Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
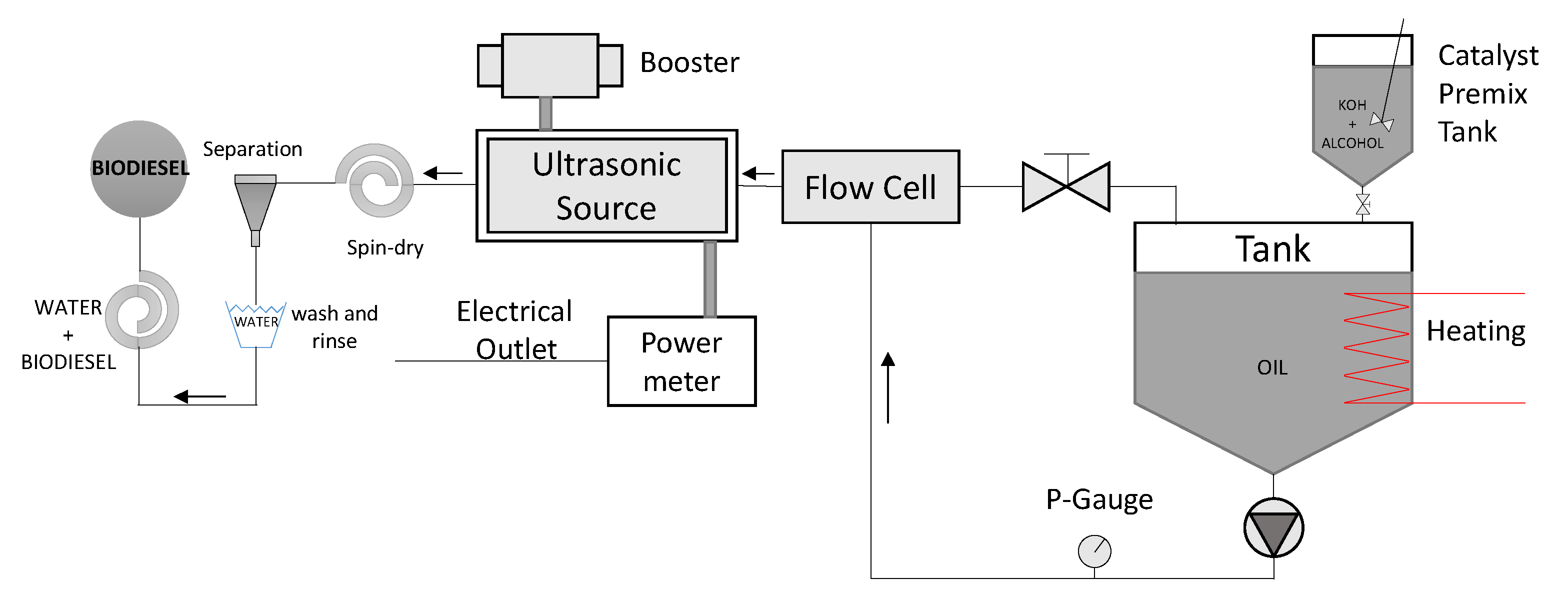
2.2. Biodiesel Production
2.3. Physicochemical Analysis
2.4. Electrochemical Tests
Electrochemical Techniques
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Electrochemical Characterization
3.2.1. Open Circuit Potential
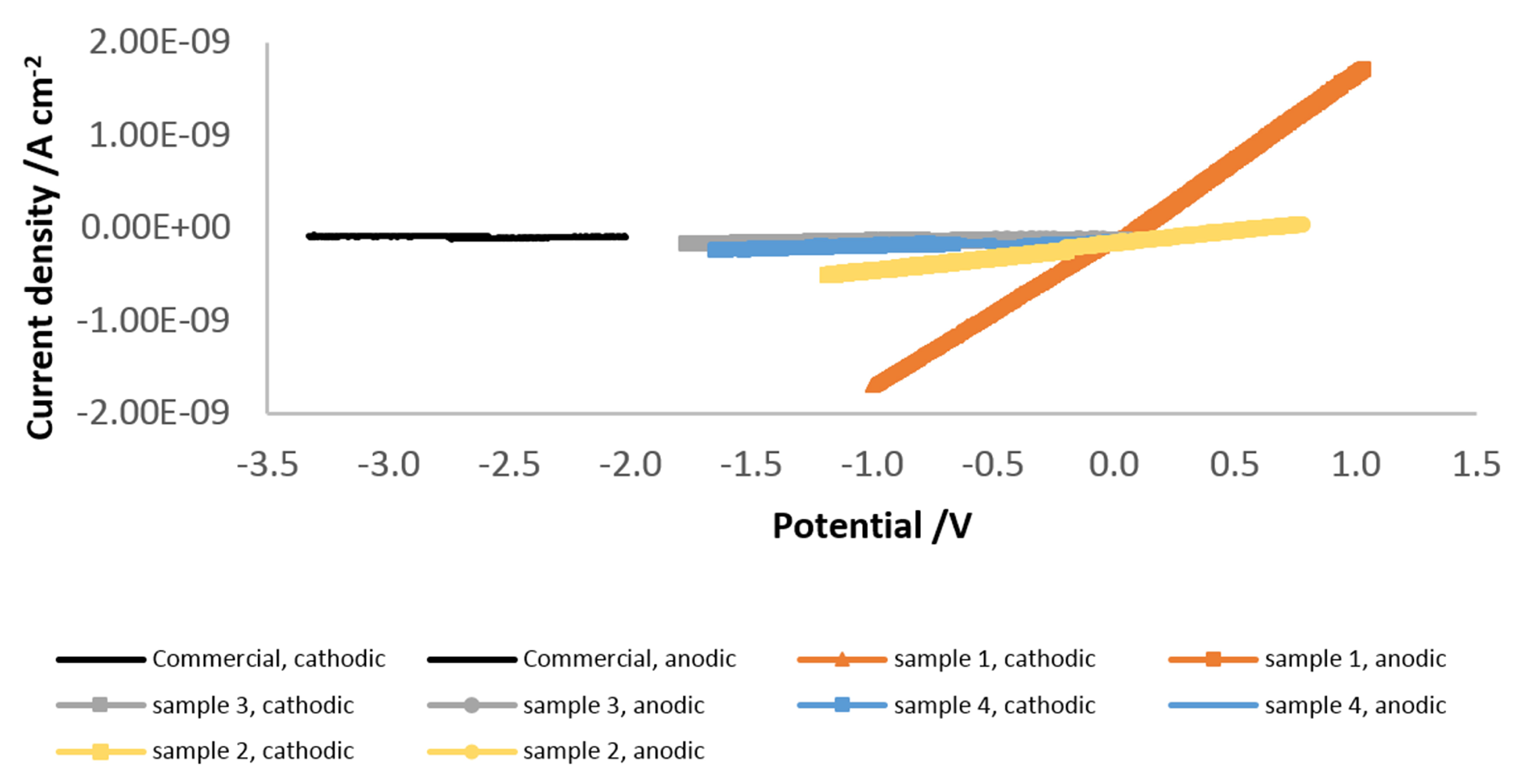
3.2.2. Linear Sweep Voltammetry
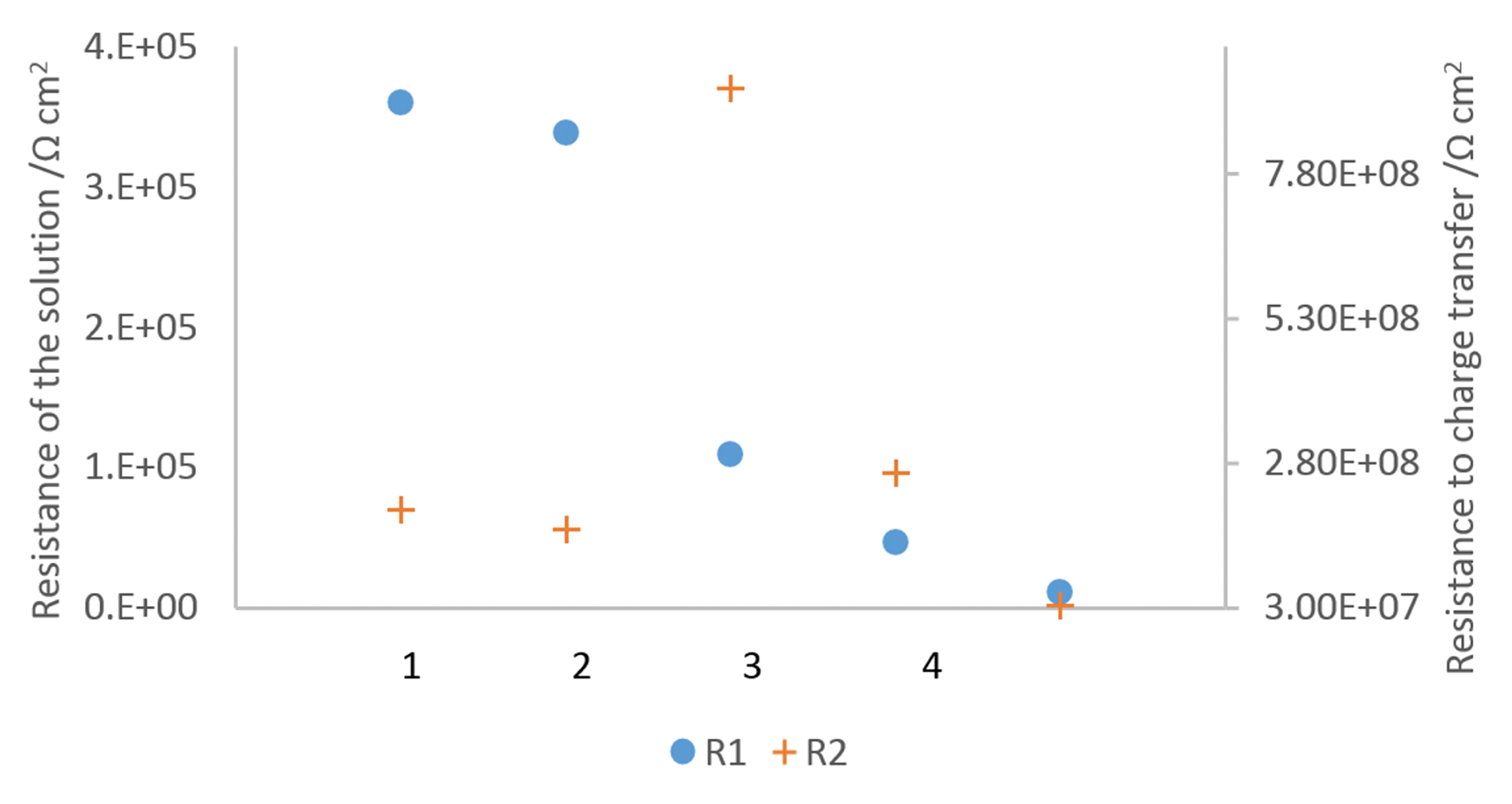
3.2.3. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del Bano, J.M.; Chica, A.; Quesada-Medina, J. Waste animal fats as feedstock for biodiesel production using non-catalytic supercritical alcohol transesterification: A perspective by the PRISMA methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.; Zularisam, A.; Sirohi, R.; Khilji, I.A.; Reddy, V.J.; Pandey, A. Bioconversion of glycerol into biofuels—Opportunities and challenges. BioEnergy Res. 2022, 15, 46–61. [Google Scholar] [CrossRef]
- Yuhendra, A.; Farghali, M.; Mohamed, I.M.; Iwasaki, M.; Tangtaweewipat, S.; Ihara, I.; Sakai, R.; Umetsu, K. Potential of biogas production from the anaerobic digestion of Sargassum fulvellum macroalgae: Influences of mechanical, chemical, and biological pretreatments. Biochem. Eng. J. 2021, 175, 108140. [Google Scholar]
- Ferreira, T.F.; Santos, P.A.; Paula, A.V.; de Castro, H.F.; Andrade, G.S. Biogas generation by hybrid treatment of dairy wastewater with lipolytic whole cell preparations and anaerobic sludge. Biochem. Eng. J. 2021, 169, 107965. [Google Scholar] [CrossRef]
- Rincón-Pérez, J.; Celis, L.B.; Morales, M.; Alatriste-Mondragón, F.; Tapia-Rodríguez, A.; Razo-Flores, E. Improvement of methane production at alkaline and neutral pH from anaerobic co-digestion of microalgal biomass and cheese whey. Biochem. Eng. J. 2021, 169, 107972. [Google Scholar] [CrossRef]
- Ma’arof, N.A.N.B.; Hindryawati, N.; Khazaai, S.N.M.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Biodiesel (Methyl Esters). Maejo Int. J. Energy Environ. Commun. 2021, 3, 30–43. [Google Scholar]
- Soudagar, M.E.M.; Khan, H.M.; Khan, T.Y.; Razzaq, L.; Asif, T.; Mujtaba, M.; Hussain, A.; Farooq, M.; Ahmed, W.; Shahapurkar, K.; et al. Experimental analysis of engine performance and exhaust pollutant on a single-cylinder diesel engine operated using moringa oleifera biodiesel. Appl. Sci. 2021, 11, 7071. [Google Scholar] [CrossRef]
- Embong, N.H.; Hindryawati, N.; Bhuyar, P.; Govindan, N.; Rahim, M.H.A.; Maniam, G.P. Enhanced biodiesel production via esterification of palm fatty acid distillate (PFAD) using rice husk ash (NiSO4)/SiO2 catalyst. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Bhuyar, P.; Sundararaju, S.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. Enhanced productivity of lipid extraction by urea stress conditions on marine microalgae Coelastrum sp. for improved biodiesel production. Bioresour. Technol. Rep. 2021, 15, 100696. [Google Scholar] [CrossRef]
- Ennetta, R.; Soyhan, H.S.; Koyunoğlu, C.; Demir, V.G. Current Technologies and Future Trends for Biodiesel Production: A Review. Arab. J. Sci. Eng. 2022, 47, 15133–15151. [Google Scholar] [CrossRef]
- Bui, H.N.; Do, H.Q.; Duong, H.T.G.; Perng, Y.S.; Dam, V.N.; Nguyen, V.T.; Bui, H.M. Taguchi optimization and life cycle assessment of biodiesel production from spent ground coffee. Environ. Dev. Sustain. 2022, 24, 12900–12916. [Google Scholar] [CrossRef]
- Fan, L.; Liu, B.; Liu, X.; Senthilkumar, N.; Wang, G.; Wen, Z. Recent progress in electrocatalytic glycerol oxidation. Energy Technol. 2021, 9, 2000804. [Google Scholar] [CrossRef]
- Kookos, I. Technoeconomic and environmental assessment of a process for biodiesel production from spent coffee grounds (SCGs). Resour. Conserv. Recycl. 2018, 134, 156–164. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdullah, A.; Patle, D.S.; Shahadat, M.; Ahmad, Z.; Athar, M.; Aslam, M.; Vo, D.V.N. Feedstocks, catalysts, process variables and techniques for biodiesel production by one-pot extraction-transesterification: A review. Environ. Chem. Lett. 2021, 20, 335–378. [Google Scholar] [CrossRef]
- Nguyen, H.U.D.; Nguyen, D.T.; Taguchi, K. A compact, membrane-less, easy-to-use soil microbial fuel cell: Generating electricity from household rice washing wastewater. Biochem. Eng. J. 2022, 179, 108338. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, J.; Yang, L.; Zhang, Z.; Li, B.; Xia, L.; Wu, L. Microalgae fuel cells enhanced biodegradation of imidacloprid by Chlorella sp. Biochem. Eng. J. 2022, 179, 108327. [Google Scholar] [CrossRef]
- Chaitanya Reddy, C.; Sakinah, A.M.; Zularisam, A. Opportunities of biodiesel industry waste conversion into value-added products. Mater. Today: Proc. 2022, 57, 1014–1020. [Google Scholar]
- Pêgas, M.M.; Amado, R.S.; de Castro, E.V.; D’Elia, E. Analysis of free glycerol in biodiesel using an electrochemical assay based on a two-enzyme platinum microelectrode system. J. Appl. Electrochem. 2010, 40, 2061–2063. [Google Scholar] [CrossRef]
- Delfino, J.R.; Pereira, T.C.; Viegas, H.D.C.; Marques, E.P.; Ferreira, A.A.P.; Zhang, L.; Zhang, J.; Marques, A.L.B. A simple and fast method to determine water content in biodiesel by electrochemical impedance spectroscopy. Talanta 2018, 179, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Castellon, J.; Mercader-Trejo, F.; Álvarez-López, A.; Larios-Durán, E.; Antaño-López, R.; Herrera-Basurto, R.; Chacón-López, A.; Rodríguez-López, A.; López-García, U. Electrochemical characterization of Biodiesel by linear voltammetry and electrochemical impedance spectroscopy. Int. J. Electrochem. Sci 2018, 13, 5452–5459. [Google Scholar] [CrossRef]
- Biernat, K.; Bocian, P.; Bukrejewski, P.; Noworyta, K.R. Application of the impedance spectroscopy as a new tool for studying biodiesel fuel aging processes. Energies 2019, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Nunes Angelis, P.; de Cássia Mendonça, J.; Rianne da Rocha, L.; Boareto Capelari, T.; Carolyne Prete, M.; Gava Segatelli, M.; Borsato, D.; Ricardo Teixeira Tarley, C. Feasibility of a Nano-Carbon Black Paste Electrode for Simultaneous Voltammetric Determination of Antioxidants in Food Samples and Biodiesel in the Presence of Surfactant. Electroanalysis 2020, 32, 1198–1207. [Google Scholar] [CrossRef]
- Hoffmann da Rocha, A.A.; Casagrande, M.; de Souza Schaumlöffel, L.; da Silva, Y.P.; Sartori Piatnicki, C.M. Simultaneous voltammetric determination of tert-butylhydroquinone and propyl gallate in biodiesel–Ethanol at a Pt ultramicroelectrode. Energy Fuels 2017, 31, 7076–7081. [Google Scholar] [CrossRef]
- Freitas, T.A.; Lima, M.J.; Marques, A.L.; Marques, E.P.; Luz, R.; Bezerra, C.W. Development of a novel and simple electroanalytical procedure for the determination of copper in biofuel employing a sensor based on vulcan functionalized with carbazone. J. Braz. Chem. Soc. 2018, 29, 671–679. [Google Scholar] [CrossRef]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K.; Murugesan, T.; Arunagiri, A. Production of biodiesel from waste bio-oil through ultrasound assisted transesterification using immobilized lipase. Environ. Technol. Innov. 2021, 21, 101199. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Reina, C.B.; Jiménez, L.N.R.; Pedraza, N.M. Obtención de biodiesel (etil-éster) mediante catálisis básica a nivel planta piloto derivado de aceites usados de la industria alimenticia. Publicaciones E Investig. 2014, 8, 99–116. [Google Scholar] [CrossRef] [Green Version]
- Man, X.; Cheung, C.S.; Ning, Z. Effect of diesel engine operating conditions on the particulate size, nanostructure and oxidation properties when using wasting cooking oil biodiesel. Energy Procedia 2015, 66, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Singh, C. Fast, easy ethanolysis of coconut oil for biodiesel production assisted by ultrasonication. Ultrason. Sonochem. 2010, 17, 555–559. [Google Scholar] [CrossRef]
- Zabot, G.L.; Viganó, J.; Silva, E.K. Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds. Molecules 2021, 26, 5117. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, I.; Zbancioc, G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules 2021, 26, 5098. [Google Scholar] [CrossRef]
- Parida, S. Improving heterogeneously catalyzed transesterification reaction for biodiesel production using ultrasound energy and petro-diesel as cosolvent. Energy Sources Part A: Recover. Util. Environ. Eff. 2021, 1–13. [Google Scholar] [CrossRef]
- Cataldo, A.; Piuzzi, E.; Cannazza, G.; De Benedetto, E. Classification and adulteration control of vegetable oils based on microwave reflectometry analysis. J. Food Eng. 2012, 112, 338–345. [Google Scholar] [CrossRef]
- Prevc, T.; Cigić, B.; Vidrih, R.; Poklar Ulrih, N.; Šegatin, N. Correlation of basic oil quality indices and electrical properties of model vegetable oil systems. J. Agric. Food Chem. 2013, 61, 11355–11362. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef] [Green Version]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.A.; Soudagar, M.E.M.; Fattah, I.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Sangarunlert, W.; Sukchai, S.; Pongtornkulpanich, A.; Nathakaranakule, A.; Luschtinetz, T. Technical and economic evaluation of a formic acid/hydrogen peroxide fuel cell system with Pt-M/C as anode catalyst. J. Fuel Cell Sci. Technol. 2011, 8, 061005. [Google Scholar] [CrossRef]
- Ha, M.G.; Na, Y.; Park, H.Y.; Kim, H.J.; Song, J.; Yoo, S.J.; Kim, Y.T.; Park, H.S.; Jang, J.H. Combined Effect of Catholyte Gap and Cell Voltage on Syngas Ratio in Continuous CO 2/H 2 O Co-electrolysis. J. Electrochem. Sci. Technol. 2021, 12, 406–414. [Google Scholar] [CrossRef]
- Garcia-Garcia, R.; Rivera, J.; Antaño-Lopez, R.; Castañeda-Olivares, F.; Orozco, G. Impedance spectra of the cathodic hydrogen evolution reaction on polycrystalline rhenium. Int. J. Hydrog. Energy 2016, 41, 4660–4669. [Google Scholar] [CrossRef]
- Piela, P.; Mitzel, J.; Rosini, S.; Tokarz, W.; Valle, F.; Pilenga, A.; Malkow, T.; Tsotridis, G. Looking Inside Polymer Electrolyte Membrane Fuel Cell Stack Using Tailored Electrochemical Methods. J. Electrochem. Energy Convers. Storage 2020, 17, 031018. [Google Scholar] [CrossRef]
- Rivera, J.; Garcia-Garcia, R.; Coutino-Gonzalez, E.; Orozco, G. Electrochemical study in acid aqueous solution and ex-situ X-ray photoelectron spectroscopy analysis of metallic rhenium surface. J. Electroanal. Chem. 2021, 893, 115297. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Pukale, D.D.; Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B.; Pratap, A.P. Ultrasound assisted transesterification of waste cooking oil using heterogeneous solid catalyst. Ultrason. Sonochem. 2015, 22, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Martınez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochem. 2010, 17, 827–832. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Angkawijaya, A.E.; Ju, Y.H.; Tran-Nguyen, P.L. A one-pot synthesis of biodiesel from leather tanning waste using supercritical ethanol: Process optimization. Biomass Bioenergy 2020, 142, 105761. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Lastras, M.; Rodríguez, F.; Cano, E.; Piquero-Cilla, J.; Osete-Cortina, L. Monitoring stabilizing procedures of archaeological iron using electrochemical impedance spectroscopy. J. Solid State Electrochem. 2014, 18, 399–409. [Google Scholar] [CrossRef]
- Rocabruno-Valdes, C.; Hernandez, J.; Munoz-Ledo, R.; Salinas-Bravo, V.; Lucio-Garcia, M.; Lopez-Sesenes, R.; Porcayo-Calderon, J.; González-Rodriguez, J. Corrosion behavior of metallic materials in chicken fat-based biodiesel. Int. J. Electrochem. Sci. 2020, 15, 334–349. [Google Scholar] [CrossRef]
- Muzaffar, A.; Muthusamy, K.; Basheer Ahamed, M. Ferrous nitrate–nickel oxide (Fe (NO3) 2–NiO) nanospheres incorporated with carbon black and polyvinylidenefluoride for supercapacitor applications. J. Electrochem. Energy Convers. Storage 2019, 16, 031008. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.; Apetrei, C.; De Saja, J. Evaluation of the polyphenolic content of extra virgin olive oils using an array of voltammetric sensors. Electrochim. Acta 2008, 53, 5867–5872. [Google Scholar] [CrossRef]
- Gambarra-Neto, F.F.; Marino, G.; Araújo, M.C.U.; Galvão, R.K.H.; Pontes, M.J.C.; de Medeiros, E.P.; Lima, R.S. Classification of edible vegetable oils using square wave voltammetry with multivariate data analysis. Talanta 2009, 77, 1660–1666. [Google Scholar] [CrossRef]
- Santos-Neto, I.S.d.; Carvalho, C.D.; Filho, G.B.A.; Andrade, C.D.S.S.; Santos, G.C.d.O.; Barros, A.K.; Neto, J.V.d.F.; Casas, V.L.P.; Alencar, L.M.R.; Lopes, A.J.O.; et al. Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends. Sensors 2021, 21, 7288. [Google Scholar] [CrossRef]


| S. num = Sample Number | t = Time | BD = Biodiesel Density | KV = Kinematic Viscosity | AI = Acidity Index | X = Conversion of Triglycerides | POY = Percentage of Oil Yield |
|---|---|---|---|---|---|---|
| 15 C (g cm) | 40 C (mm s) | (mg KOH g) | (wt.%) | (wt.%) | ||
| 1 | 15 | 0.91 ± 0.01 | 4.92 ± 0.12 | 0.42 ± 0.05 | 97.79 ± 0.05 | 63.04 ± 0.25 |
| 2 | 30 | 0.88 ± 0.02 | 4.64 ± 0.11 | 0.40 ± 0.02 | 98.96 ± 0.04 | 65.32 ± 0.35 |
| 3 | 45 | 0.87 ± 0.02 | 4.49 ± 0.10 | 0.37 ± 0.03 | 99.12 ± 0.03 | 66.42 ± 0.73 |
| 4 | 60 | 0.86 ± 0.02 | 4.285 ± 0.09 | 0.35 ± 0.03 | 99.23 ± 0.03 | 68.03 ± 0.49 |
| S. num = Sample Number | t = Time | BD = Biodiesel Density | KV = Kinematic Viscosity | AI = Acidity Index | X = Conversion of Triglycerides | POY = Percentage of Oil Yield |
|---|---|---|---|---|---|---|
| 15 C (g cm) | 40 C (mm s) | (mg KOH g) | (wt.%) | (wt.%) | ||
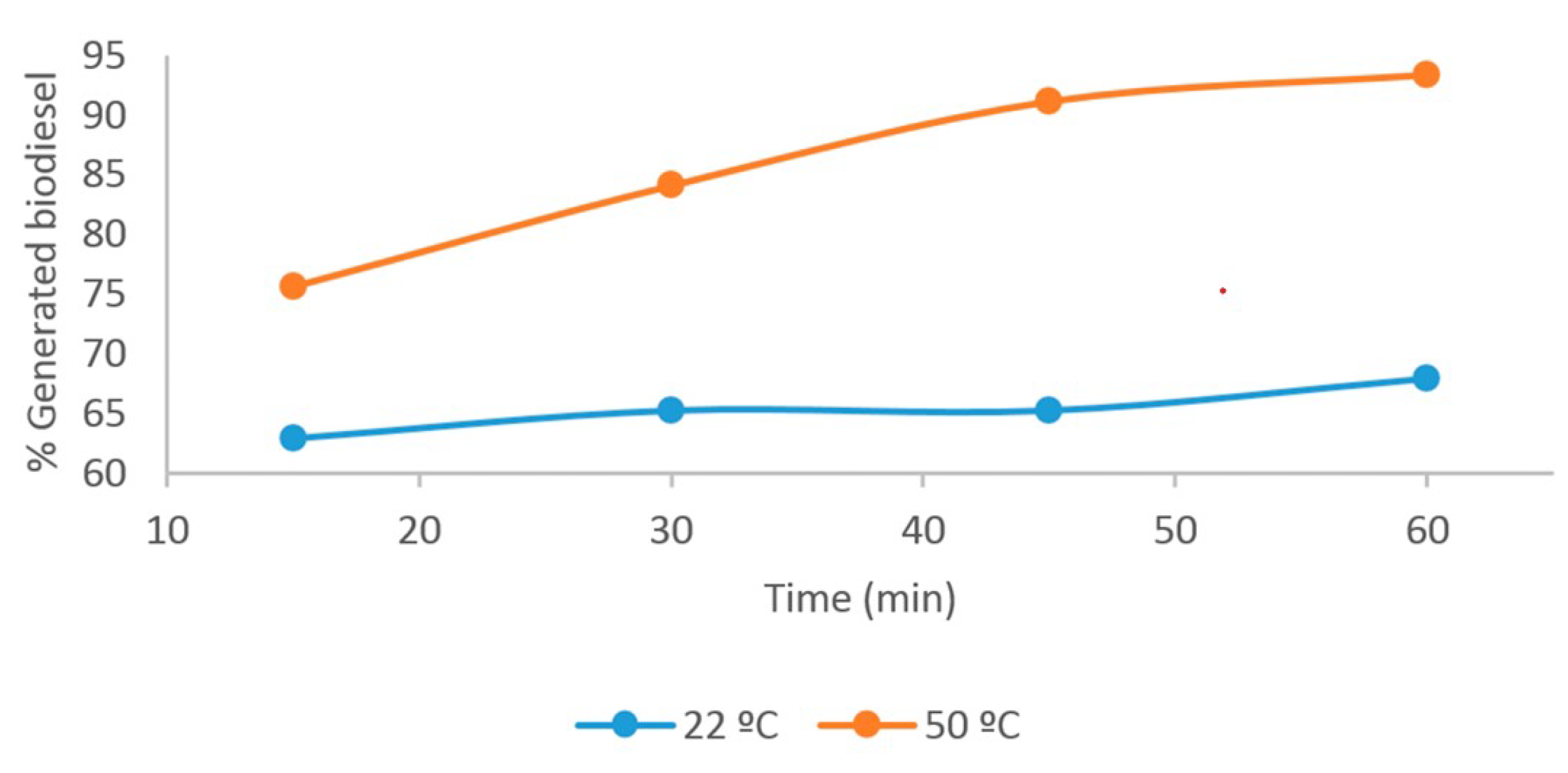
| 1 | 15 | 0.89 ± 0.01 | 4.17 ± 0.11 | 0.22 ± 0.05 | 99.519 ± 0.04 | 75.70 ± 0.71 |
| 2 | 30 | 0.87 ± 0.02 | 4.04 ± 0.08 | 0.17 ± 0.03 | 99.61 ± 0.04 | 84.14 ± 0.65 |
| 3 | 45 | 0.86 ± 0.02 | 3.89 ± 0.08 | 0.16 ± 0.03 | 99.67 ± 0.02 | 91.17 ± 0.38 |
| 4 | 60 | 0.85 ± 0.02 | 3.79 ± 0.08 | 0.15 ± 0.05 | 99.79 ± 0.01 | 93.40 ± 0.09 |
| Vegetable Oil | Ultrasonic Power (W) | Frequency (kHz) | Ultrasound Time (min) | Temperature in C | Molar Ratio Methanol/Oil | KOH Catalyst (wt.%) | Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Waste cooking | 200 | 20 | 40 | 45 | 06-ene | 1 | 89.5 | [49] |
| Sunflower | 200 | 24 | 60 | 50 | 06-ene | 1 | 99.79 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital-López, L.; Mercader-Trejo, F.; Rodríguez-Reséndiz, J.; Zamora-Antuñano, M.A.; Rodríguez-López, A.; Esquerre-Verastegui, J.E.; Farrera Vázquez, N.; García-García, R. Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound. Processes 2023, 11, 94. https://doi.org/10.3390/pr11010094
Vital-López L, Mercader-Trejo F, Rodríguez-Reséndiz J, Zamora-Antuñano MA, Rodríguez-López A, Esquerre-Verastegui JE, Farrera Vázquez N, García-García R. Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound. Processes. 2023; 11(1):94. https://doi.org/10.3390/pr11010094
Chicago/Turabian StyleVital-López, Lourdes, Flora Mercader-Trejo, Juvenal Rodríguez-Reséndiz, Marco Antonio Zamora-Antuñano, Aarón Rodríguez-López, Jorge Eduardo Esquerre-Verastegui, Neín Farrera Vázquez, and Raul García-García. 2023. "Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound" Processes 11, no. 1: 94. https://doi.org/10.3390/pr11010094








