Tomasson, T.; Xiong, S.; Li, X.; Gudjónsdóttir, M. Thermal-Induced Autolysis Enzymes Inactivation, Protein Degradation and Physical Properties of Sea Cucumber, Cucumaria frondosa. Processes 2022, 10, 847. https://doi.org/10.3390/pr10050847
Zhang Q, Liu R, Geirsdóttir M, Li S, Tomasson T, Xiong S, Li X, Gudjónsdóttir M. Thermal-Induced Autolysis Enzymes Inactivation, Protein Degradation and Physical Properties of Sea Cucumber, Cucumaria frondosa. Processes. 2022; 10(5):847. https://doi.org/10.3390/pr10050847
Chicago/Turabian StyleZhang, Qian, Ru Liu, Margrét Geirsdóttir, Shiyu Li, Tumi Tomasson, Shanbai Xiong, Xiuchen Li, and María Gudjónsdóttir. 2022. "Thermal-Induced Autolysis Enzymes Inactivation, Protein Degradation and Physical Properties of Sea Cucumber, Cucumaria frondosa" Processes 10, no. 5: 847. https://doi.org/10.3390/pr10050847
APA StyleZhang, Q., Liu, R., Geirsdóttir, M., Li, S., Tomasson, T., Xiong, S., Li, X., & Gudjónsdóttir, M. (2022). Thermal-Induced Autolysis Enzymes Inactivation, Protein Degradation and Physical Properties of Sea Cucumber, Cucumaria frondosa. Processes, 10(5), 847. https://doi.org/10.3390/pr10050847






