Leaching Kinetics of Aluminum from Alkali-Fused Spent Cathode Carbon Using Hydrochloric Acid and Sodium Fluoride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure
2.3. Characterization
3. Results and Discussion
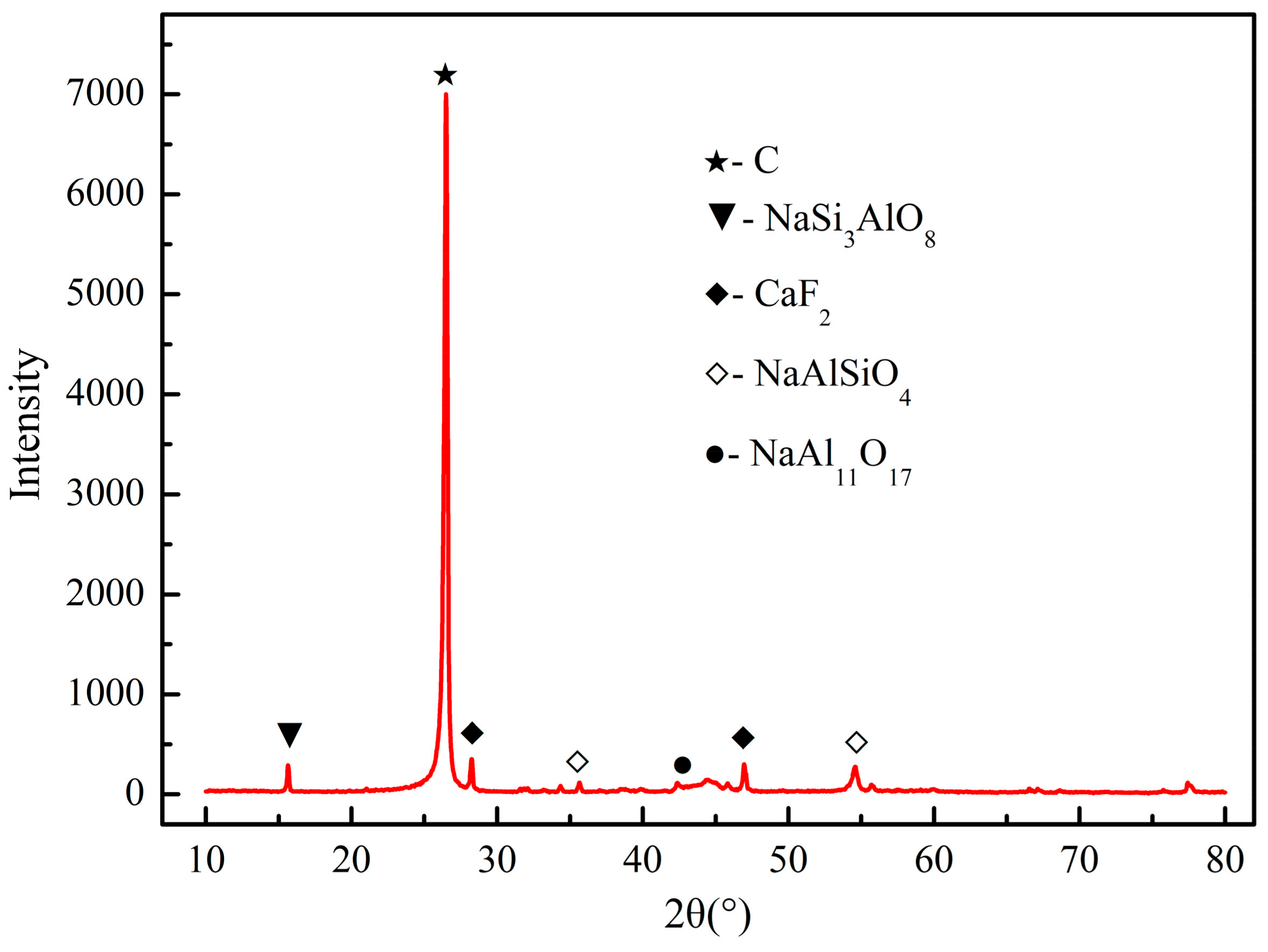
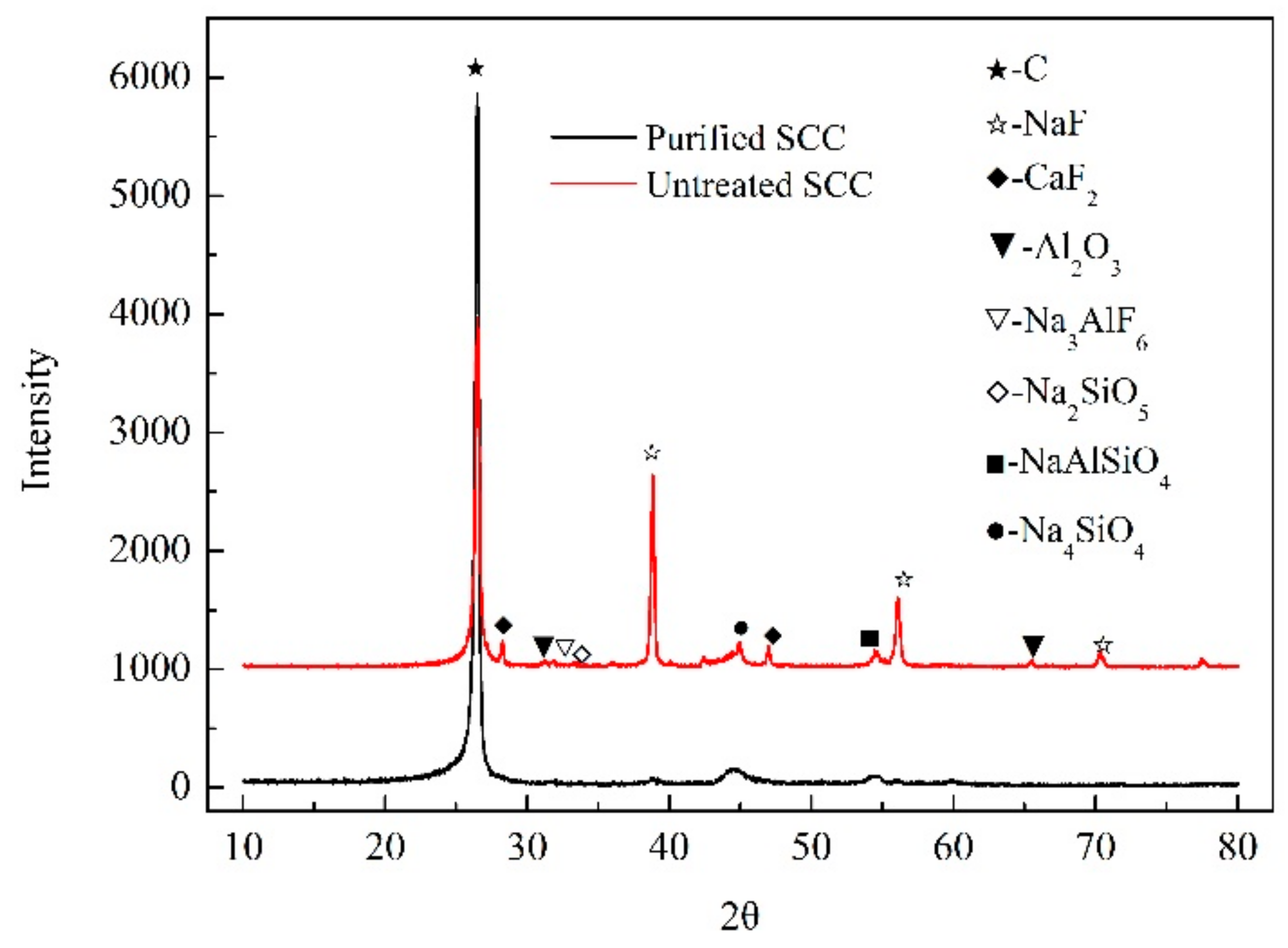
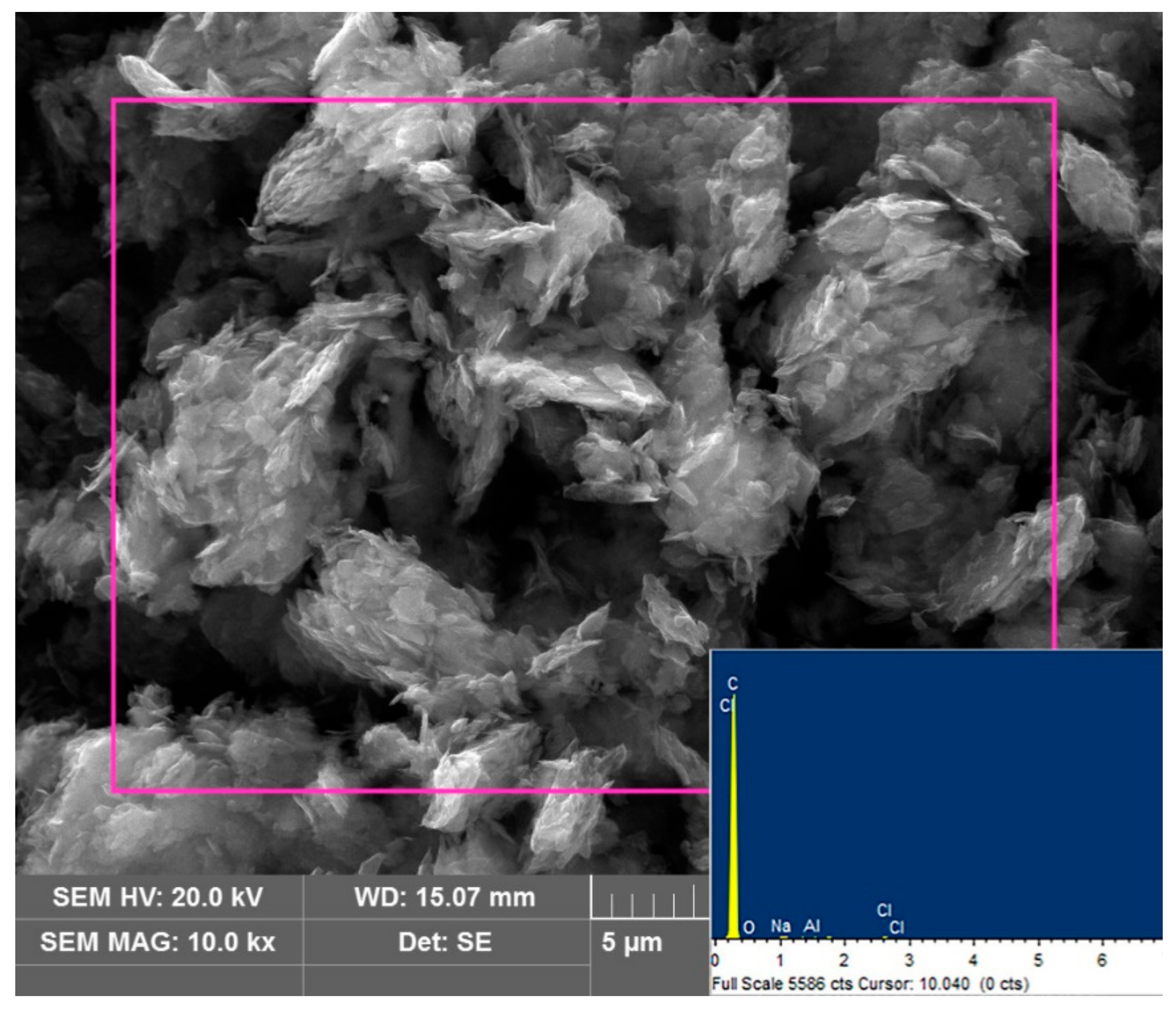
3.1. Characterization and Analysis
3.2. Thermodynamic Calculations
3.3. Effect of Experimental Factors on Leaching Efficiency
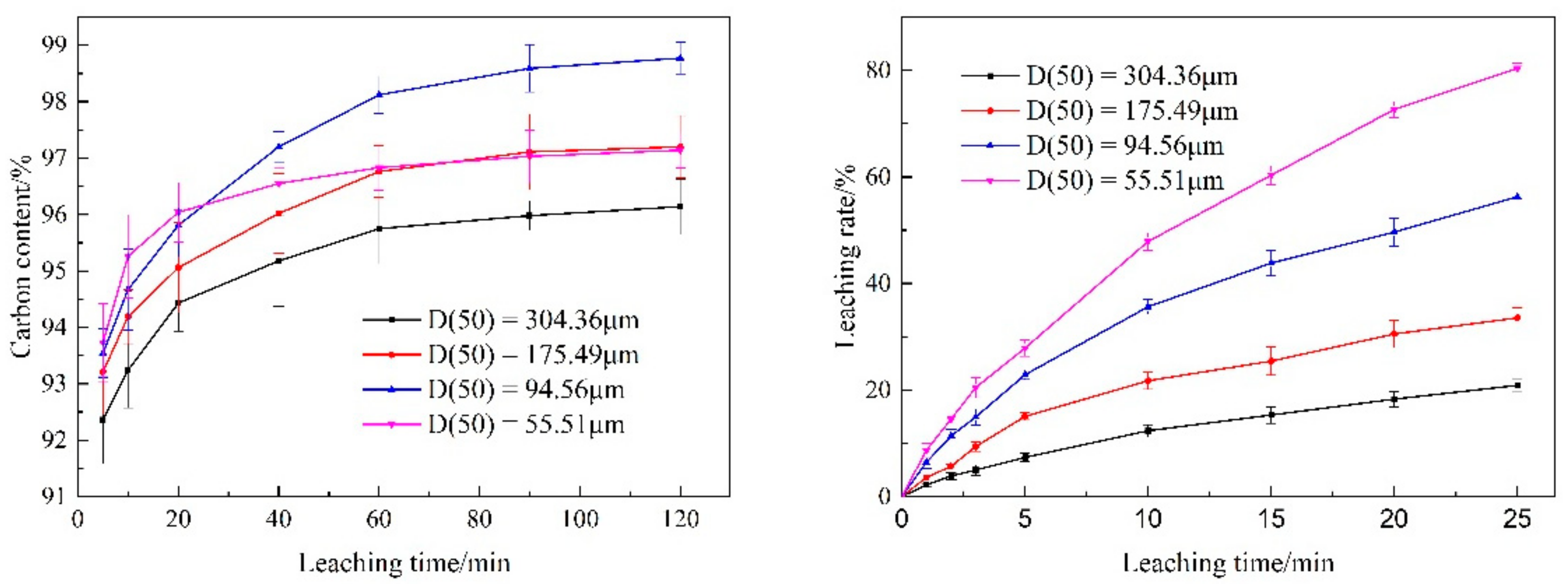
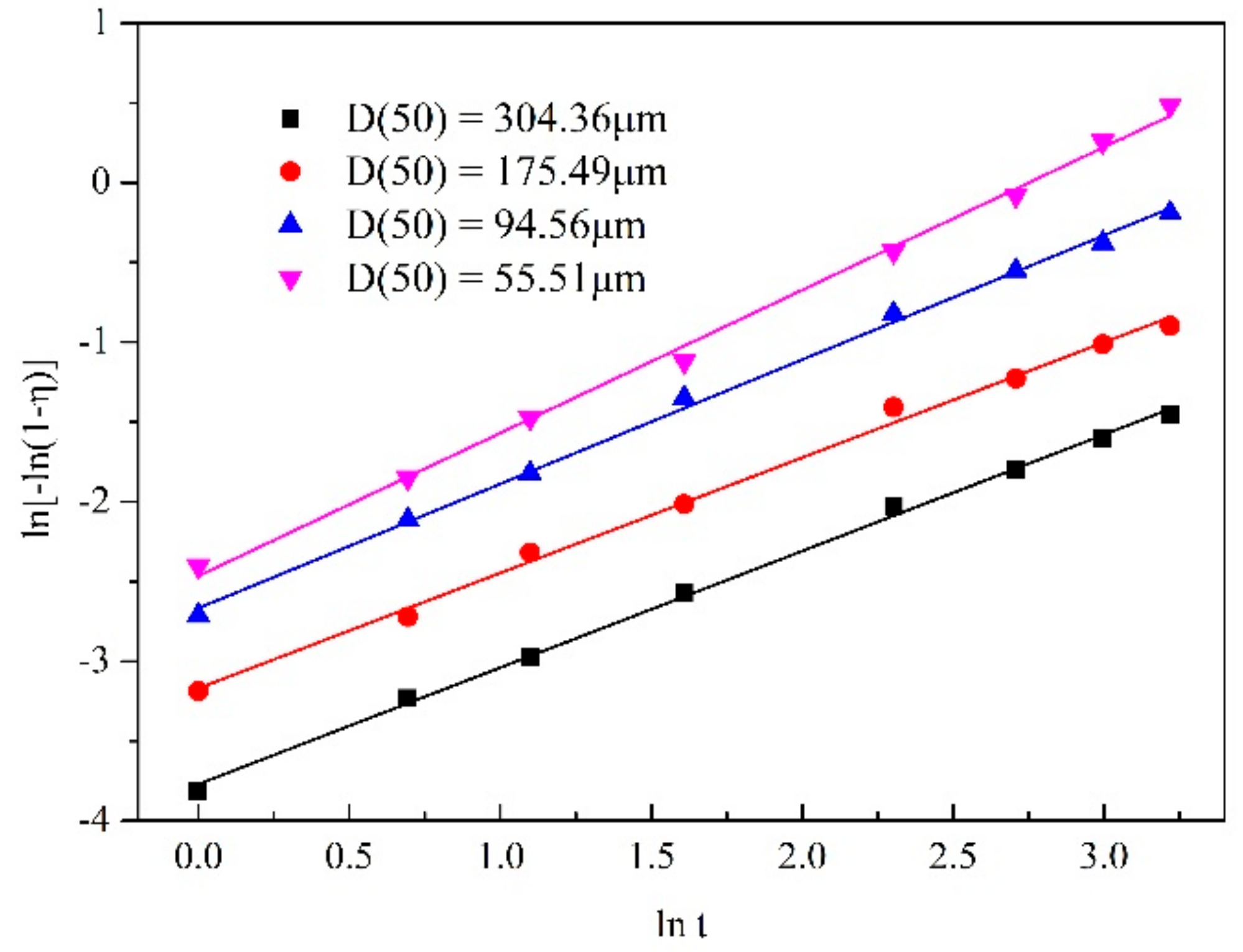
3.3.1. Particle Size
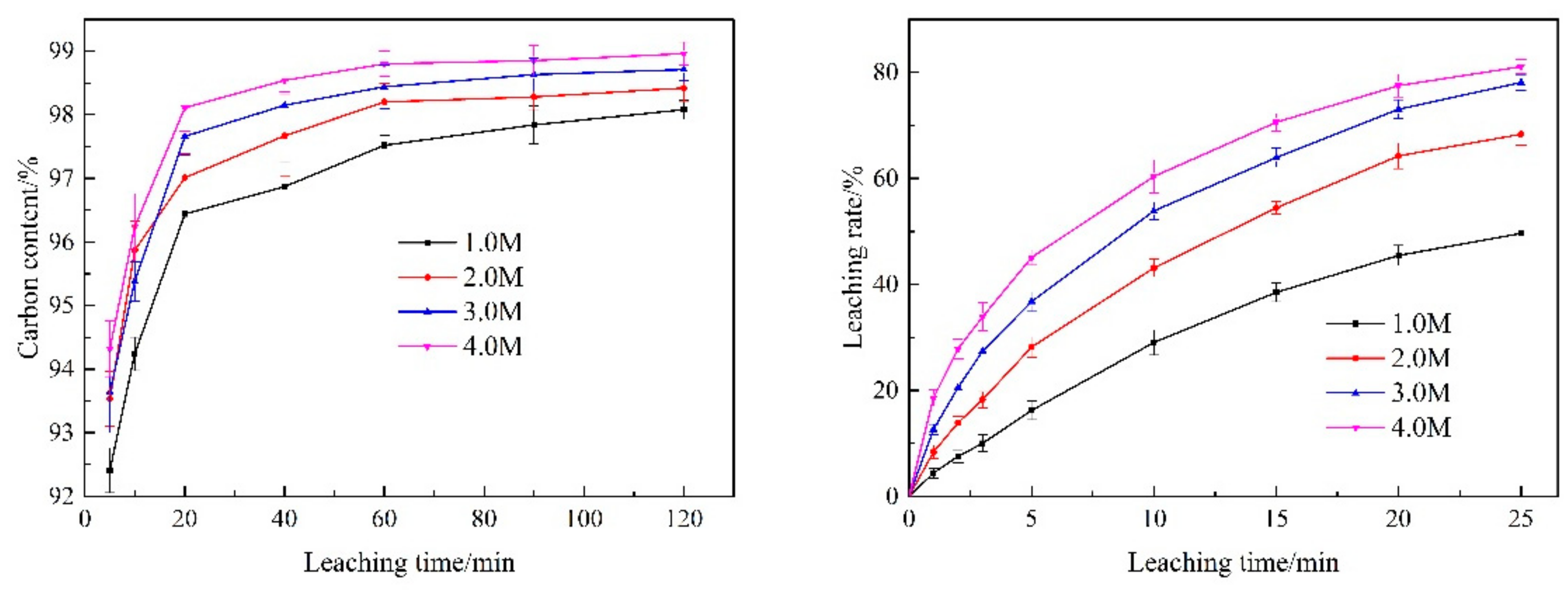
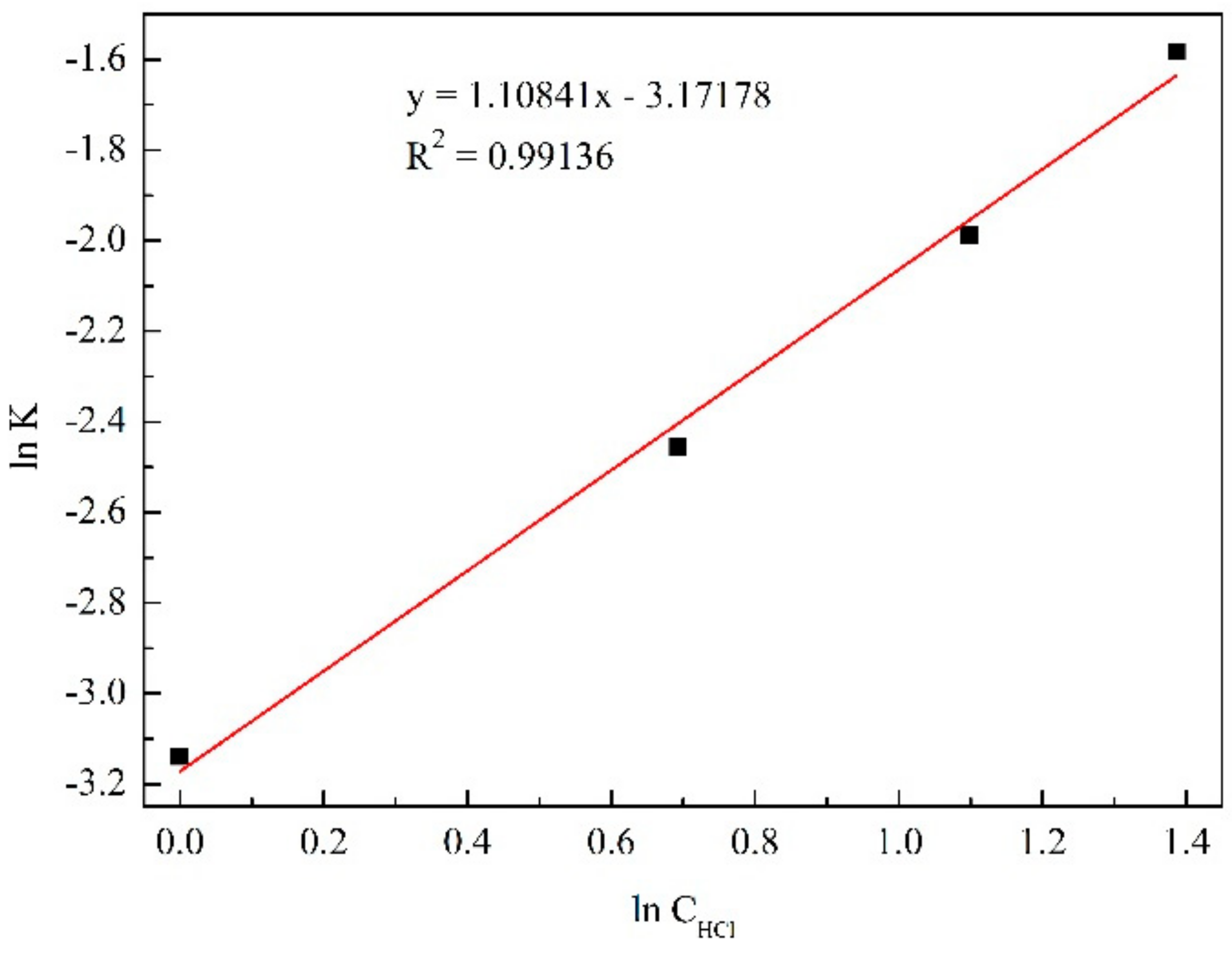
3.3.2. Initial HCl Concentration
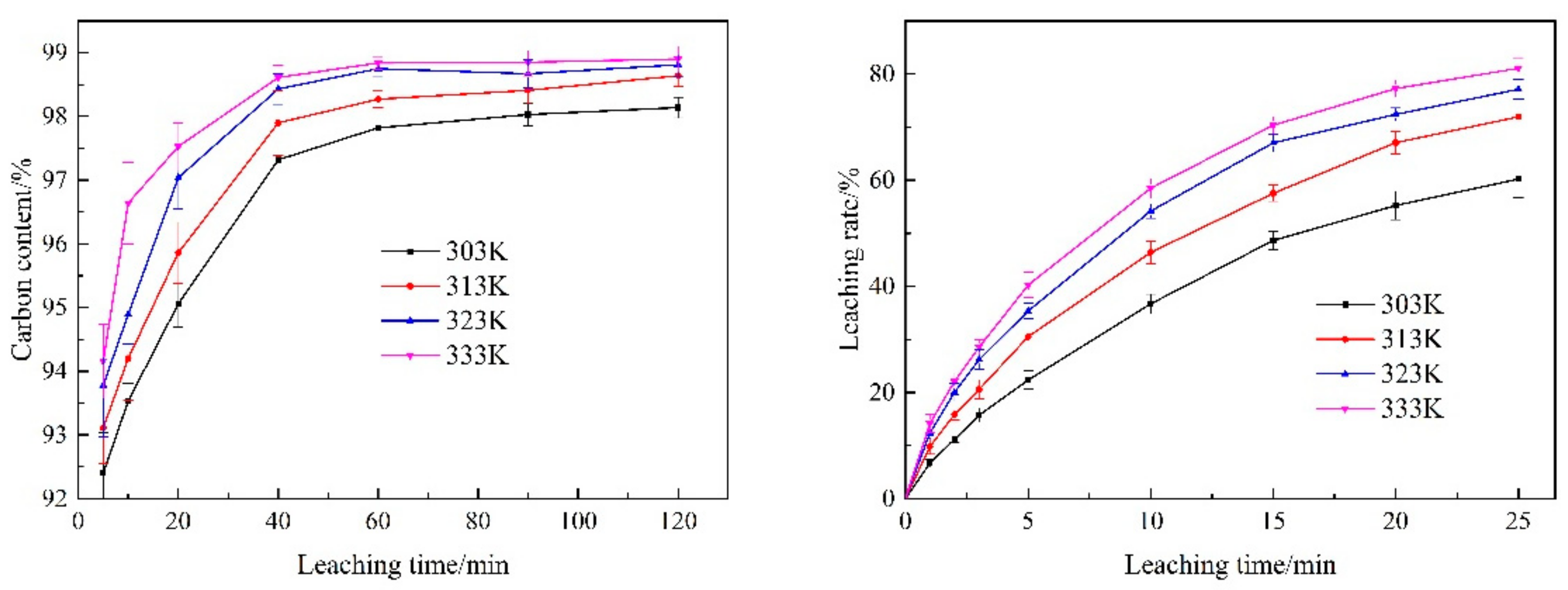
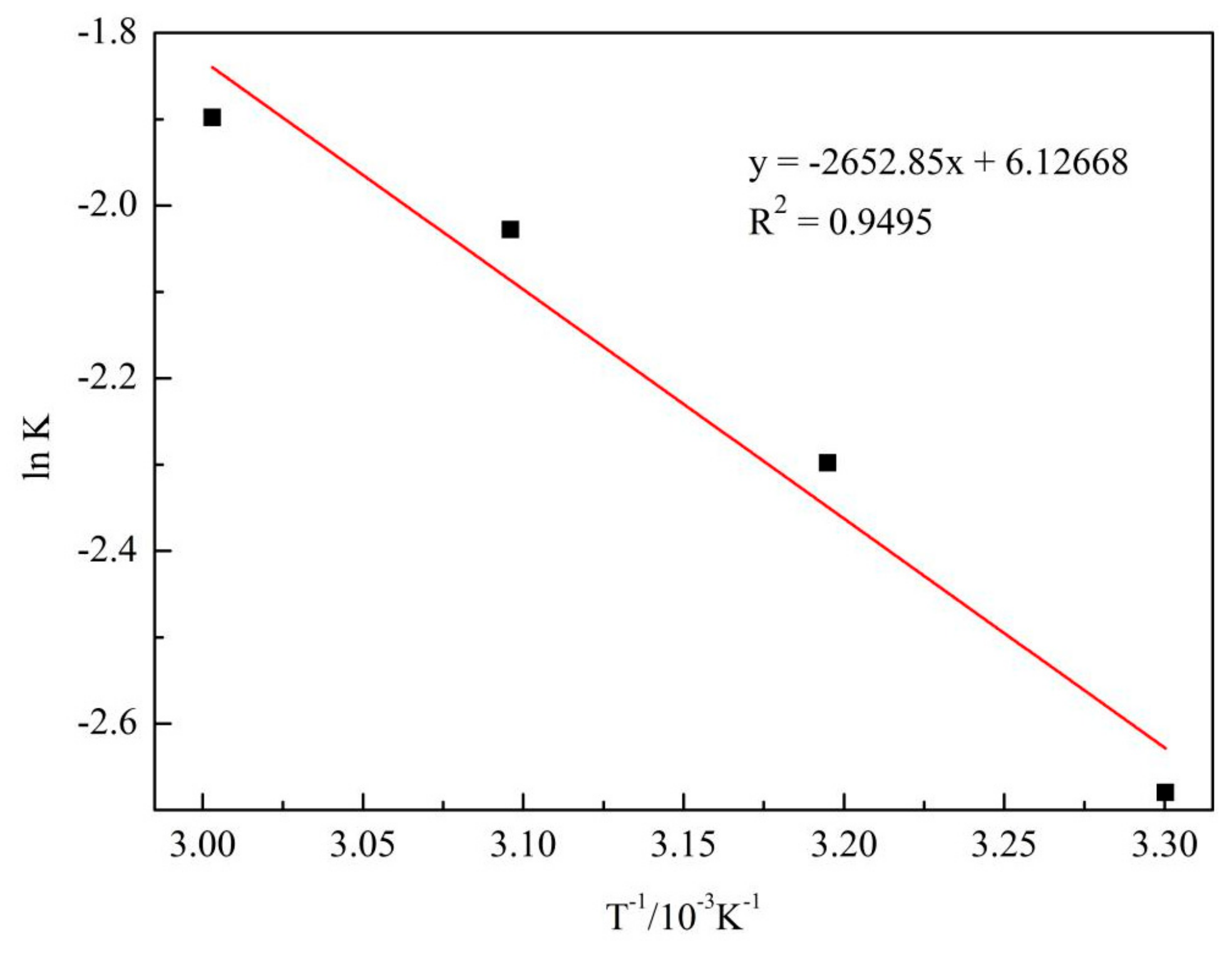
3.3.3. Temperature
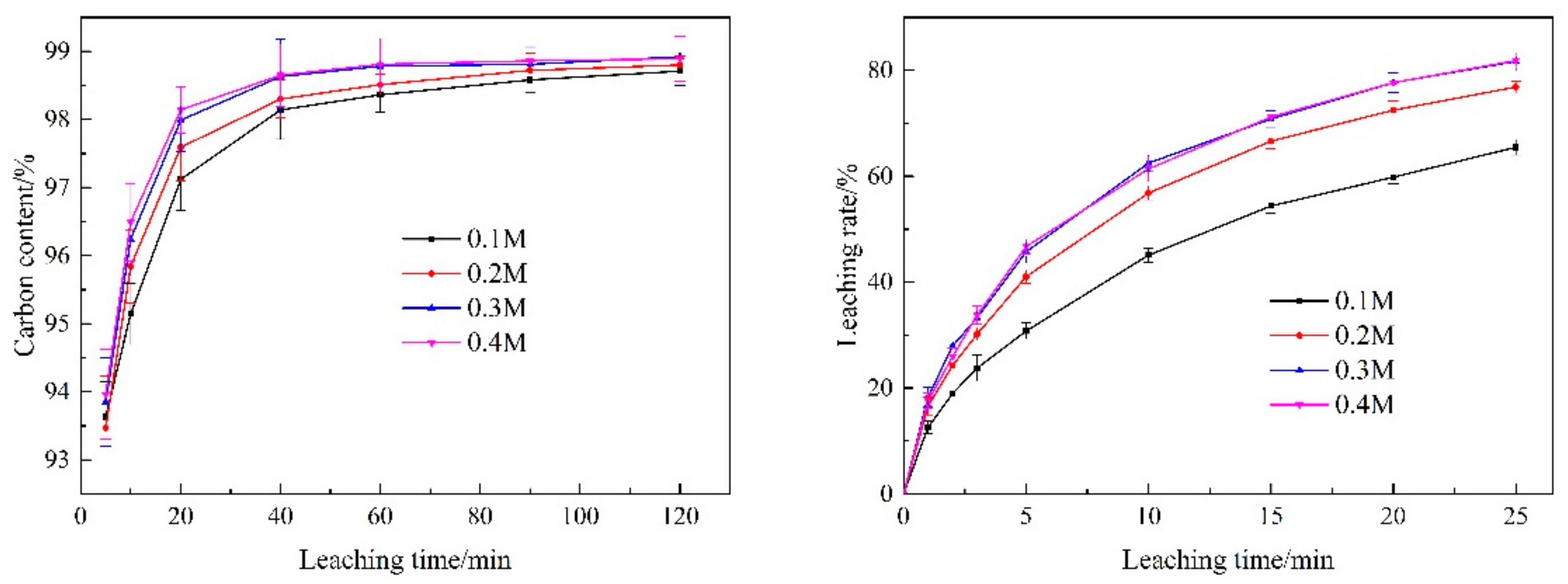
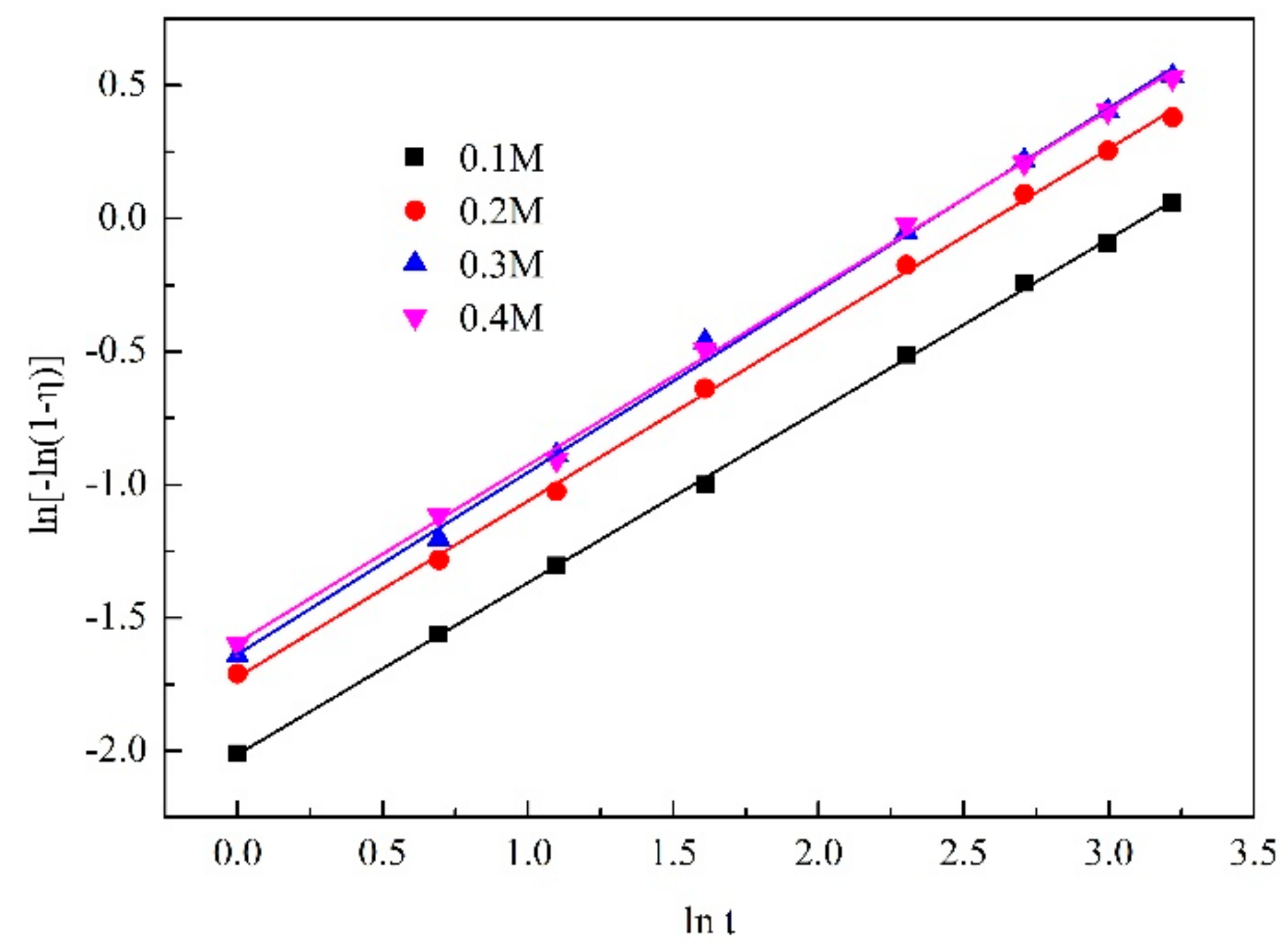
3.3.4. Sodium Fluoride Addition
3.3.5. Purified SCC Analysis
3.4. Leaching Kinetic Analysis
3.4.1. Kinetic Model
3.4.2. Determination of Leaching Model Parameters
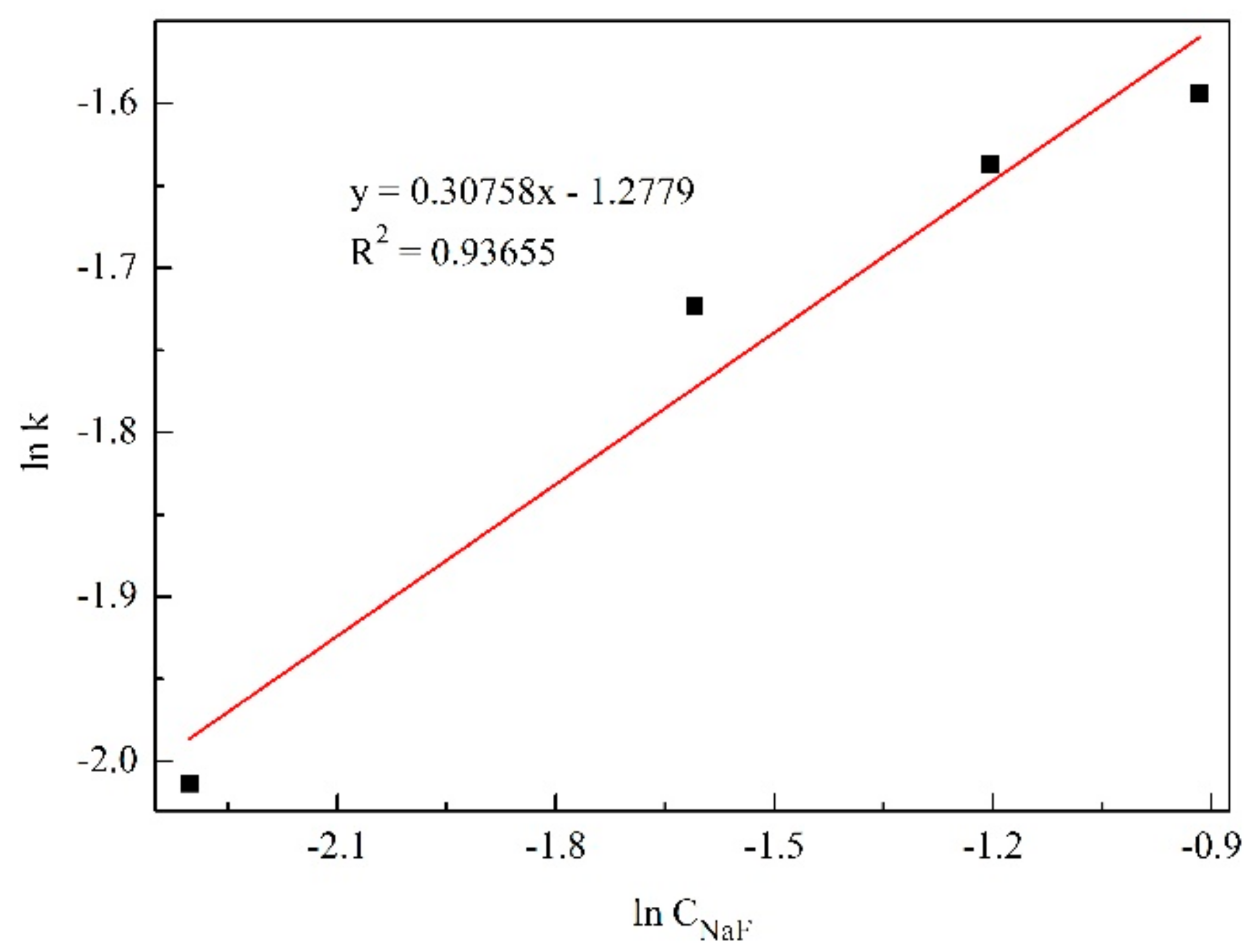
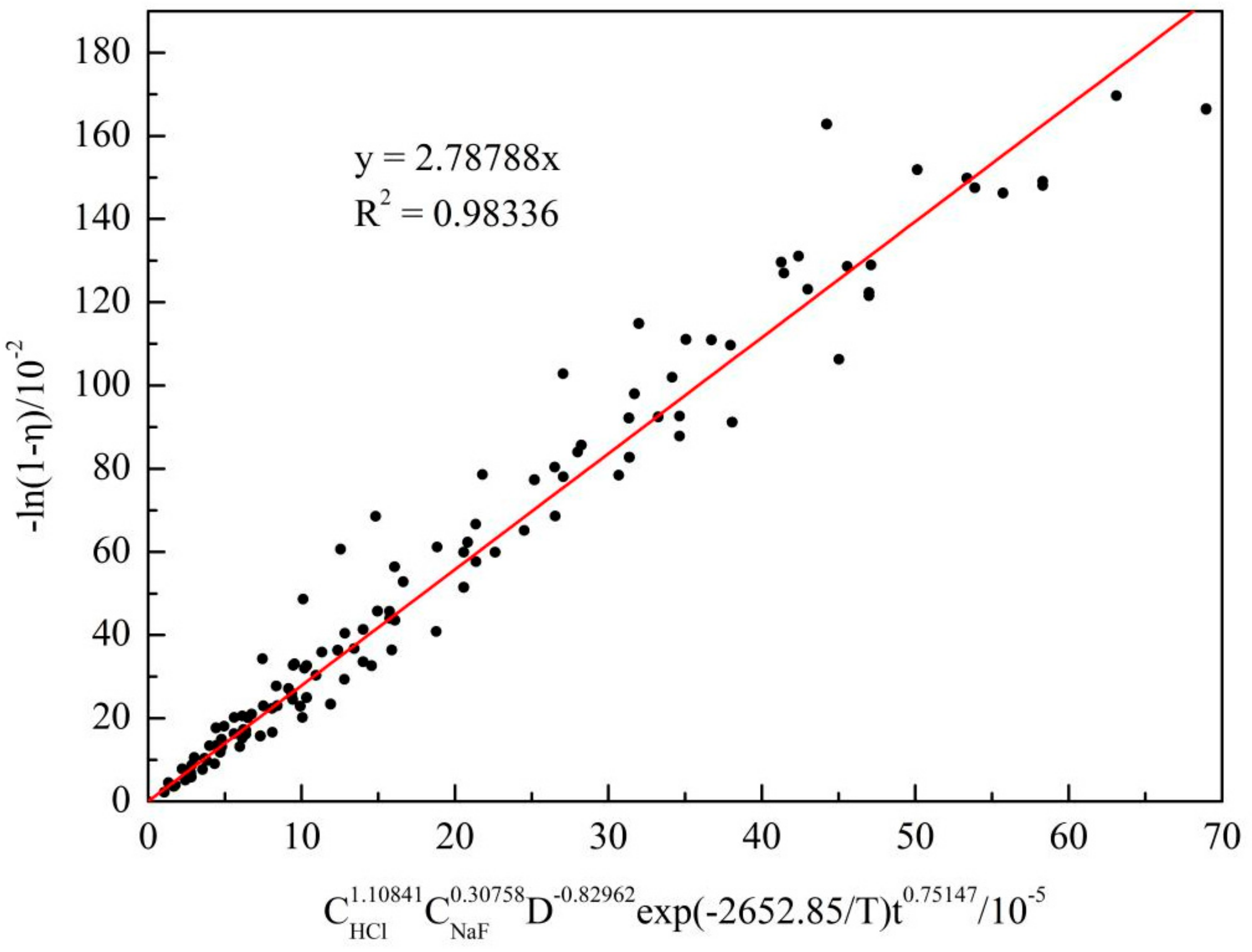
3.4.3. Determination of the Kinetic Equation
4. Conclusions
- (1)
- Alkali-fused SCC powders from aluminum electrolysis were purified in a mixed solution of hydrochloric acid and sodium fluoride; the residual ash content was <1%.
- (2)
- Leaching of alkali fusion treated SCC powder in a hydrochloric acid and sodium fluoride system is described by the Avrami equation. The reaction characteristic parameter was 0.75147 and the apparent activation energy was 22.056 kJ/mol. Leaching was controlled by a mixed mechanism of chemical reaction and diffusion.
- (3)
- Kinetic equation of aluminum extraction was: −ln(1 − η) = 2.D−0.82962exp(−2652.85/T)t0.75147.
Author Contributions
Funding
Conflicts of Interest
References
- Lu, F.; Su, X.; Huang, F.; Wang, J.; Wang, H. Co-Treatment of Spent Pot-Lining and Red Mud for Carbon Reutilization and Recovery of Iron, Aluminum and Sodium by Reductive Roasting Process. Metall. Mater. Trans. B 2020, 51, 1564–1575. [Google Scholar] [CrossRef]
- Liu, F.; Xie, M.; Liu, W.; Zhao, H. Footprint of harmful substances in spent pot lining of aluminum reduction cell. Trans. Nonferrous Met. Soc. 2020, 30, 1956–1963. [Google Scholar] [CrossRef]
- Nunez, P. Developing Guidance to Support Sustainable Spent Pot Lining (SPL) Management Across the Aluminum Industry. JOM 2020, 72, 3334–3340. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, H.; Li, T.; Xiao, J. Structural characteristics and sodium penetration behaviors in anthracite cathodes: A combination study using Monte Carlo and molecular dynamics simulations. Carbon Lett. 2019, 30, 259–269. [Google Scholar] [CrossRef]
- Holywell, G.; Breault, R. An Overview of Useful Methods to Treat, Recover, or Recycle Spent Potlining. JOM 2013, 65, 1441–1451. [Google Scholar] [CrossRef]
- Senanu, S.; Wang, Z.; Ratvik, A.; Grande, T. Carbon Cathode Wear in Aluminium Electrolysis Cells. JOM 2019, 72, 210–217. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, G.; Liu, J.; Xie, W.; Evrendilek, F.; Buyukada, M. (Co-)combustion behaviors and products of spent potlining and textile dyeing sludge. J. Clean. Prod. 2019, 224, 384–395. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, C.; Chang, Q.; Cheng, H.; Li, D.; Zhang, D. Optimization of flotation conditions for spent pot lining carbon of aluminum reduction. Light Met. 2017, 26–31. [Google Scholar]
- Yang, K.; Gong, P.; Tian, Z.; Lai, Y.; Li, J. Recycling spent carbon cathode by a roasting method and its application in Li-ion batteries anodes. J. Clean. Prod. 2020, 261, 121090. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, J.; Tian, Z.; Yang, K. Comparison of ultrasound-assisted and traditional caustic leaching of spent cathode carbon (SCC) from aluminum electrolysis. Ultrason. Sonochem. 2018, 40, 21–29. [Google Scholar] [CrossRef]
- Ghenai, C.; Inayat, A.; Shanableh, A.; Al-Saraira, E.; Janajreh, I. Combustion and emissions analysis of Spent Pot lining (SPL) as alternative fuel in cement industry. Sci. Total Environ. 2019, 684, 519–526. [Google Scholar] [CrossRef]
- Flores, I.V.; Fraiz, F.; Lopes Junior, R.A.; Bagatini, M.C. Evaluation of spent pot lining (SPL) as an alternative carbonaceous material in ironmaking processes. J. Mater. Res. Technol. 2019, 8, 33–40. [Google Scholar] [CrossRef]
- Li, R.; Lu, T.; Xie, M.; Liu, F. Analysis on thermal behavior of fluorides and cyanides for heat-treating spent cathode carbon blocks from aluminum smelters by TG/DSC-MS & ECSA(R). Ecotox. Environ. Safe 2020, 189, 110015. [Google Scholar]
- Yao, Z.; Xiao, J.; Mao, Q.; Wang, G.; Tang, L.; You, Z.; Zhong, Q. Detoxification and Recovery of Spent Carbon Cathodes via NaOH–Na2CO3 Binary Molten Salt Roasting-Water Leaching: Toward a Circular Economy for Hazardous Solid Waste from Aluminum Electrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16912–16923. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, L.; Yuan, J.; Yao, Z.; Tang, L.; Wang, Z.; Zhang, Z. Co-utilization of spent pot-lining and coal gangue by hydrothermal acid-leaching method to prepare silicon carbide powder. J. Clean. Prod. 2018, 204, 848–860. [Google Scholar] [CrossRef]
- Li, X.; Yin, W.; Fang, Z.; Liu, Q.; Cui, Y.; Zhao, J.; Jia, H. Recovery of Carbon and Valuable Components from Spent Pot Lining by Leaching with Acidic Aluminum Anodizing Wastewaters. Metall. Mater. Trans. B 2019, 50, 914–923. [Google Scholar] [CrossRef]
- Lisbona, D.F.; Somerfield, C.; Steel, K.M. Leaching of spent pot-lining with aluminium nitrate and nitric acid: Effect of reaction conditions and thermodynamic modelling of solution speciation. Hydrometallurgy 2013, 134–135, 132–143. [Google Scholar] [CrossRef]
- Nie, Y.; Guo, X.; Guo, Z.; Tang, J.; Xiao, X.; Xin, L. Defluorination of spent pot lining from aluminum electrolysis using acidic iron-containing solution. Hydrometallurgy 2020, 194, 105319. [Google Scholar] [CrossRef]
- Birry, L.; Leclerc, S.; Poirier, S. The LCL&L process: A sustainable solution for the treatment and recycling of Spent potling. TMS 2016, 467–471. [Google Scholar]
- Xie, M.; Li, R.; Zhao, H.; Liu, W.; Lu, T.; Liu, F. Detoxification of spent cathode carbon blocks from aluminum smelters by joint controlling temperature-vacuum process. J. Clean. Prod. 2020, 249, 119370. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Di, Y. Separation and Recycling of Spent Carbon Cathode Blocks in the Aluminum Industry by the Vacuum Distillation Process. JOM 2018, 70, 1877–1882. [Google Scholar]
- Tang, H. High-Temperature Continuous Electric Calciner. Patent CN201711147639, 17 November 2017. [Google Scholar]
- Yuan, J.; Xiao, J.; Tian, Z.; Yang, K.; Yao, Z.; Zhang, L. Optimization of purification treatment of spent cathode carbon from aluminum electrolysis using response surface methodology (RSM). Asia-Pac. J. Chem. Eng. 2018, 13, e2164. [Google Scholar] [CrossRef]
- Hodge, H.; Hayes, P.C.; Hawker, W.; Vaughan, J. The DSP concentrate sinter-leach process for aluminium and sodium recovery 2: Leaching behaviour. Miner. Process. Extr. Metall. 2020, 131, 341–353. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, J.; Tian, Z.; Yang, K. Optimization of spent cathode carbon purification process under ultrasonic action using Taguchi method. Ind. Eng. Chem. Res. 2018, 57, 7700–7710. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Cai, X.; Fu, J.; Yu, H. The leaching behavior of copper and iron recovery from reduction roasting pyrite cinder. J. Hazard. Mater. 2021, 420, 126561. [Google Scholar] [CrossRef]
- Wang, B. Study on High-Purity Aluminum Oxide Recovered from Aluminum Dross by Acid Leaching. Master’s Thesis, Zhengzhou University, Zhengzhou, China, May 2016. [Google Scholar]
- Guía-Tello, J.C.; Garay-Reyes, C.G.; Ruiz-Esparza-Rodríguez, M.A. Effect of plastic deformation on the precipitation reaction in 2024 alloys. Mater. Chem. Phys. 2021, 271, 124927. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Li, D. Leaching kinetics of tellurium-bearing materials in alkaline sulfide solutions. Miner. Process. Extr. Metall. Rev. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Turunen, K.; Yazdani, M.R.; Santasalo-Aarnio, A.; Seppl, A. Exceptional cold-crystallization kinetics of erythritol-polyelectrolyte enables long-term thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111273. [Google Scholar] [CrossRef]



| Element | C | F | Na | Al | Ca | Si | O | Others | |
|---|---|---|---|---|---|---|---|---|---|
| content | untreated | 61.06 | 14.37 | 8.71 | 7.09 | 1.35 | 0.43 | 5.47 | 1.52 |
| treated | 93.26 | 0.74 | 0.48 | 1.46 | 2.38 | 0.57 | 0.73 | 0.38 |
| Particle Size | Regression Equation | R2 |
|---|---|---|
| D(50) = 304.36 μm | ln[−ln(1 − η)] = 0.73058lnt − 3.76902 | 0.99799 |
| D(50) = 175.49 μm | ln[−ln(1 − η)] = 0.72284lnt − 3.16824 | 0.99552 |
| D(50) = 94.56 μm | ln[−ln(1 − η)] = 0.77899lnt − 2.66602 | 0.99755 |
| D(50) = 55.51 μm | ln[−ln(1 − η)] = 0.89611lnt − 2.46394 | 0.99666 |
| Initial Acid Concentration | Regression Equation | R2 |
|---|---|---|
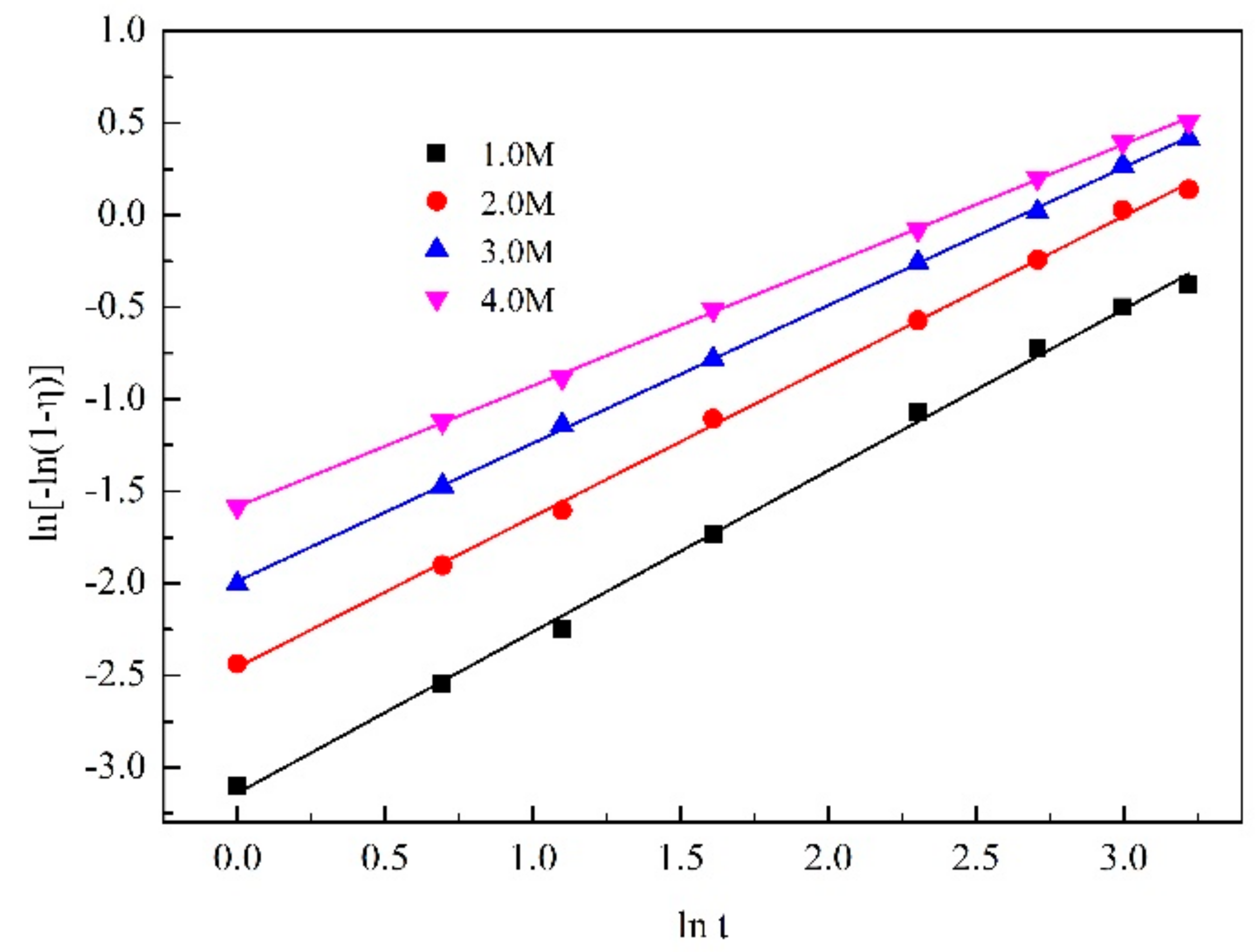
| 1 mol/L | y = 0.87611x − 3.13885 | 0.99762 |
| 2 mol/L | y = 0.81689x − 2.45518 | 0.99885 |
| 3 mol/L | y = 0.74951x − 1.98818 | 0.99964 |
| 4 mol/L | y = 0.65595x − 1.58231 | 0.99957 |
| Temperature | Regression Equation | R2 |
|---|---|---|
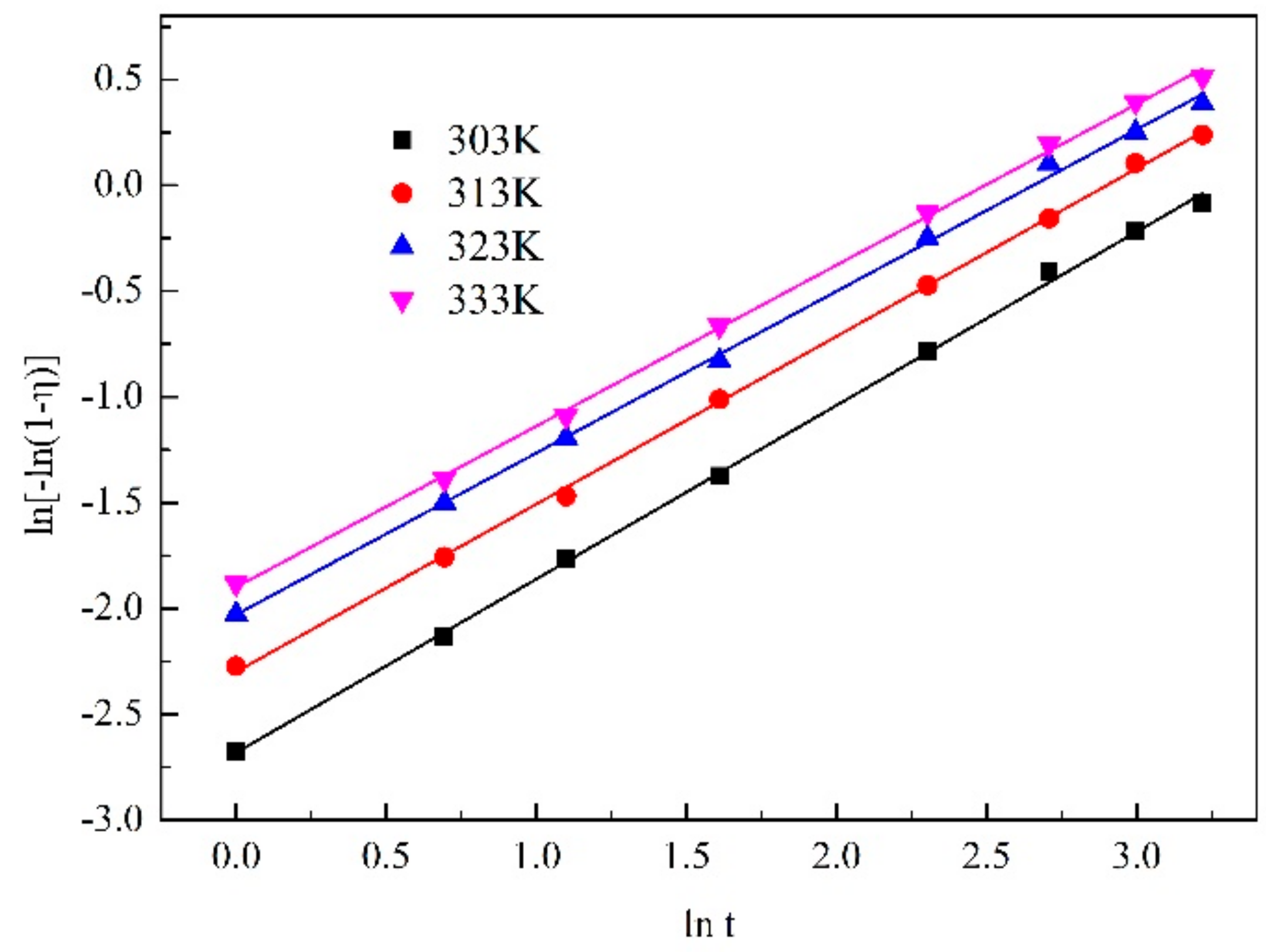
| 303 K | y = 0.82056x − 2.67984 | 0.99899 |
| 313 K | y = 0.79246x − 2.29784 | 0.9993 |
| 323 K | y = 0.76404x − 2.02802 | 0.99847 |
| 333 K | y = 0.7609x − 1.89813 | 0.99900 |
| NaF Concentration | Regression Equation | R2 |
|---|---|---|
| 0.1 mol/L | y = 0.64577x − 2.01349 | 0.99951 |
| 0.2 mol/L | y = 0.66183x − 1.72284 | 0.99899 |
| 0.3 mol/L | y = 0.68402x − 1.63707 | 0.99782 |
| 0.4 mol/L | y = 0.66698x − 1.59361 | 0.99858 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Li, H.; Ding, S. Leaching Kinetics of Aluminum from Alkali-Fused Spent Cathode Carbon Using Hydrochloric Acid and Sodium Fluoride. Processes 2022, 10, 849. https://doi.org/10.3390/pr10050849
Yuan J, Li H, Ding S. Leaching Kinetics of Aluminum from Alkali-Fused Spent Cathode Carbon Using Hydrochloric Acid and Sodium Fluoride. Processes. 2022; 10(5):849. https://doi.org/10.3390/pr10050849
Chicago/Turabian StyleYuan, Jie, Huijin Li, and Shuang Ding. 2022. "Leaching Kinetics of Aluminum from Alkali-Fused Spent Cathode Carbon Using Hydrochloric Acid and Sodium Fluoride" Processes 10, no. 5: 849. https://doi.org/10.3390/pr10050849





