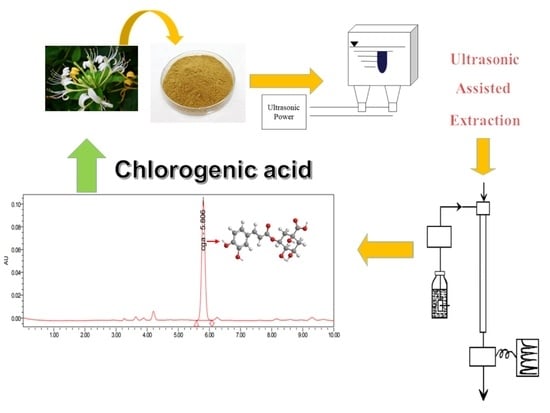
Optimization of Glycerol Extraction of Chlorogenic Acid from Honeysuckle by Response Surface Methodology
Abstract
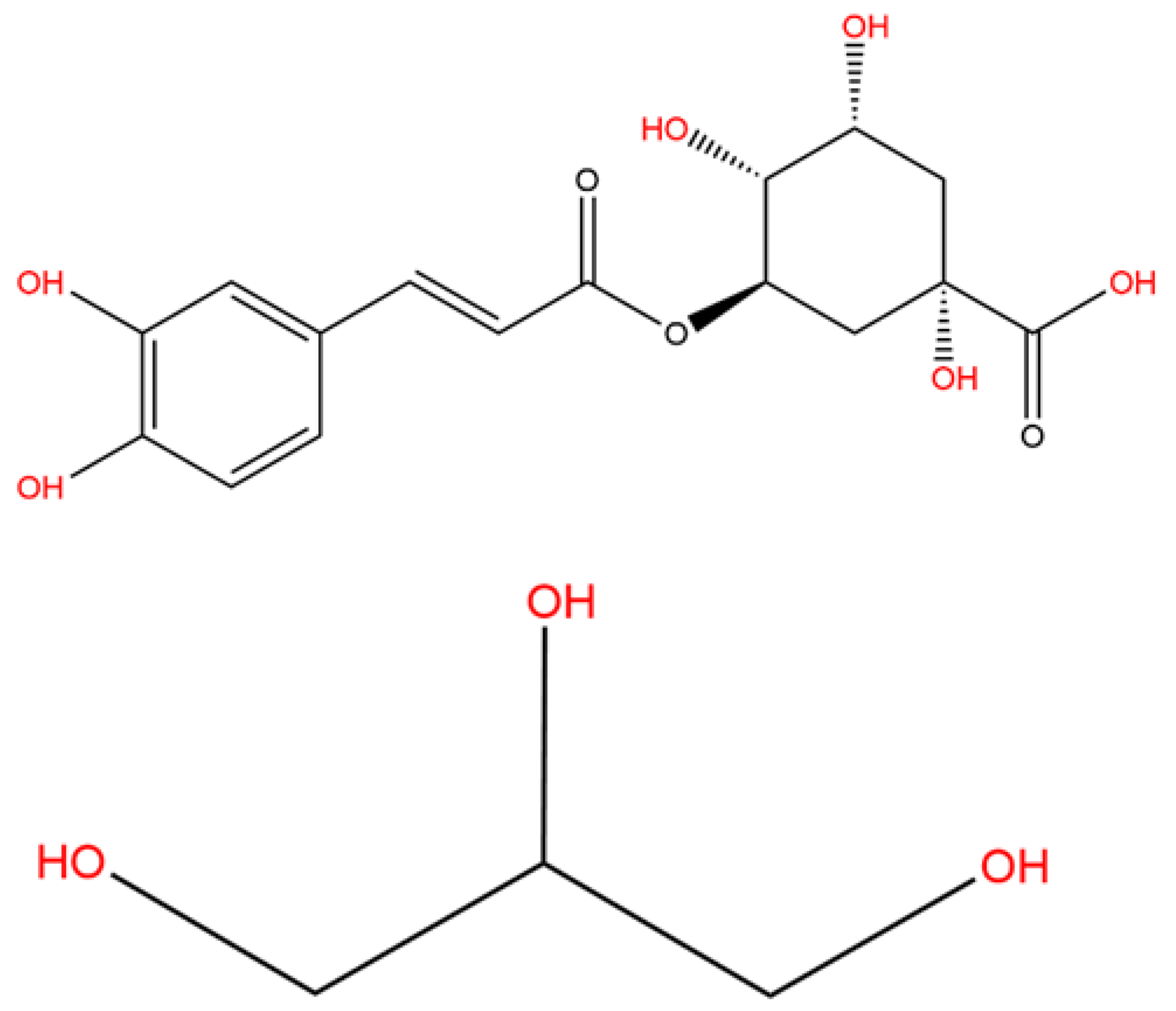
1. Introduction
2. Experimental
Reagents and Materials
3. Results and Discussion
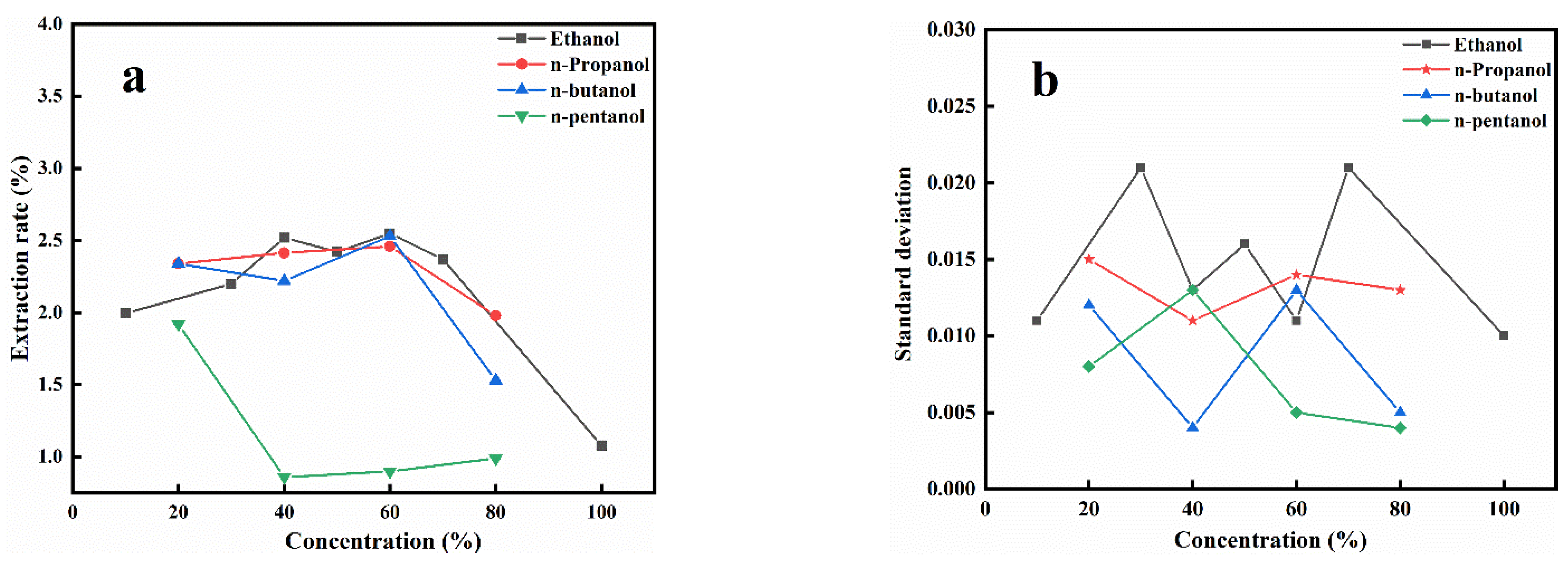
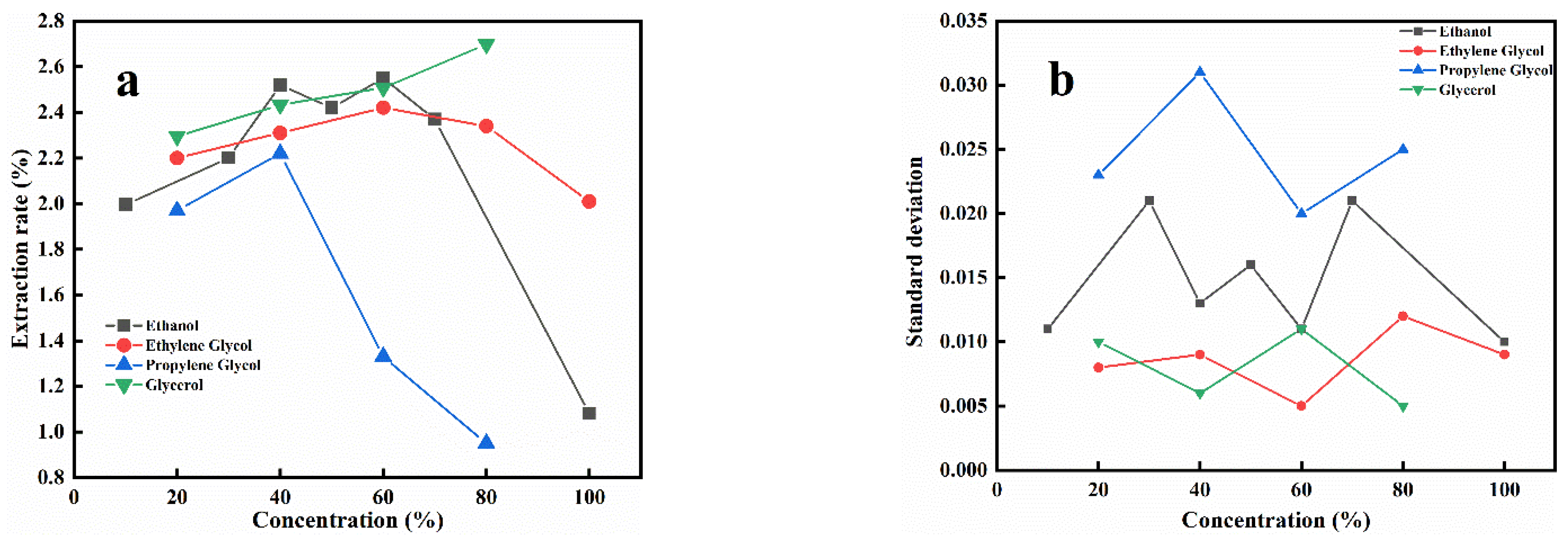
3.1. Extraction Using Different Alcohols
3.1.1. Effects of Alcohol Types
3.1.2. The Effect of Hydroxyl Number
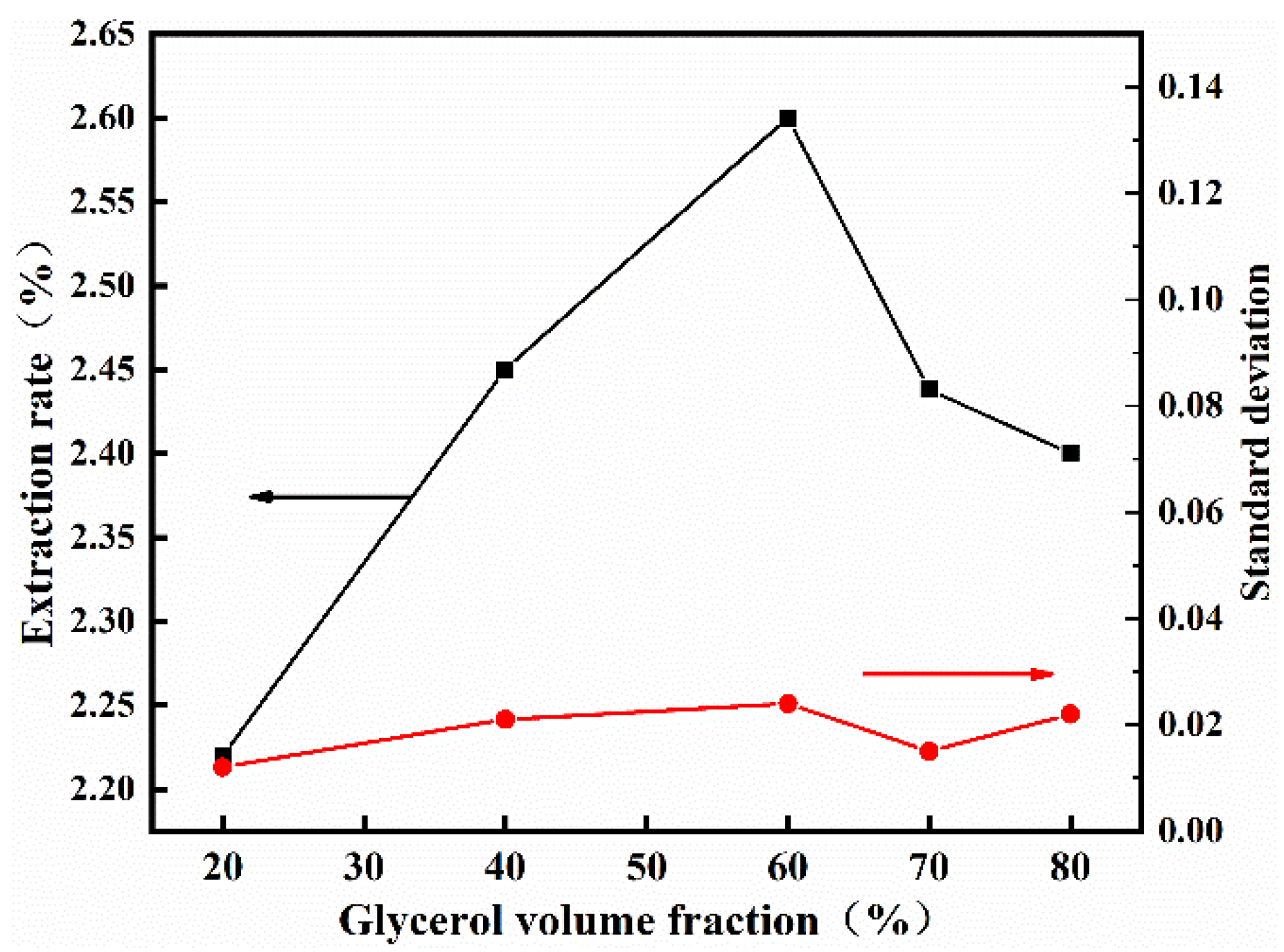
3.2. Single−Factor Experimental Design
3.2.1. Effect of Solid/Liquid Ratio on Extraction Rate
3.2.2. Effect of Glycerol Volume Fraction
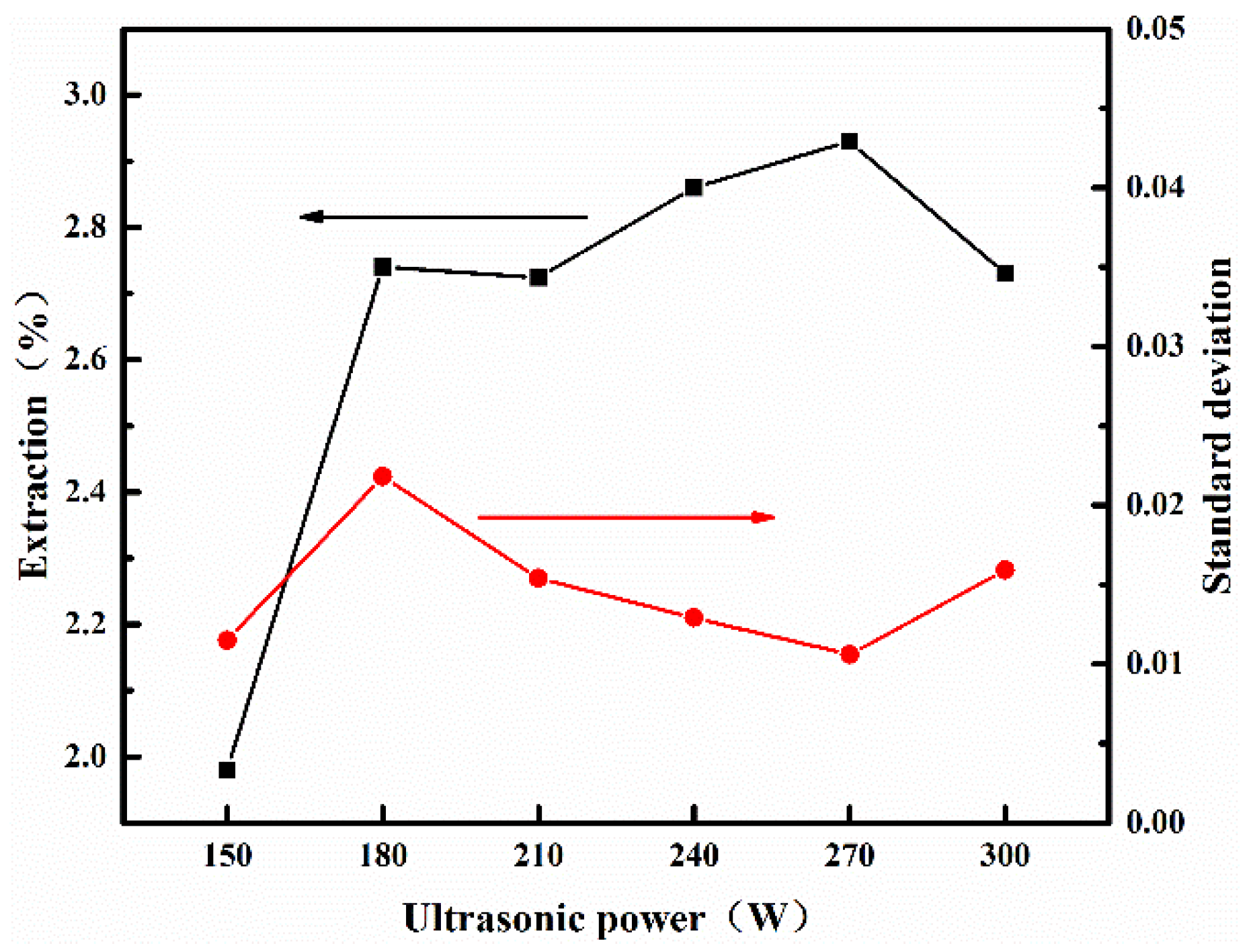
3.2.3. The Effect of Ultrasonic Vibrator
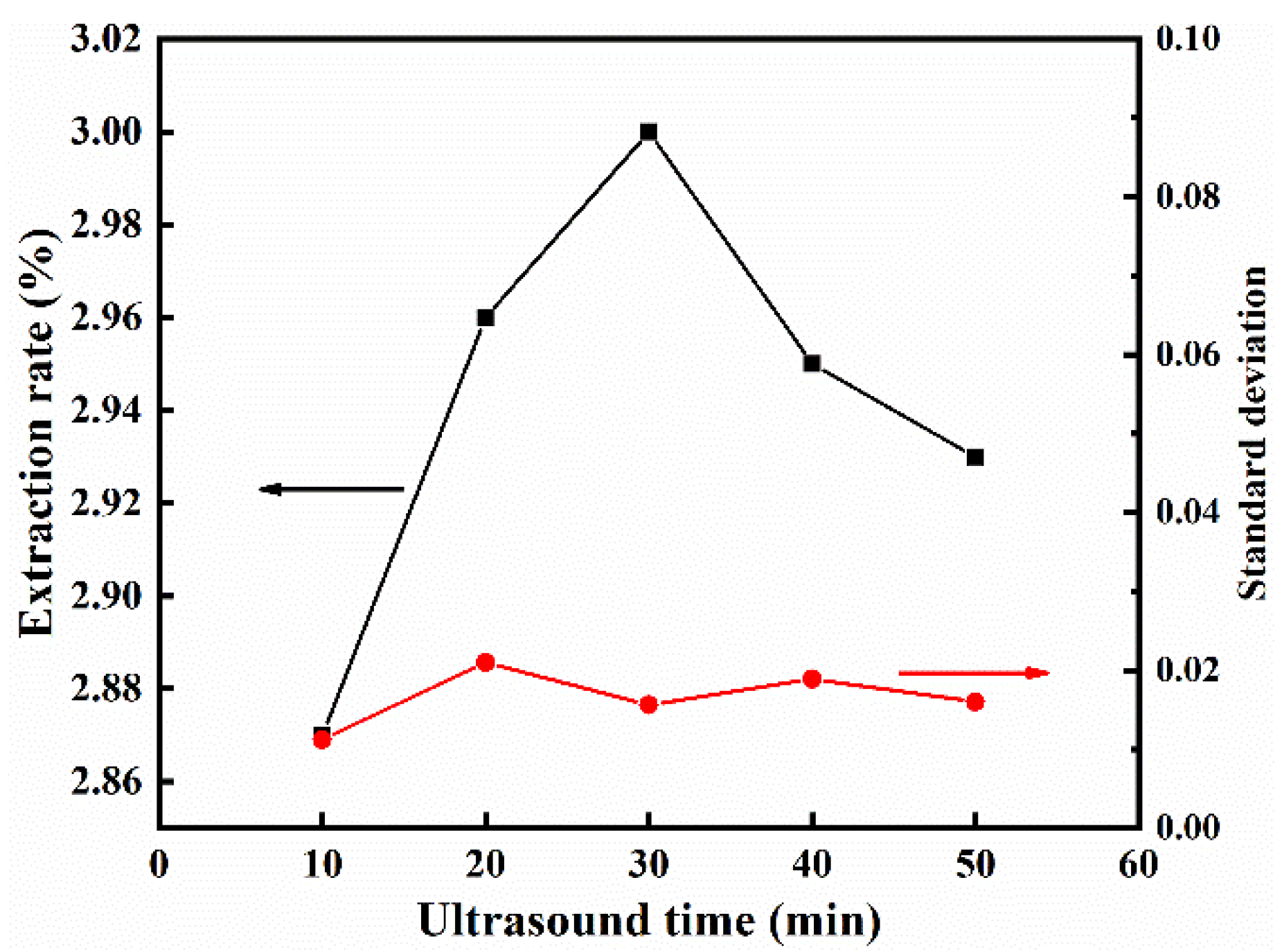
3.2.4. Effect of Ultrasonic Time
3.3. Optimization of the Extraction Parameters by Response Surface Methodology
3.3.1. Response Surface Experimental Design
3.3.2. Model Evaluation
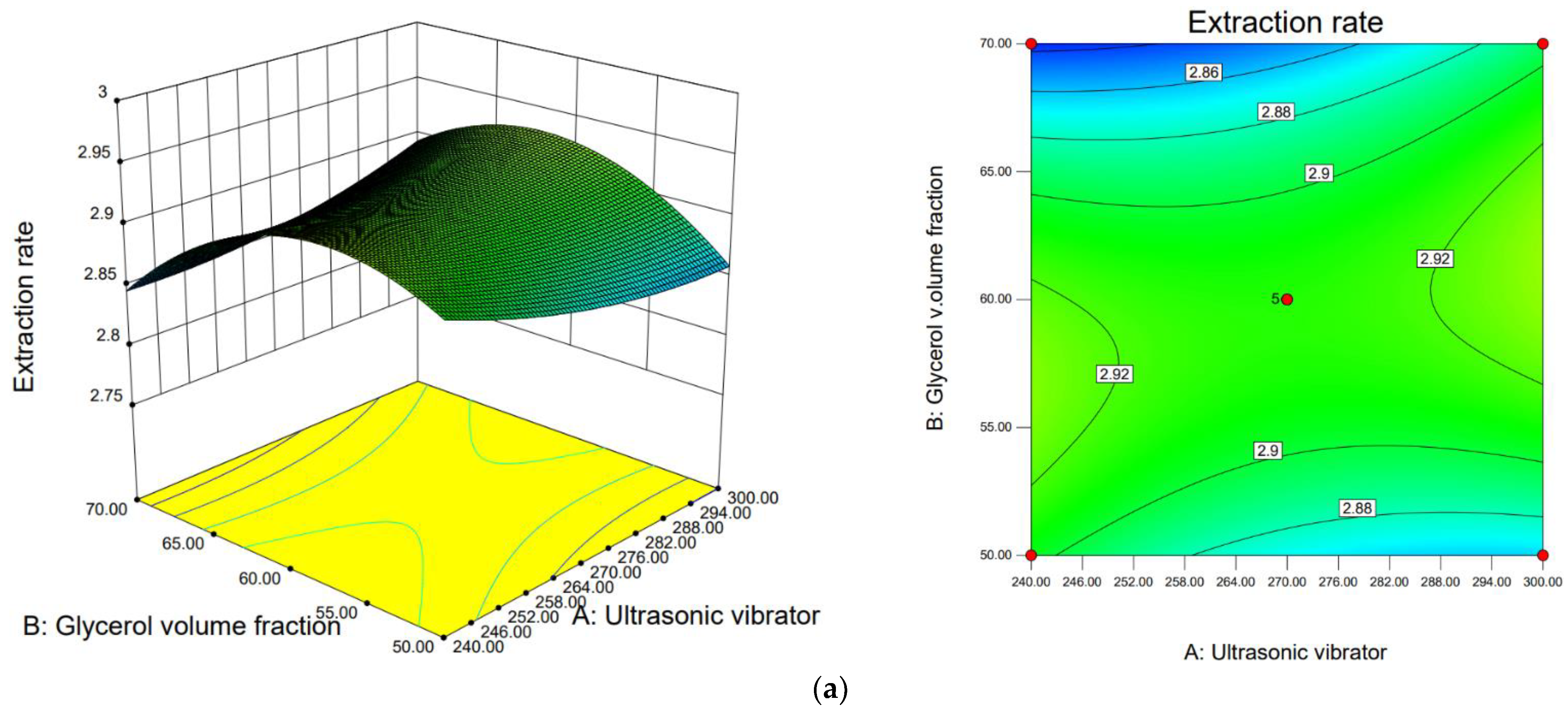
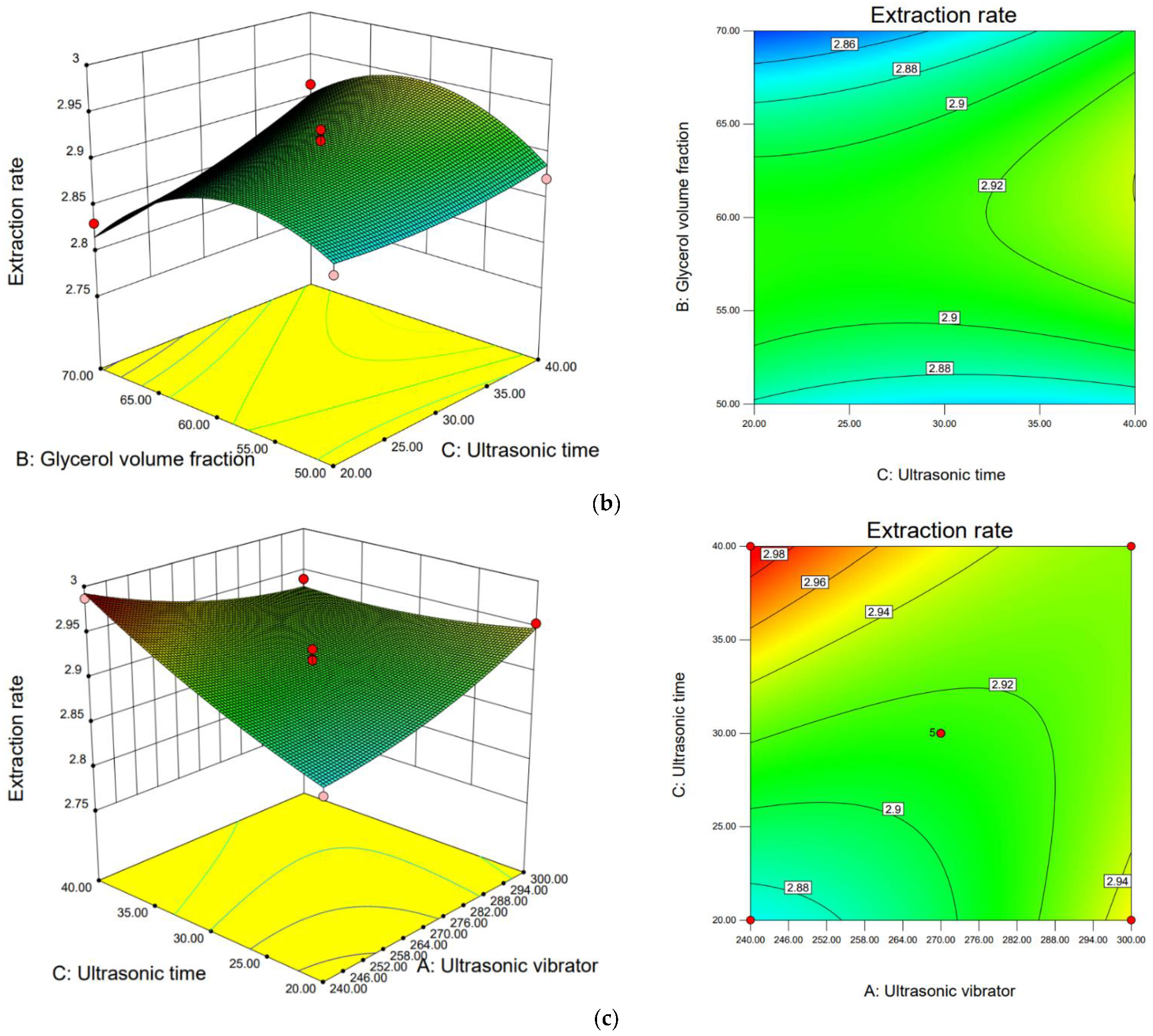
3.3.3. Response Surface Analysis
3.3.4. Verification of Experimental Data with Predicted Modeling Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jurikova, T.; Rop, O.; Mlček, J.; Sochor, J.; Balla, Š.; Szekeres, L.; Hegedȕsová, A.; Hubálek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (Genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.Y.; Song, Z.J.; Ruan, L.J.; Yan, B.X.; Wu, Q.H.; He, L.L.; Wu, Y.Q.; Liu, X.H.; Peng, Y.D.; Miao, J.H. A new methoxylated flavone from Lonicera hypoglauca and its chemotaxonomic significance. Biochem. Syst. Ecol. 2021, 97, 104249. [Google Scholar] [CrossRef]
- Tang, Y.R.; Zeng, T.; Zafar, S.; Yuan, H.W.; Li, B.; Peng, C.Y.; Wang, S.C.; Jian, Y.Q.; Qin, Y.; Choudhary, M.I.; et al. Lonicerae Flos: A review of chemical constituents and biological activities. Digit. Chin. Med. 2018, 1, 173–188. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wei, F.Y.; Wei, Z.F.; Zhang, L.; Luo, M.; Zhang, Y.H.; Zu, Y.G.; Fu, Y.J. Homogenate-assisted negative-pressure cavitation extraction for determination of organic acids and flavonoids in honeysuckle (Lonicera japonica Thunb.) by LC–MS/MS. Sep. Purif. Technol. 2014, 135, 80–87. [Google Scholar] [CrossRef]
- Bao, T.; Karim, N.; Xie, L.H.; Xie, J.H.; Chen, W. Simulated gastrointestinal digestion and colonic fermentation of blue honeysuckle: Phenolic profile and protectivity on ethyl carbamate-induced oxidative damage. Process Biochem. 2022, 120, 74–84. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Health properties and composition of honeysuckle berry Lonicera caerulea L. An update on recent studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, R.X.; Liu, B.G.; Fan, W.X.; Zhang, H. Extracting characteristics of trace elements in honeysuckle flower tea. Agro Food Ind. Hi-Tech. 2008, 19, 53–55. [Google Scholar]
- Zhang, S.B.; Li, J.Y.; Guo, Y.S. Study on determination of metal elements in honeysuckle by Carbon Pick-Up acid dissolvation digestion with ICP-AES. Spectrosc. Spectr. Anal. 2007, 27, 1222–1224. [Google Scholar]
- Visan, D.C.; Radulescu, V.; Duţu, L.E. Volatile constituents from the flowers of two species of honeysuckle (Lonicera japonica and Lonicera caprifolium). Farmacia 2014, 62, 194–201. [Google Scholar]
- Muturi, E.J.; Doll, K.; Berhow, M.; Flor-Weiler, L.B.; Rooney, A.P. Honeysuckle essential oil as a potential source of ecofriendly larvicides for mosquito control. Pest Manag. Sci. 2019, 75, 2043–2048. [Google Scholar] [CrossRef]
- Wu, S.S.; He, X.; Wu, X.S.; Qin, S.; He, J.H.; Zhang, S.R.; Hou, D.X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Liu, M.; Tan, J.; He, Z.; He, X.; Hou, D.X.; He, J.; Wu, S. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice. Anim. Nutr. 2018, 11, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.L.; Li, J.M.; Wan, H.Q.; Zhang, K.D.; Wu, W.D.; Zou, X.T.; Wu, S.P.; Zhou, B.P.; Tian, J.; Zeng, X.B. Novel flavonoids from Lonicera japonica flower buds and validation of their anti-hepatoma and hepatoprotective activity in vitro studies. Ind. Crops Prod. 2018, 125, 114–122. [Google Scholar] [CrossRef]
- Liu, M.Z.; Yu, Q.; Yi, Y.; Xiao, H.H.; Putra, D.F.; Ke, K.; Zhang, Q.; Li, P.F. Antiviral activities of Lonicera japonica Thunb. Components against grouper iridovirus in vitro and in vivo. Aquaculture 2020, 519, 734882. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.H.; Yin, Z.M.; Luo, L. CGA protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Golubev, D.; Zemskaya, N.; Shevchenko, O.; Shaposhnikov, M.; Kukuman, D.; Patov, S.; Punegov, V.; Moskalev, A. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of Drosophila melanogaster. Biogerontology 2022, 23, 215–235. [Google Scholar] [CrossRef]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol and supercritical fluid extraction techniques. Ind. Crops Prod. 2016, 89, 543–549. [Google Scholar] [CrossRef]
- Meng, X.L.; Cao, H.; Li, H.; Li, K.K.; Yang, G.K.; Zhang, Y.M.; Chang, X.L.; Zhang, X.D.; Zhang, J.X. Effect of dietary honeysuckle (Lonicera caerulea L.) supplementation on lipid metabolism, immunity and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquac. Rep. 2022, 23, 101063. [Google Scholar] [CrossRef]
- Feng, J.C.; Chang, X.L.; Zhang, Y.R.; Lu, R.H.; Meng, X.L.; Song, D.Y.; Yan, X.; Zhang, J.X.; Nie, G.X. Characterization of a polysaccharide HP-02 from honeysuckle flowers and its immunoregulatory and anti-Aeromonas hydrophila effects in Cyprinus carpio L. Int. J. Biol. Macromol. 2019, 140, 477–483. [Google Scholar] [CrossRef]
- Li, H.J.; Li, Y.; Luo, C.X.; Liang, X.Y.; Liu, Z.X.; Liu, Y.Z.; Ling, Y.Z. New approach for targeted treatment of mild COVID-19 by honeysuckle through network pharmacology analysis. Comput. Math. Methods Med. 2022, 21, 9604456. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Łyko, L.; Nowak, R. Influence of accelerated solvent extraction conditions on the LC-ESI-MS/MS polyphenolic profile, triterpenoid content, and antioxidant and anti-lipoxygenase activity of Rhododendron luteum sweet leaves. Antioxidants 2022, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Muthukumaran, C.; Suriya, E.; Keerthana, R.M.; Kamatchi, M.; Kumar, N.M.; Anbarasan, T.; Jeyanthi, J. Ultrasound aided extraction of yellow pigment from Tecoma castanifolia floral petals: Optimization by response surface method and evaluation of the antioxidant activity. Ind. Crops Prod. 2019, 130, 467–477. [Google Scholar] [CrossRef]
- Huang, T.Y.; Ho, J.S.; Goh, S.W.; Chong, T.H. Ethanol recovery from dilute aqueous solution by perstraction using supported ionic liquid membrane (SILM). J. Clean. Prod. 2021, 298, 126811. [Google Scholar] [CrossRef]
- Beatriz, M.G.; Sandra, P.M.; Ana, M.G.; David, A.R.; Antonio, S.C. Box-Behnken experimental design for a green extraction method of phenolic compounds from olive leaves. Ind. Crops Prod. 2020, 154, 112741. [Google Scholar]
- Yang, Z.Z.; Tan, Z.J.; Li, F.F.; Li, X.D. An effective method for the extraction and purification of CGA from ramie (Boehmeria nivea L.) leaves using acidic ionic liquids. Ind. Crops Prod. 2016, 89, 78–86. [Google Scholar] [CrossRef]
- Ribeiro, L.F. Differences between isoamyl alcohol and ethanol on the metabolism and DNA ethylation of N-nitrosodiethylamine in the rat. Toxicology 2000, 151, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, E.; Clubbs, E.; Shellhammer, T.H.; Vodovotz, Y. Effect of high-pressure processing and addition of glycerol and salt on the properties of water in corn tortillas. J. Cereal Sci. 2004, 39, 109–117. [Google Scholar] [CrossRef]
- Sripad, G.; Prakash, V.; Rao, M.N. Extractability of polyphenols of sunflower seed in various solvents. J. Biosci. 1982, 4, 145–152. [Google Scholar] [CrossRef]
- Daraee, A.; Ghoreishi, S.M.; Hedayati, A. Supercritical CO2 extraction of CGA from sunflower (Helianthus annuus) seed kernels: Modeling and optimization by response surface methodology. J. Supercrit. Fluids 2019, 144, 19–27. [Google Scholar] [CrossRef]
- Haque, S.K.M. Box–Behnken experimental design for optimizing the HPLC method to determine hydrochlorothiazide in pharmaceutical formulations and biological fluid. J. Mol. Liq. 2022, 352, 118708. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Heidari, E. Extraction of Epigallocatechin-3-gallate from green tea via supercritical fluid technology: Neural network modeling and response surface optimization. J. Supercrit. Fluids 2013, 74, 128–136. [Google Scholar] [CrossRef]

| Raw Materials and Reagents | Manufacturer |
|---|---|
| Honeysuckle pollen | Bozhou Celebration Medicine Hall, Anhui, China |
| CGA standard | Beijing InnoChem Science & Technology Co., Ltd., Beijing, China |
| Anhydrous ethanol (analytical grade) | Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Phosphoric acid (analytical grade) | Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Sodium hydroxide (analytical grade) | Beijing Chemical Works, Beijing, China |
| Acetonitrile (chromatographic grade) | Beijing Mindray Technology Co., Ltd., Beijing, China |
| Distilled water | Watsons Distilled Water Co., Ltd., Beijing, China |
| Chromatographic Conditions | Parameter |
|---|---|
| Waters 2487 UV detector | 2 channel, tunable UV/Vis |
| C18 Column (Kromasil 100–5 4.6 mm × 250 mm) | AkzoNobel Sweden |
| Mobile phase | V(acetonitrile): V (0.4% phosphoric acid) = 1:9 |
| Detection wavelength | 327 nm |
| Column temperature | 25 °C |
| Flow rate | 1 mL/min |
| Injection volume | 5 μL |
| Level | A Ultrasonic Vibrator (W) | B Glycerol Volume Fraction (%) | C Ultrasound Time (min) |
|---|---|---|---|
| −1 | 240 | 50 | 20 |
| 0 | 270 | 60 | 30 |
| 1 | 300 | 70 | 40 |
| Test Number | A Ultrasonic Vibrator (W) | B Glycerol Volume Fraction (%) | C Ultrasound Time (min) | Extraction Rate (%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 2.925 |
| 2 | 1 | −1 | 0 | 2.869 |
| 3 | −1 | 1 | 0 | 2.830 |
| 4 | 1 | 1 | 0 | 2.872 |
| 5 | −1 | 0 | −1 | 2.862 |
| 6 | 1 | 0 | −1 | 2.954 |
| 7 | −1 | 0 | 1 | 2.987 |
| 8 | 1 | 0 | 1 | 2.940 |
| 9 | 0 | −1 | −1 | 2.860 |
| 10 | 0 | 1 | −1 | 2.831 |
| 11 | 0 | −1 | 1 | 2.872 |
| 12 | 0 | 1 | 1 | 2.915 |
| 13 | 0 | 0 | 0 | 2.904 |
| 14 | 0 | 0 | 0 | 2.918 |
| 15 | 0 | 0 | 0 | 2.917 |
| 16 | 0 | 0 | 0 | 2.929 |
| 17 | 0 | 0 | 0 | 2.900 |
| Source of Variance | Sum of Square | Degrees of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 2.700 × 10−2 | 9 | 3.022 × 10−3 | 8.79 | 0.0046 |
| A | 1.178 × 10−4 | 1 | 1.178 × 10−4 | 0.34 | 0.5767 |
| B | 7.605 × 10−4 | 1 | 7.605 × 10−4 | 2.21 | 0.1806 |
| C | 5.341 × 10−3 | 1 | 5.341 × 10−3 | 15.53 | 0.0056 |
| AB | 2.401 × 10−3 | 1 | 2.401 × 10−3 | 6.98 | 0.0333 |
| AC | 4.851 × 10−3 | 1 | 4.851 × 10−3 | 14.11 | 0.0071 |
| BC | 1.296 × 10−3 | 1 | 1.296 × 10−3 | 3.77 | 0.0934 |
| A2 | 7.434 × 10−4 | 1 | 7.434 × 10−4 | 2.16 | 0.1850 |
| B2 | 1.2 × 10−2 | 1 | 1.200 × 10−2 | 34.24 | 0.0006 |
| C2 | 3.251 × 10−4 | 1 | 3.251 × 10−4 | 0.95 | 0.3633 |
| Residual | 2.407 × 10−3 | 7 | 3.439 × 10−4 | ||
| Lack of fit | 1.862 × 10−3 | 3 | 6.207 × 10−4 | 4.55 | 0.0886 |
| Pure error | 5.452 × 10−4 | 4 | 1.363 × 10−4 | ||
| Sum | 3.000 × 10−2 | 16 |
| Extraction Cycles | CGA Extraction Rate/% | Average Extraction Yield/% |
|---|---|---|
| 1 | 2.99 | 2.98 |
| 2 | 2.98 | |
| 3 | 2.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Liu, X.; Zhao, Z.; Wang, F.; Shao, C. Optimization of Glycerol Extraction of Chlorogenic Acid from Honeysuckle by Response Surface Methodology. Processes 2023, 11, 110. https://doi.org/10.3390/pr11010110
Luo M, Liu X, Zhao Z, Wang F, Shao C. Optimization of Glycerol Extraction of Chlorogenic Acid from Honeysuckle by Response Surface Methodology. Processes. 2023; 11(1):110. https://doi.org/10.3390/pr11010110
Chicago/Turabian StyleLuo, Mingsheng, Xinyue Liu, Zhijun Zhao, Fengli Wang, and Changke Shao. 2023. "Optimization of Glycerol Extraction of Chlorogenic Acid from Honeysuckle by Response Surface Methodology" Processes 11, no. 1: 110. https://doi.org/10.3390/pr11010110
APA StyleLuo, M., Liu, X., Zhao, Z., Wang, F., & Shao, C. (2023). Optimization of Glycerol Extraction of Chlorogenic Acid from Honeysuckle by Response Surface Methodology. Processes, 11(1), 110. https://doi.org/10.3390/pr11010110