Abstract
This work investigated the effect of coal blending on ash fusibility and slurryability of Xinjiang low-rank coal. The results showed that Xinjiang low-rank coals were characterized by high internal water content, high volatile content, high ash fusing point, and poor slurryability, which can not be directly used in coal water slurry gasification. The blending method not only reduced the ash fusibility but also improved the slurryability of these low-rank coals. When the coals with low calcium and high silicon contents (KG and YK) were blended with coal with high calcium content (SH), the ash fusion temperatures of the blended coal were significantly reduced. Moreover, the SH coal showed the worst slurryability performance with a concentration of 48.56%. The slurryability of HS coal can be dramatically improved by blending with KG. When the mass fraction of KG coal reached 70%, the concentration of coal water slurry increased by 11%. For the blended coal of KG and YK, the concentration and stability of coal water slurry gradually increase with the increasing mass ratio of KG. The coal blending method can effectively improve the concentration of coal water slurry for the low-rank coals, which were difficult-to-prepare slurry.
1. Introduction
Coal water slurry gasification technology is an important way for the efficient and clean utilization of coal resources, which is characterized by low pollution and high efficiency. The feedstock for the water slurry gasifier is developed towards low-rank coal, coal blending, and industrial and domestic waste [1,2]. Coal water slurry (CSW) is a highly concentrated suspension of coal particles in water, which has high solid loading in the range of 60–75% (by weight). The high viscosity of the slurry always leads to an increase in pumping energy consumption. Therefore, in order to easy handling and pumping of these highly loaded mixtures, the viscosity of coal waste slurry should be low. The technical requirements of raw coal for coal water slurry gasification include internal water, ash content, ash fusibility, viscosity–temperature characteristics, total sulfur content, slurryability, Hastelloy grindability index, etc. Among them, slurryability, ash content, and ash fusibility are the most important parameters that are widely used to select feedstock coal.
It is difficult to prepare highly loaded CWS from low-rank coal. Many scholars have performed a lot of research to improve the slurryability of low-rank coals by particle size controlling [3,4], microwave or ultrasonic irradiation [5,6], thermal treatment [5], development of high-efficient dispersant agent [7,8], hydrothermal dewater process [9,10], mixing with petroleum coke [11] and coal blending, etc. Coal blending is a simple and effective method to adjust the physical and chemical properties of coal. Wang et al. [12] studied the blending of coal with high and low viscosity to improve its ash fusibility. Lv et al. [13] blended the direct coal liquefaction residues with four kinds of low-rank coals, and the slurryability of low-rank coals was significantly improved. Gu et al. [14] investigated the effect of three different grades of blending coals on the preparation of coal water slurry. Compared to the two coals, the coal concentration in the slurry can be effectively improved and generally increased by 3–5%. Liu et al. [15] mixed two different anthracite and bituminous coals into Shenmu coal, and the CWS concentration of Shenmu coal was increased by 6%.
Xinjiang contains a large number of coal resources. A total of 391.5 billion tons of reserves has been found, accounting for 24.5% of the national total [16]. The distribution of coal in Xinjiang shows significant spatial zoning and age differences. Moreover, these coals are generally low-rank coals with the characteristics of high water content, young coal quality, high ash fusing point, and well-developed pores [17], which are widely used as fuel coal. However, it has insufficient advantages in the coal chemical industry, especially in the development of coal-water slurry and liquid slag gasification technology. In order to improve the rational development and utilization of coal resources in Xinjiang, the coal blending method was employed to reduce the ash fusion temperatures and improve the CWS property of Xinjiang low-rank coal.
2. Materials and Methods
2.1. Materials
Four low-rank coals from Xinjiang, including Kuangou coal (denoted as KG), Heishan open coal (denoted as HS), Yankuang open coal (denoted as YK), and Shenhua open coal (denoted as SH), were chosen in this work. These coal samples were ground to a particle size of less than 0.200 mm and dried at 105 °C for 24 h in an N2 atmosphere. According to Chinese standards for coal analyses (GB/T212-2001, GB/T476-2001, GB/T213-2003, and GB/T214-1996), the proximate analyses, ultimate analyses, higher heating values on the basis of air dry (HHV), and total sulfur contents of the four samples were analyzed. Table 1 shows the analysis results of the coals. The four low-rank coals have high internal moisture and high volatile content. The internal water of SH was 6.34%, and the volatile content of HS was 37.44%.

Table 1.
Analysis results of Xinjiang low rank coals.
The coal ash samples were prepared according to Chinese standard procedures (GB/T 212-2008). Basically, the coal samples were placed in a muffle furnace, heated to 500 °C within 30 min and kept at this temperature for a further 30 min, and then the temperature was increased to 815 °C and kept at this level for 2 h. The chemical compositions of coal ashes were analyzed by Bruker X-ray Fluorescence Analyzer (S8 Tiger, Bruker Corp., Saarbrücken, Germany) according to Chinese standard GB/T1574-2011, and the results of four low-rank coals are given in Table 2. The KG and YK coal ashes have high contents of silicon and aluminum, and the HS and SH coals were typically high-calcium coal.

Table 2.
Ash composition of four low-rank coals (%).
The coal ash is usually discharged from the bottom as molten slag. A high ash fusion temperature easily led to the heavier corrosion of refractory bricks and the high consumption of energy. Therefore, the ash fusion point is very important for liquid slagging gasifiers, and a comparatively low ash fusion temperature coal is preferred [18].
2.2. Preparation of the Coal Water Slurry
The KG coal with the best slurryability was mixed with other three low-rank coals to prepare coal water slurry. The additive used in this work was obtained from Shanxi Linyi Kehua Co., Ltd. (Shanghai, China), of which its main component is sodium lignosulfonate. The coal water slurry samples were prepared by dry grinding and wet pulping. The coal is pulverized into a certain particle size with a jaw crusher and then pulverized with a small rod mill. Based on the grindability index of the coal and the particle size requirements of the coal water slurry gasification, the grinding time is controlled to obtain the coal powder with the required particle size distribution. According to the required ratio, the pulverized coal, additives (Dry agent/Dry coal = 0.2%) and primary water were mixed and then stirred for 10 min with an electronic stirrer at 1000 r/min to ensure the formation of a homogenization coal-water slurry [19,20]. The coal water slurry was then placed for 3 min to release any entrapped air for further tests to measure performance parameters.
The coal samples with different properties were blended according to different proportions. The mass fractions were 30%, 40%, 50%, 60%, and 70%, respectively. The particle size distributions of coals used in this work are given in Table 3.

Table 3.
Particle size distribution of coals for slurry preparation.
2.3. Measurement of Coal Water Slurry Property
2.3.1. Ash Fusion Temperatures of Coal (AFTs)
The AFTs of the blended coal ashes were measured by SDAF105a ash fusion analyzer (Hunan Sande Technology Development Co., Ltd., Changsha, China) under a reducing atmosphere according to Chinese standard GB/T 219-2008. Coal ash with a particle size less than 0.1 mm with an agate mortar was mixed with gummeline and then shaped into triangular ash cones (a equilateral triangle with a height of 20 mm and a base length of 7 mm). This procedure involves heating the ash cone at a rate of 15 °C/min up to 900 °C and then changing the heating rate to 5 °C/min. During this process, four characteristic temperatures were recorded for four low-rank coal ashes: deformation temperature (DT), softening temperature (ST), hemispherical temperature (HT), and flow temperature (FT). The error for the AFTs measurement was ±40 °C according to the standard. In order to ensure the accuracy of the experimental data, the measurement was repeated three times for each sample, and the average value of the respective characteristic temperatures was selected as the final measured value to reduce measurement error. Table 4 gives the ash fusion temperatures (AFTs) of four coal samples, and the FT of YK was 1400 °C.

Table 4.
Ash fusion temperatures of four low-rank coals (%).
2.3.2. CWS Concentration
The infrared drying method, which was performed by HX-204 (Mettler-Toledo National Trading (Shanghai) Co., Ltd., Shanghai, China) fast moisture analyzer was used to measure the coal water slurry concentration according to Chinese standard GB/T 18856.2-2008.
2.3.3. CWS Viscosity
The CWS viscosity was measured by NXS-4C (Chengdu Corp., Chengdu, China) viscometer according to Chinese standard GB/T 18856.4-2008. Appropriate amount of uniform CWS sample was put into the measuring container, and the shear rate increased from 0 s−1 to 100 s−1. Six groups of test data were collected once every 12 s at the shear rate of 100 s−1, which was defined as the apparent viscosity. Three times were also repeated for each measurement, and the repeatability of the measurement results was within 0.5%. Finally, the average value was the viscosity of CWS. The maximum solid loading of slurry was designated as the solid loading when the apparent viscosity of slurries was 1000 mPa·s at 20 °C [21].
2.3.4. CWS Stability
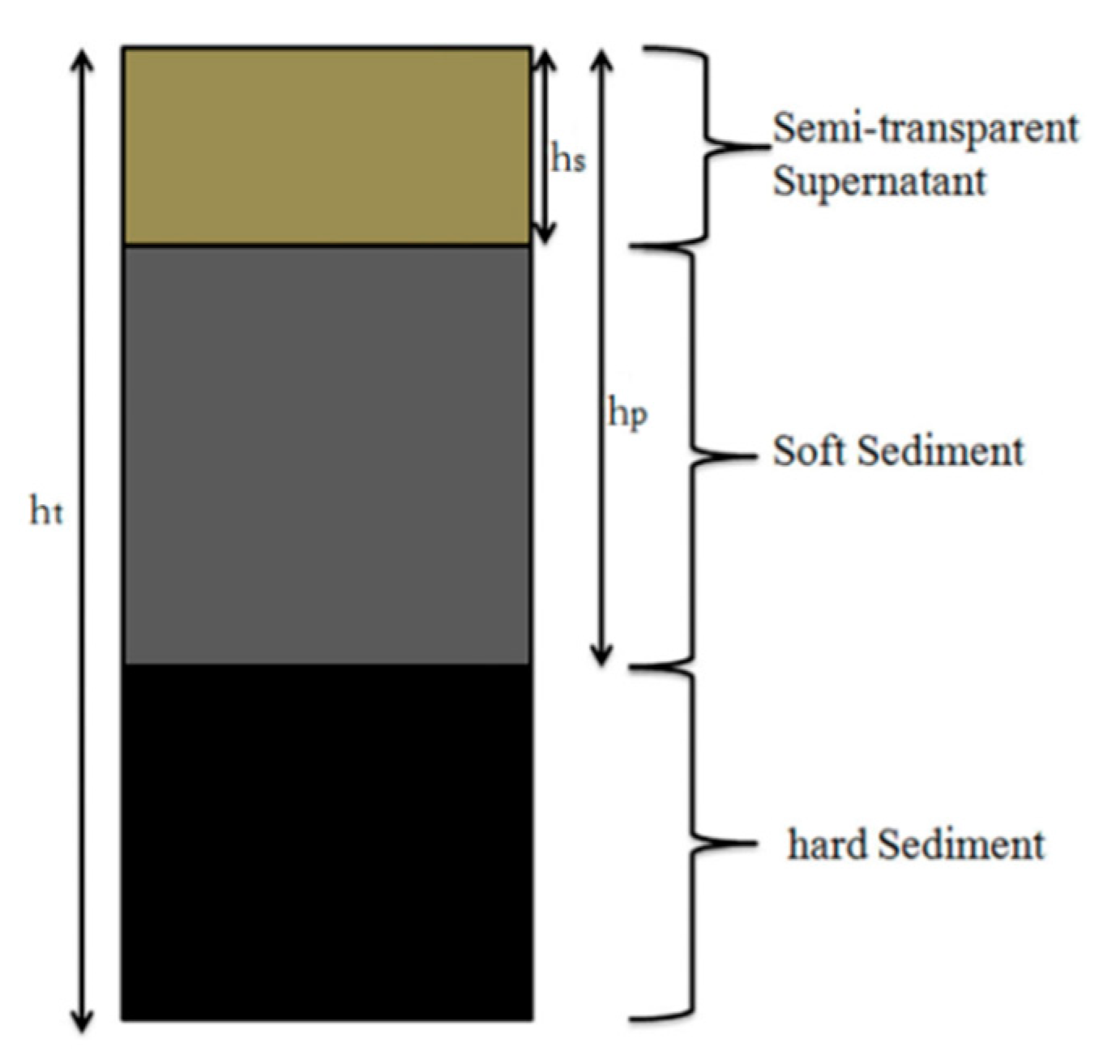
A glass rod penetration test was employed to evaluate the stability of the coal water slurry by measuring the change of penetration ratio (%) with the storage time (h). The prepared CWS was stored in a 100 mL sealed and graduated cylinder (coal water slurry layer with the height of 100 mL) at room temperature for 48 h. The schematic diagram of the coal water slurry in the measuring cylinder is presented in Figure 1.
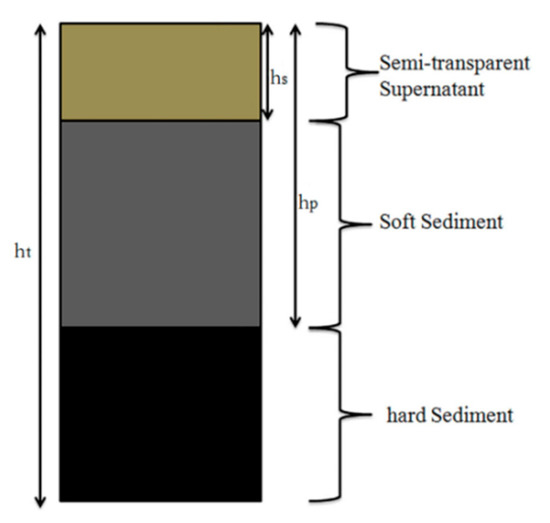
Figure 1.
The schematic diagram of the coal water slurry in the measuring cylinder.
A glass rod of 30 cm in length, 6 mm in diameter, and 27.2 g in weight was allowed to fall freely from the surface of the coal water slurry to the bottom and stopped when the tip touched the hard deposit.
The penetration ratio was calculated as follows:
where hp is the effective traveled distance (cm) by the glass rod, and ht is the total height (cm) of the CWS in the measuring cylinder.
Penetration ratio (%) = hp/ht × 100
The separation ratio was calculated below:
where hs was the water in the suspension layer in the CWS after 48 h and ht is the total height (cm) of the CWS in the measuring cylinder.
Separation ratio (%) = hs/ht × 100
2.3.5. CWS Fluidity
The fluidity of CWS was determined by the time it takes when 100 mL of coal water slurry pass through the funnel (diameter 120 mm in diameter, 18 mm in lower mouth diameter, 45 mm in tube height, 130 mm in total height).
2.4. FactSage Calculations
In order to explain the effect of coal blending on the ash fusibility, thermodynamic software package FactSage based on minimization of Gibbs energy was applied to calculate mineral composition of the blended coal ashed at high temperatures. In this work, FactSage 8.1 (GTT-Technologies, Herzogenrath, Germany) with FToxid and FactPS was used to calculate mineral compositions and viscosity [22]. Eight components, including SiO2, Al2O3, Fe2O3, CaO, and MgO, were input into calculation. Calculations were carried out from 800 to 1500 °C with an interval of 50 °C under the reducing atmosphere (60% CO and 40% CO2, volume ratio). Equilib was used to calculate phase transformation.
3. Results and Discussion
3.1. Influence of Coal Blending on Ash Fusibility
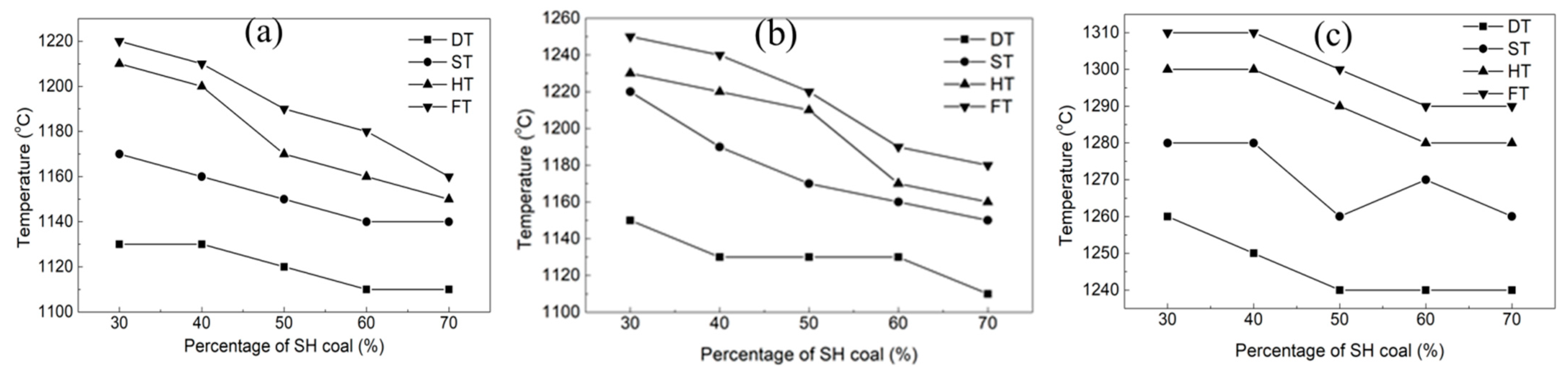
The SH coal with a lower ash fusion point is blended with the other three coals with high ash fusion point (KG, YK, HS). The mass fraction of SH coal was 30%, 40%, 50%, 60%, and 70%, respectively. Figure 2 shows the effect of coal blending on the ash fusion temperatures, and the blended ash composition is given in Table 4. As the SH coal mass fraction increased, the DT, ST, HT, and FT of the blended coal ashes showed different changes. When the SH coal was blended with KG and YK coals, the ash fusion temperatures gradually decreased with the increasing SH coal fraction. When the SH mass fraction reached 70%, the FT of the blended coal ash dropped to 1160 °C and 1180 °C, respectively, for KG and YK, while its effect was not obvious when the SH coal was blended with the HS coal. For the coal water slurry gasification, the FT of the feedstock is usually required to be lower than 1380 °C. As the FT of YK was 1400 °C, it cannot be directly used in the coal water slurry gasification. The blending method was an effective way to produce a feed with appropriate ash fusibility.
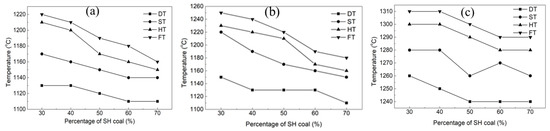
Figure 2.
Ash fusion temperature of the coal blended with SH coal. (a) KG; (b) YK; (c) HS.
Table 5 presents the ash composition of the blended coal, which was calculated by ash content, ash composition, and blending ratio of the coal sample. The result presented additivity, which showed a close relationship with the fusion temperature of coal ash. The calcium content of SH coal ash was high, while the contents of silicon and aluminum were low. However, the contents of silicon and aluminum in KG and YK coal ashes were high, and the content of calcium was low. When the SH coal was blended with KG and YK coals, the content of silicon and aluminum gradually decreased, and the content of calcium gradually increased with the increasing SH coal fraction. For the KG coal, when the SH fraction reached 70%, the contents of silicon and aluminum decreased to 39.83% and 15.80%, while the calcium content decreased to 14.88%. For the YK coal, the contents of silicon and aluminum were lowered to 40.36% and 16.90%, and the calcium content increased to 13.38%. This illustrated that the blending coal method balanced the ash composition, resulting in a decrease in the ash fusion temperatures. Moreover, the ash composition of HS coal was similar to that of SH coal, and both of them belonged to high calcium coal. As a result, the blending of coal did not significantly affect the contents of calcium, silicon, and aluminum in the coal ash. Therefore, the ash fusion temperatures were not significantly reduced when the HS coal was blended with the SH coal.

Table 5.
Ash compositions of the blended coals (%).
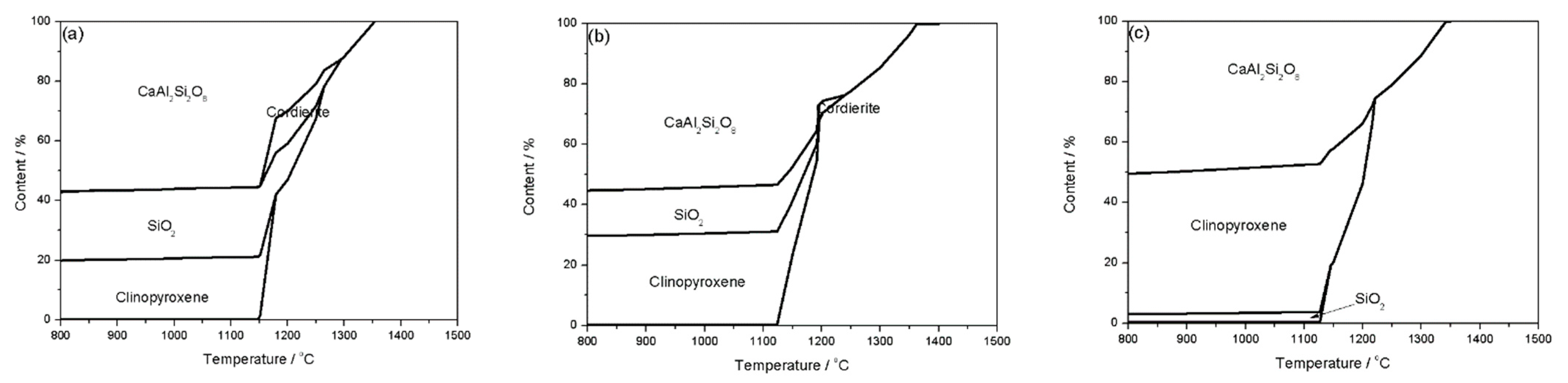
It is well accepted that mineral components interacted and fused into a liquid during the process of ash fusion, leading to variations in the mineral components and their contents [23]. Figure 3 shows the mineral transformation of the blended coal of KG and SH. When the mass fraction of SH coal was 30–70%, anorthite (CaAl2Si2O8), cordierite, clinopyroxene, and quartz (SiO2) were the main mineral formed in the blended coal ash at high temperature. As the mass fraction of SH coal increased, the contents of anorthite and quartz decreased, especially the content of quartz was remarkably lowered from more than 20% (SH:KG = 3:7) to around 4% (SH:KG = 7:3) at 1100 °C, whereas the content of clinopyroxene significantly increased. The anorthite and quartz were high melting point minerals, and their melting point was 1550 °C and 1750 °C, respectively, and the clinopyroxene had a low melting point of 1391 °C [24]. Moreover, the low eutectic can be formed between anorthite and clinopyroxene [25]. All these transformations contributed to the decrease in the AFTs of the blended coal with the increasing SH mass fraction.
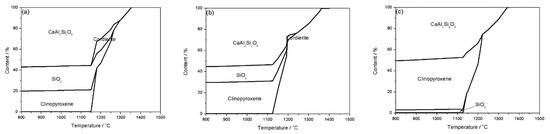
Figure 3.
Mineral transformation of the blended coal of KG and SH. (a) SH:KG = 3:7; (b) SH:KG = 5:5; (c) SH:KG = 7:3.
3.2. Slurryability of Four Low-Rank Coals
The slurryability of the four low-rank coals, as well as the stability of coal water slurry samples, including the penetration ratio and separation ratio, is presented in Table 6, which is used to estimate the coal concentration required to prepare CWS with a viscosity of 600 mPa·s. The KG coal showed a high slurryability performance with a concentration of up to 69.72%, and the SH coal exhibited the worst slurryability performance with a concentration of 48.56%. The YK and SH samples showed phase separation between the semi-transparent supernatant and sediments. The two phases separated quickly in the first 48 h and then reached a plateau. The top was the semi-transparent supernatant, and the middle was the soft sediment, which can be re-dispersed easily. The bottom was the hard sediment, which looked like a solid layer and was hard to re-disperse. In Table 6, the concentration of YK coal was acceptable, but the separation ratio was up to 4%, and the penetration rate was only 95%, which suggested that the stability of the CWS was poor. The separation ratio of SH coal was up to 3%, but the penetration rate was 100%. In addition, no apparent trend of separation ratio was observed for KG and HS coals.

Table 6.
Slurry properties of the four low-rank coals.
The slurryability of the coal was mainly dependent on the physical properties of the coal. The surface properties of the coals varied, which was dependent upon the rank of the coal. As the low-rank coals had higher oxygen and moisture contents, the viscosities of the coal water slurries were different for the same particle size distribution and same solids loading because of the differences in surface chemistry. The O/C atomic ratio was commonly used to account for the slurryability of the coal, which suggested the content of oxygen-containing functional groups in the coal. For the coal with a high O/C atomic ratio, the slurryability was poor. As the O/C atomic ratio decreased with the coal rank, the number of polar functional groups increased, resulting in a decrease in the slurryability [13]. The O/C atomic ratio in KG coal was 0.14, while it was 0.21 for the SH coal. Moreover, the SH coal had the highest internal moisture content. Because the surface of the coal with the high internal moisture content easily absorbed water molecules, the SH coal presented poor slurryability. In addition, for a fixed mass concentration of coal in the slurry, the density of the coal increased as the ash content of the coal increased. Because the KG and YK coals have higher ash content, they showed a low volume concentration and a better fluidity of coal in the CWS, which also implies that the slurry has a low viscosity.
3.3. Influence of Blending on Slurryability of Coal
Table 7 shows the properties of CWS of KG coal with different mass fractions of the other three coals. When the KG coal was blended with the SH coal, the slurryability performance was gradually improved with the increasing KG coal ratio. As the mass fraction of KG coal reached 70%, the slurry concentration increased to 59%. The internal moisture content was 2.38% and 6.34%, respectively, for KG and SH coals. The O/C atomic ratio in KG coal was 0.14, while it was 0.21 for the SH coal. The ash content of KG coal was 21.7% and 9.01%. When the SH was blended with SH coals, the internal moisture content and O/C atomic ratio in blended coal were significantly lowered, and the ash content was clearly increased. All these were conducive to improving the slurry formation of coal. Therefore, when the ratio of KG to SH was 7:3, the slurry concentration of mixed coal increased by 11%.

Table 7.
Properties of CWS of KG coal with other three low rank coals.
When the YK coal was blended with the KG, with the increasing KG coal proportion, the slurry concentration and stability gradually improved, and the separation ratio gradually decreased. As the mass proportion of KG coal reached 40%, the penetration ratio was up to 100%, and the separation rate dropped to 1%. Moreover, the soft sediment was over 99 vol%. However, for YK coal, it was only 91 vol%, and the hard sediment occupied over 5 vol%. The soft sediment was probably formed by the weak flocculation/aggregation of coal particles with attractive forces between particles. In the dense coal water slurry, the aggregations of coal particles are distributed in the whole slurry, which generates enough strength to support the particle and prevent it from settling into hard sediment.
4. Conclusions
The four low-rank coals from Xinjiang had the characteristics of high internal water content, high volatile content, high ash fusion temperatures, and poor slurryability. In order to improve the utilization of the low-rank coal, the blending method was used to improve the properties of coal water slurry, including ash composition, ash fusion temperature, slurryability.
The ash fusion temperatures of the blended coal largely depended on the ash chemical composition. The ash composition of the two blended coal exhibited additivity. When the SH coal with high calcium content was blended with the KG or YK coal with high silicon and aluminum contents, the ash fusion temperatures of the blended coal obviously reduced due to the balanced content of silicon, aluminum, and calcium. However, for the blended coal of SH and another high calcium content HS coal, the ash fusion temperatures did not clearly reduce because the contents of calcium, silicon, and aluminum in the coal ash did not change significantly.
The coal blending method can effectively improve the concentration of coal water slurry for the low-rank coals, which were difficult-to-prepare slurry. The KG coal exhibited good slurryability. The slurryability of SH coals can be dramatically improved by blending with the KG coal. As the blended ratio of KG and SH was 7:3, the concentration of coal water slurry increased by 11%. When the KG and YK coal were blended, the concentration and stability of coal water slurry gradually increased with the increasing mass ratio of KG coal.
Author Contributions
H.L. (Hui Li) carried out the experiments, analyzed the data, and wrote the initial draft of the manuscript; X.S. and G.L. conceived and designed the experiments; H.L. (Huaizhu Li) used software to valid the AFTs result; L.K., J.B. and W.L. reviewed and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duan, Q. Application status and development prospect of coal water mixture technology in china. Coal Sci. Technol. 2015, 43, 129–133. [Google Scholar]
- Das, D.; Panigrahi, S.; Misra, P.K.; Nayak, A. Effect of organized assemblies. Part 4. Formulation of highly concentrated coal-water slurry using a natural surfactant. Energy Fuels 2008, 22, 1865–1872. [Google Scholar]
- Su, X. Experimental Study on the Effect of Particle Size Distribution on the slurryability of Shenhua Coal. Coal Chem. Ind. 2020, 48, 36–40. [Google Scholar]
- Zhou, Y. Three peak fractal grading coal water slurry concentration technology. Clean Coal Technol. 2018, 24, 63–68, 73. [Google Scholar]
- Cheng, J.; Zhou, J.; Li, Y.; Liu, J.; Cen, K. Improvement of coal water slurry property through coal physicochemical modifications by microwave irradiation and thermal heat. Energy Fuels 2008, 22, 2422–2428. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, R.; Zheng, Y.; Fu, X. Improvement in properties of coal water slurry by combined use of new additive and ultrasonic irradiation. Ultrason Sonochem. 2007, 14, 583–588. [Google Scholar] [CrossRef]
- Das, D.; Dash, U.; Meher, J.; Misra, P.K. Improving stability of concentrated coal–water slurry using mixture of a natural and synthetic surfactants. Fuel Process. Technol. 2013, 113, 41–51. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Li, J.; Zhao, F. Synthesis, adsorption and dispersion of a dispersant based on starch for coal–water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 165–171. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Cen, K. Properties of coal water slurry prepared with the solid and liquid products of hydrothermal dewatering of brown coal. Ind. Eng. Chem. Res. 2014, 53, 4511–4517. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Liu, Q.; Zhang, J. Effects of Hydrothermal Dewatering of Lignite on Rheology of Coal Water Slurry. Can. J. Chem. Eng. 2019, 97, 323–329. [Google Scholar] [CrossRef]
- Xu, R.; He, Q.; Cai, J.; Pan, Y.; Shen, J.; Hu, B. Effects of chemicals and blending petroleum coke on the properties of low-rank Indonesian coal water mixtures. Fuel Process. Technol. 2008, 89, 249–253. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Q.; Gong, X.; Liu, H.; Liu, X. Influence of coal blending on ash fusion property and viscosity. Fuel 2017, 189, 15–22. [Google Scholar] [CrossRef]
- Lv, D.; Yuchi, W.; Bai, Z.; Bai, J.; Kong, L.; Guo, Z.; Yan, J.; Li, W. An approach for utilization of direct coal liquefaction residue: Blending with low-rank coal to prepare slurries for gasification. Fuel 2015, 145, 143–145. [Google Scholar] [CrossRef]
- Gu, T.; Wu, G.; Li, Q.; Sun, Z.; Zeng, F.; Wang, G.; Meng, X. Blended coals for improved coal water slurries. J. China Univ. Min. Technol. 2008, 18, 50–54. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Zhou, J.; Du, C.; Ye, L.; Cheng, J.; Cen, K. Study on the Effects of Coal Blending on the Slurry Ability of Shenmu Coals. Manuf. Sci. Technol. 2012, 383–390, 3011–3016. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Wufuer, Y.; Lu, Q.; Wang, W.; Feng, S.; Zhang, B. Analysis on development trend and prospects of scientific mining for coal resources in Xinjiang. Coal Eng. 2017, 49, 20–22. [Google Scholar]
- Ge, L.; Zhang, Y.; Wang, Z.; Zhou, J.; Cen, K. Effects of microwave irradiation treatment on physicochemical characteristics of Chinese low-rank coals. Energy Convers. Manag. 2013, 71, 84–91. [Google Scholar] [CrossRef]
- Kobayashi, N.; Fujimori, A.; Tanaka, M.; Piao, G.; Itaya, Y. Study of Coal Gasification Using High Ash Fusion Temperature Coal in an Entrained Flow Gasifier. J. Chem. Eng. Jpn. 2015, 48, 22–28. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Zhou, J.; Cheng, J.; Zhang, G.; Feng, Y.; Cen, K. An investigation on the rheological and sulfur-retention character istics of desulfurizing coal water slurry with calcium-based additives. Fuel Process. Technol. 2009, 90, 91–98. [Google Scholar] [CrossRef]
- Bouhamed, H.; Boufi, S.; Magnin, A. Alumina interaction with AMPS−MPEG copolymers produced by RAFT polymerization: Stability and rheological behavior. J. Colloid Interface Sci. 2009, 333, 209–220. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Wang, R.; Zhou, J.; Cen, K. Effect of hydrothermal dewatering on theslurryability of brown coals. Energy Convers. Manag. 2012, 57, 8–12. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Kong, L.; Bai, J.; Ni, Z.; Li, H.; Bai, Z.; Li, W. Comparison study of fusibility between coal ash and synthetic ash. Fuel Process. Technol. 2021, 211, 106593. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Li, H.; Chen, X.; Wang, J.; Bai, Z.; Guo, Z.; Li, W. The mineral evolution during coal washing and its effect on ash fusion characteristics of Shanxi high ash coals. Fuel 2018, 212, 268–273. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Fang, Y.; Wang, Y. The effects of leaching and floatation on the ash fusion temperatures of three selected lignites. Fuel 2011, 90, 2377–2383. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Li, B. Characterization of low-temperature coal ash behaviors at high temperatures under reducing atmosphere. Fuel 2008, 87, 583–591. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).