Adsorption–Desorption Process to Separate Dyes from Tanning Wastewaters
Abstract
:1. Introduction
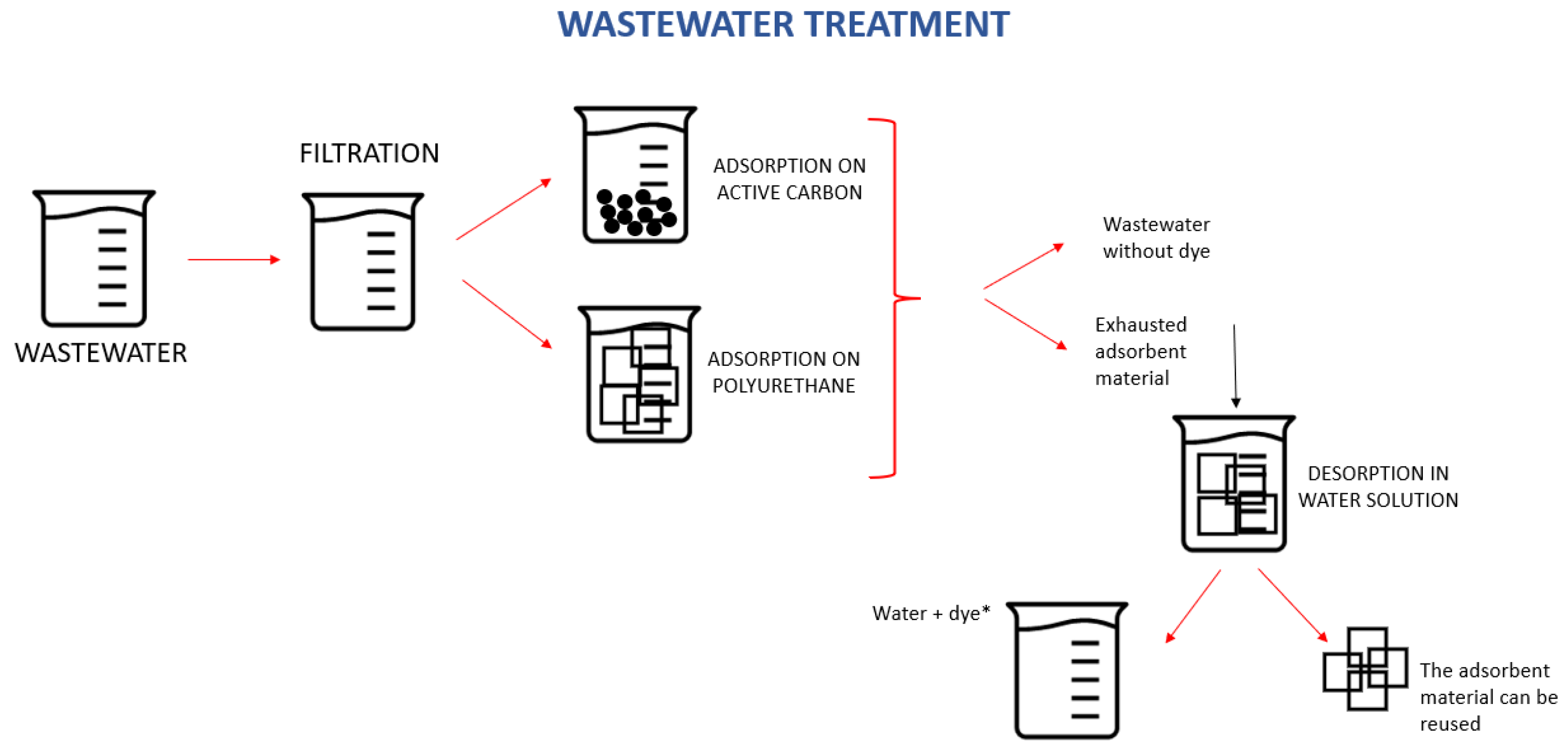
2. Materials and Methods
2.1. Materials
2.2. Methods
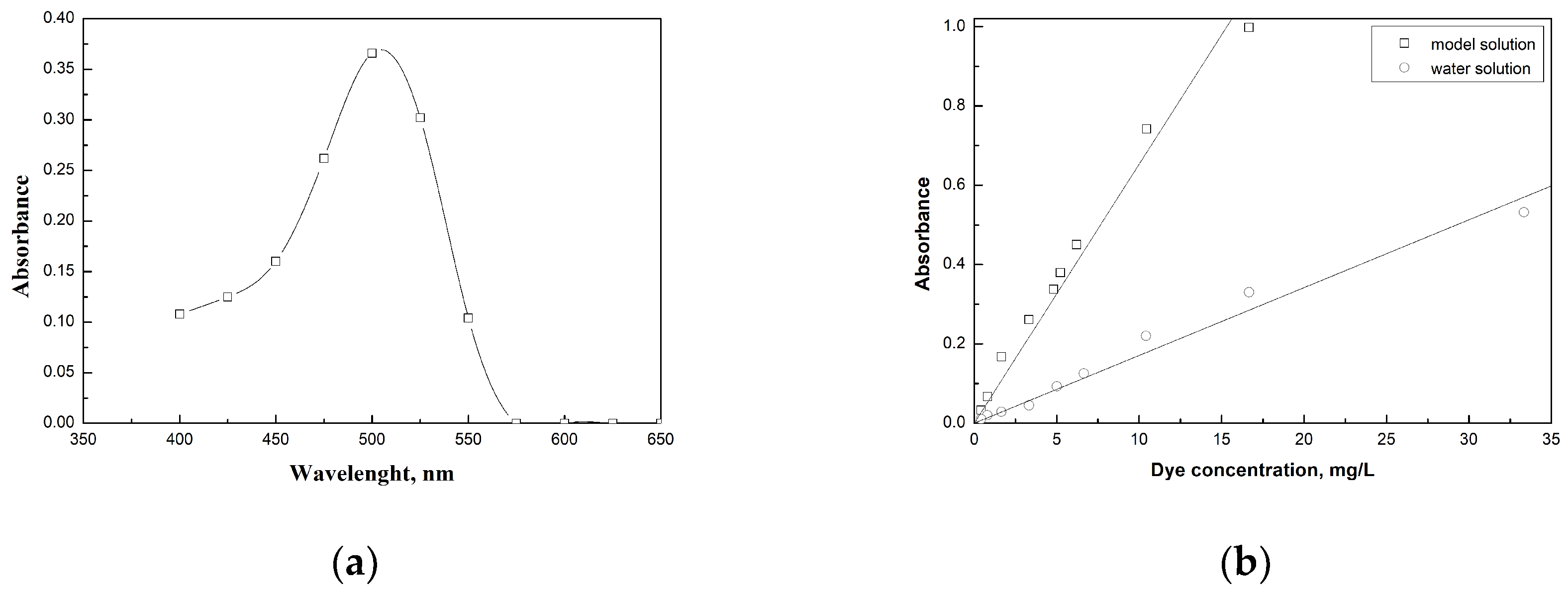
2.2.1. Dye Preparation Solutions
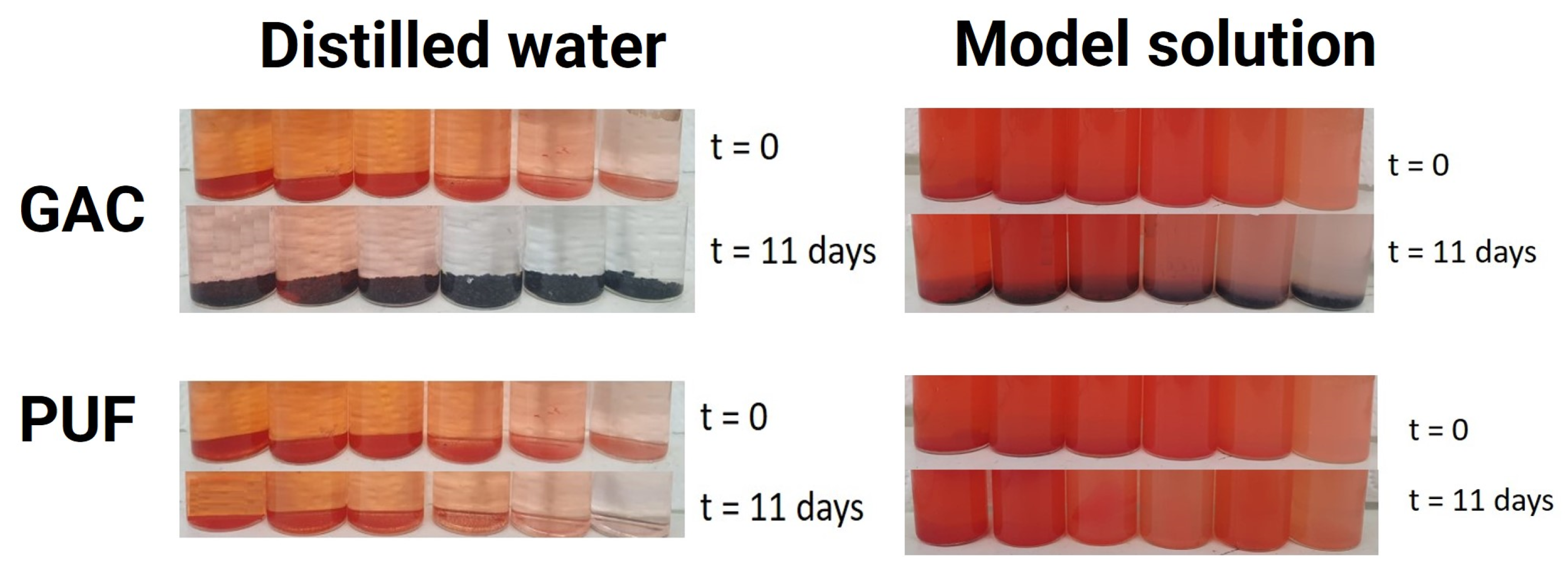
2.2.2. Adsorption Experiments
2.2.3. Desorption Experiments
3. Results and Discussion
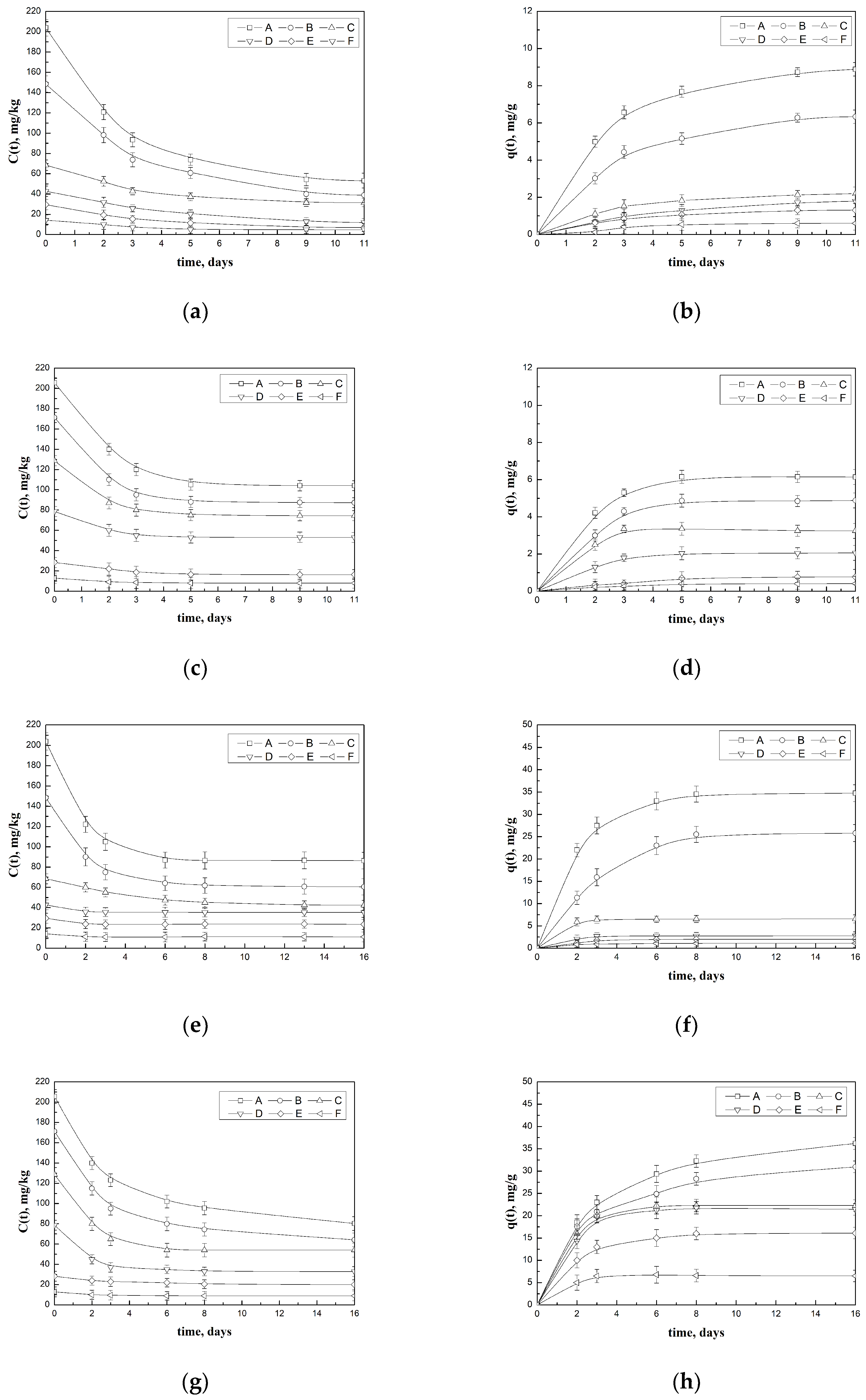
- (A)
- A preliminary model solution of 30 mL containing an initial dye concentration of 80 mg/kg was prepared. In these conditions, without putting the solution in contact with GAC, the initial uptake capacity is referred to as 0 mg/kg. Then, 2 g of GAC (4 times larger than the previous adsorption experiments) was added to the solution in order to obtain a solid/liquid ratio of around 67 g/L.
- (B)
- The equilibrium adsorption uptake was reached at 3.53 mg/g, at a concentration of 10.9 mg/kg at the temperature of 20 °C.
- (C)
- The GAC adsorbent spent was then separated from the solution, dried, and put in contact with a fresh solution to perform the desorption step (in this case, the c(t) becomes equal to zero).
- (D)
- One-third of the spent GAC was added to the desorption liquid (volume of 6 mL), reaching a final concentration of 172 mg/kg, corresponding to about 1.47 mg/g residual uptake.
- (E)
- One-third of the spent GAC was added to the desorption liquid (volume of 9 mL), reaching a final concentration of 174 mg/kg, corresponding to about 0.39 mg/g residual uptake.
- (F)
- One-third of the spent GAC was added to the desorption liquid (volume of 12 mL), reaching a final concentration of 173 mg/kg, corresponding to almost no residual uptake.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laing, R.M.; Sleivert, G.G. Clothing, textiles, and human performance. Text. Prog. 2002, 32, 1–122. [Google Scholar] [CrossRef]
- Bonetti, E. Strategic groups in the fashion industry. Int. J. Bus. Glob. 2014, 13, 153–172. [Google Scholar] [CrossRef]
- Mabeleng, L.B. An Assessment of the Role of the Textile and Clothing Industry in the South Africa Economy. Doctoral Dissertation, North-West University, Potchefstroom, South Africa, 2021. [Google Scholar]
- Burns, L.D.; Mullet, K.K.; Bryant, N.O. The Business of Fashion: Designing, Manufacturing, and Marketing; Bloomsbury Publishing USA: New York, NY, USA, 2016. [Google Scholar]
- Ledezma, V. Globalization and fashion: Too fast, too furious. Laurier Undergrad. J. Arts 2017, 4, 9. [Google Scholar]
- Narumi, H. Fashion orientalism and the limits of counter culture. Postcolonial Stud. Cult. Politics Econ. 2000, 3, 311–330. [Google Scholar] [CrossRef]
- Kim, J.O.; Traore, M.K.; Warfield, C. The textile and apparel industry in developing countries. Text. Prog. 2006, 38, 1–64. [Google Scholar] [CrossRef]
- Yu, H.L.; Kim, C.; Lee, J.; Hong, N. An analysis of modern fashion designs as influenced by Asian ethnic dress. Int. J. Consum. Stud. 2001, 25, 309–321. [Google Scholar] [CrossRef]
- Elson, D.; Pearson, R. ‘Nimble fingers make cheap workers’: An analysis of women’s employment in third world export manufacturing. Fem. Rev. 1981, 7, 87–107. [Google Scholar] [CrossRef]
- Pollin, R.; Burns, J.; Heintz, J. Global apparel production and sweatshop labour: Can raising retail prices finance living wages? Camb. J. Econ. 2004, 28, 153–171. [Google Scholar] [CrossRef]
- Barbaresco, G.; Salerno, E. Mid-Sized Companies According to Mediobanca-Unioncamere; Mid-Sized Manufacturing Companies: The New Driver of Italian Competitiveness; Springer: Milano, Italy, 2013; pp. 13–40. [Google Scholar] [CrossRef]
- Martina, M. How do we talk about fashion in Italy? The big players and independent publishers from 2000 to today: Transformations and orientations. Film Fash. Consum. 2016, 5, 83–102. [Google Scholar] [CrossRef]
- Radwan, M.; Kamal, M.; Khavarinezhad, S.; Calandra, D. Influencing Factors on Modest Fashion Market: A Case Study. International. J. Appl. Res. Manag. Econ. 2019, 2, 12–22. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2020, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Le, Q.V.; Meng, L.; Yang, H.; Gu, H.; Yang, Y.; Chen, X.; Lam, S.S.; Sonne, C.; Peng, W. Environmental perspectives of textile waste, environmental pollution and recycling. Environ. Technol. Rev. 2022, 11, 62–71. [Google Scholar] [CrossRef]
- Dissanayake, D.G.K.; Sinha, P. Sustainable waste management strategies in the fashion industry sector. Int. J. Environ. Sustain. 2012, 8, 77–90. [Google Scholar] [CrossRef]
- Targosz-Wrona, E. Ecolabelling as a Confirmation of the Application of Sustainable Materials in Textiles. Fibres Text. East. Eur. 2009, 17, 75. [Google Scholar]
- Chavero, S.T. The unsustainability of fast fashion. Datatèxtil 2017, 36, 56–62. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Iiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Parmar, S.; Daki, S.; Bhattacharya, S.; Shrivastav, A. Microorganism: An ecofriendly tool for waste management and environmental safety. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–193. [Google Scholar] [CrossRef]
- Bullon, J.; González Arrieta, A.; Hernández Encinas, A.; Queiruga Dios, A. Manufacturing processes in the textile industry. Expert Systems for fabrics production. ADCAIJ Adv. Distrib. Comput. Articial Intell. J. 2017, 6, 17–23. [Google Scholar]
- Macchion, L.; Moretto, A.; Caniato, F.; Caridi, M.; Danese, P.; Vinelli, A. Production and supply network strategies within the fashion industry. Int. J. Prod. Econ. 2015, 163, 173–188. [Google Scholar] [CrossRef]
- Hauser, P.J. Reducing Pollution and Energy Requirements in Cotton Dyeing. Text. Chem. Color. Am. Dyest. Report. 2000, 32, 44–48. [Google Scholar]
- Mukherjee, S. Environmental and social impact of fashion: Towards an eco-friendly, ethical fashion. Int. J. Interdiscip. Multidiscip. Stud. 2015, 2, 22–35. [Google Scholar]
- Correia, V.M.; Stephenson, T.; Judd, S.J. Characterisation of textile wastewaters—A review. Environ. Technol. 1994, 15, 917–929. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Couras, C.; Karim, A.V.; Nadais, H. A review of integrated advanced oxidation processes and biological processes for organic pollutant removal. Chem. Eng. Commun. 2022, 209, 390–432. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Phillips, B.J.; McQuarrie, E.F. Contesting the social impact of marketing: A re-characterization of women’s fashion advertising. Mark. Theory 2011, 11, 99–126. [Google Scholar] [CrossRef]
- Karthik, T.; Gopalakrishnan, D. Environmental analysis of textile value chain: An overview. In Roadmap to Sustainable Textiles and Clothing; Springer: Singapore, 2014; pp. 153–188. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. Environmental impacts of the textile industry and its assessment through life cycle assessment. In Roadmap to Sustainable Textiles and Clothing; Springer: Singapore, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Ding, X. Textile supply chain waste management in China. J. Clean. Prod. 2021, 289, 125147. [Google Scholar] [CrossRef]
- Kamau, E.C. Pollution Control in Developing Countries with a case study on Kenya. A need for consistent and stable regimes. Rev. Int. Direito Cid. 2010, 9, 29–42. [Google Scholar]
- Bhuiya, M.H. Upcycling the Garment Solid Waste in Bangladesh. Ph.D. Dissertation, Tallinn University of Technology, Tallinn, Estonia, 2017. [Google Scholar]
- Zhang, Y.Q.; Lykaki, M.; Markiewicz, M.; Alrajoula, M.T.; Kraas, C.; Stolte, S. Environmental contamination by microplastics originating from textiles: Emission, transport, fate and toxicity. J. Hazard. Mater. 2022, 430, 128453. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef]
- Atangana, E.; Oberholster, P.J. Using heavy metal pollution indices to assess water quality of surface and groundwater on catchment levels in South Africa. J. Afr. Earth Sci. 2021, 182, 104254. [Google Scholar] [CrossRef]
- Hellawell, J.M. Toxic substances in rivers and streams. Environ. Pollut. 1988, 50, 61–85. [Google Scholar] [CrossRef]
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; De Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar] [CrossRef]
- Chen, H.L.; Burns, L.D. Environmental analysis of textile products. Cloth. Text. Res. J. 2006, 24, 248–261. [Google Scholar] [CrossRef]
- Hussain, T.; Wahab, A. A critical review of the current water conservation practices in textile wet processing. J. Clean. Prod. 2018, 198, 806–819. [Google Scholar] [CrossRef]
- Thadepalli, S.; Roy, S. Implications of Sustainability on Textile Fibres and Wet Processing, Barriers in Implementation. In Sustainable Approaches in Textiles and Fashion; Springer: Singapore, 2018; pp. 133–156. [Google Scholar]
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S.R. Harmful environmental effects for textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles; Woodhead Publishing: Cambridge, UK, 2021; pp. 153–164. [Google Scholar]
- Verma, R.K.; Sankhla, M.S.; Rathod, N.V.; Sonone, S.S.; Parihar, K.; Singh, G.K. Eradication of fatal textile industrial dyes by wastewater treatment. Biointerface Res. Appl. Chem. 2021, 12, 567–587. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Islam, A.; Mustafa, A.I. Characterization of dye industry effluent and assessment of its suitability for irrigation purpose. J. Bangladesh Acad. Sci. 2009, 33, 99–106. [Google Scholar] [CrossRef]
- Sen, S.K.; Patra, P.; Das, C.R.; Raut, S.; Raut, S. Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: An impact on its use in irrigation of wheat crop. Water Resour. Ind. 2019, 21, 100106. [Google Scholar] [CrossRef]
- Lin, S.H.; Peng, C.F. Treatment of textile wastewater by electrochemical method. Water Res. 1994, 28, 277–282. [Google Scholar] [CrossRef]
- Malliaros, C.; Guitonas, A. Pre-treatment and elimination systems of toxic industrial waste and sludges. The case study of the department of Attika. Water Sci. Technol. 1997, 36, 91–100. [Google Scholar] [CrossRef]
- Babu, B.R.; Parande, A.; Raghu, S.; Kumar, T.P. Textile technology. Technology 1995, 11, 110–122. [Google Scholar]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Haddad, M.; Abid, S.; Hamdi, M.; Bouallagui, H. Reduction of adsorbed dyes content in the discharged sludge coming from an industrial textile wastewater treatment plant using aerobic activated sludge process. J. Environ. Manag. 2018, 223, 936–946. [Google Scholar] [CrossRef]
- Ciardelli, G.; Corsi, L.; Marcucci, M. Membrane separation for wastewater reuse in the textile industry. Resour. Conserv. Recycl. 2001, 31, 189–197. [Google Scholar] [CrossRef]
- Ciardelli, G.; Capannelli, G.; Bottino, A. Ozone treatment of textile wastewaters for reuse. Water Sci. Technol. 2001, 44, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Zhou, X.; He, X.; Zhou, M.; Jia, K.; Liu, X. Design of polymer composite-based porous membrane for in-situ photocatalytic degradation of adsorbed organic dyes. J. Phys. Chem. Solids 2021, 154, 110094. [Google Scholar] [CrossRef]
- Hu, F.; Fang, C.; Wang, Z.; Liu, C.; Zhu, B.; Zhu, L. Poly (N-vinyl imidazole) gel composite porous membranes for rapid separation of dyes through permeating adsorption. Sep. Purif. Technol. 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A. Membranes for wastewater treatment. Nanostructured Polym. Membr. 2016, 2, 159–208. [Google Scholar] [CrossRef]
- Vatanpour, V.; Nekouhi, G.N.; Esmaeili, M. Preparation, characterization and performance evaluation of ZnO deposited polyethylene ultrafiltration membranes for dye and protein separation. J. Taiwan Inst. Chem. Eng. 2020, 114, 153–167. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile dyeing wastewater treatment. Adv. Treat. Text. Effl. 2011, 5, 91–116. [Google Scholar] [CrossRef]
- Singh, K.; Arora, S. Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Särkkä, H.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter (NOM) removal by electrochemical methods—A review. J. Electroanal. Chem. 2015, 755, 100–108. [Google Scholar] [CrossRef]
- Kobya, M.; Bayramoglu, M.; Eyvaz, M. Techno-economical evaluation of electrocoagulation for the textile wastewater using different electrode connections. J. Hazard. Mater. 2007, 148, 311–318. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Kreetachart, T.; Sirilamduan, C.; Rioyo, J.; Wongphat, A. Evaluation of the Microsoft Excel Solver Spreadsheet-Based Program for nonlinear expressions of adsorption Isotherm models onto magnetic nanosorbent. Appl. Sci. 2021, 11, 7432. [Google Scholar] [CrossRef]
- Russo, M.E.; Di Natale, F.; Prigione, V.; Tigini, V.; Marzocchella, A.; Varese, G.C. Adsorption of acid dyes on fungal biomass: Equilibrium and kinetics characterization. Chem. Eng. J. 2010, 162, 537–545. [Google Scholar] [CrossRef]
- Russo, M.E.; Di Natale, F.; Marzocchella, A.; Varese, G. Dyes biosorption of textile wastewaters in a lab-scale fluidised bed. In Proceedings of the MFIP12: 12th International Conference on Multiphase Flow in Industrial Plants, Ischia, Italy, 21–23 September 2011. [Google Scholar]
- Buckley, T.; Xu, X.; Rudolph, V.; Firouzi, M.; Shukla, P. Review of foam fractionation as a water treatment technology. Sep. Sci. Technol. 2022, 57, 929–958. [Google Scholar] [CrossRef]
- Hailan, S.M.; Ponnamma, D.; Krupa, I. The separation of oil/water mixtures by modified melamine and polyurethane foams: A review. Polymers 2021, 13, 4142. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 825910. [Google Scholar] [CrossRef]
- Dasa, P.; Duttab, S. Sustainable membranes with FNs: Current and emerging research trends. In Membranes with Functionalized Nanomaterials: Current and Emerging Research Trends in Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2022; p. 159. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Simpson, D.R. Biofilm processes in biologically active carbon water purification. Water Res. 2018, 42, 2839–2848. [Google Scholar] [CrossRef]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Biological activated carbon treatment of industrial wastewater in stirred tank reactors. Chem. Eng. J. 1999, 75, 201–206. [Google Scholar] [CrossRef]
- Simón, D.; De Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Glycolysis of high resilience flexible polyurethane foams containing polyurethane dispersion polyol. Polym. Degrad. Stab. 2016, 133, 119–130. [Google Scholar] [CrossRef]
- Cuq, M.H.; Benjelloun-Mlayah, B.; Delmas, M. Oil extracted from seal hides: Characterization and use as leather fat liquor. J. Am. Oil Chem. Soc. 1998, 75, 1015–1019. [Google Scholar] [CrossRef]
- Sardari, A.; Sabbagh Alvani, A.A.; Ghaffarian, S.R. Preparation of castor oil-based fatliquoring agent via a Pickering emulsion method for use in leather coating. J. Coat. Technol. Res. 2019, 16, 1765–1772. [Google Scholar] [CrossRef]
- Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated carbon for dyes removal: Modeling and understanding the adsorption process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Maddalena, R.L.; McKone, T.E.; Kado, N.Y. Simple and rapid extraction of polycyclic aromatic hydrocarbons collected on polyurethane foam adsorbent. Atmos. Environ. 1998, 32, 2497–2503. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; de Luis, A.; Lombraña, J.I. Analysis of the effect of the operational conditions in a combined adsorption–ozonation process with granular activated carbon for the treatment of phenol wastewater. React. Chem. Eng. 2020, 5, 760–778. [Google Scholar] [CrossRef]
- Ying, W.C.; Zhang, W.; Chang, Q.G.; Jiang, W.X.; Li, G.H. Improved methods for carbon adsorption studies for water and wastewater treatment. Environ. Prog. 2006, 25, 110–120. [Google Scholar] [CrossRef]
- Ozsoy, H.D.; van Leeuwen, J.H. Removal of color from fruit candy waste by activated carbon adsorption. J. Food Eng. 2010, 101, 106–112. [Google Scholar] [CrossRef]
- Vona, A.; Di Martino, F.; Garcia-Ivars, J.; Picó, Y.; Mendoza-Roca, J.A.; Iborra-Clar, M.I. Comparison of different removal techniques for selected pharmaceuticals. J. Water Process Eng. 2015, 5, 48–57. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamil, S.N.A.M.; Shean Yaw Choong, T.; Abdullah, A.H.; Mastuli, M.S.; Othman, N.; Jiman, N. Green flexible polyurethane foam as a potent support for Fe-Si adsorbent. Polymers 2019, 11, 2011. [Google Scholar] [CrossRef]
- Lefebvre, L.; Kelber, J.; Jierry, L.; Ritleng, V.; Edouard, D. Polydopamine-coated open cell polyurethane foam as an efficient and easy-to-regenerate soft structured catalytic support (S2CS) for the reduction of dye. J. Environ. Chem. Eng. 2017, 5, 79–85. [Google Scholar] [CrossRef]
- Selvasembian, R.; Gwenzi, W.; Chaukura, N.; Mthembu, S. Recent advances in the polyurethane-based adsorbents for the decontamination of hazardous wastewater pollutants. J. Hazard. Mater. 2021, 417, 125960. [Google Scholar] [CrossRef]
- Anglada, A.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2019, 84, 1747–1755. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. Available online: https://hal.science/hal-02082916 (accessed on 1 January 2020). [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sirilamduan, C.; Deepatana, A.; Kreetachat, T.; Wongcharee, S. Characterization and optimization of polymeric bispicolamine chelating resin: Performance evaluation via RSM using copper in acid liquors as a model substrate through ion exchange method. Molecules 2022, 27, 7210. [Google Scholar] [CrossRef]
- Trucillo, P.; Lancia, A.; Di Natale, F. Recovery of platinum from diesel catalysts by combined use of H2O2/HCl leaching and adsorption. J. Environ. Chem. Eng. 2022, 10, 107730. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Agarwala, R.; Mulky, L. Adsorption of Dyes from Wastewater: A Comprehensive Review. ChemBioEng Rev. 2023, 10, 326–335. [Google Scholar] [CrossRef]



| Material | Molecular Weight [g/mol] | Color | CAS Number | Bulk Density [kg/m3] | Water Solubility |
|---|---|---|---|---|---|
| Acid Red 97 | 699 | red-yellowish | 10169-02-5 | 1579 | 8.85 g/L |
| Lipoderm N | * | opaque white | * | * | * |
| Ammonia | 17 | colorless | 7664-41-7 | 0.73 | 31% w/w |
| Formic acid | 46 | colorless | 64-18-6 | 1.22 | Highly miscible |
| Filtercarb GCC 1240 | * | black | * | 530 | Not miscible |
| Polyurethane | 312 | red-yellowish | 25036-33-3 | 870 | Not miscible |
| Coripol DX 1202 | * | opaque white | * | * | * |
| Mass Ratio | Steps of Preparation | Role of Additive |
|---|---|---|
| 1st step—60 min stirring at 200 rpm * | ||
| 600% w/w | Distilled water | Diluent |
| 1% w/w | Lipoderm N | Emulsifier |
| 3% w/w | Ammonia | pH corrector |
| 2nd step—addition of dye with 30 min stirring at 200 rpm | ||
| 3% w/w | Acid Red 97 | Coloring agent |
| 3rd step—pre-fixing with 30 min stirring at 200 rpm | ||
| 3% w/w | Coripol DX 1202 | Emulsifier |
| 4th step—fixing with 60 min stirring at 200 rpm | ||
| 5% w/w | Formic acid diluted in water 1:1 v/v | pH corrector |
| Solution | Ads* Material | Temperature, °C | Henry Constant | R2, % |
|---|---|---|---|---|
| Aqueous | GAC | 20 | 0.121 | 93.4 |
| Model | GAC | 20 | 0.056 | 98.5 |
| Model | GAC | 40 | 0.012 | 96.7 |
| Aqueous | PUF | 20 | 0.459 | 98.6 |
| Model | PUF | 20 | 0.469 | 95.7 |
| Model | PUF | 40 | 0.296 | 96.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trucillo, P.; Lancia, A.; Di Natale, F. Adsorption–Desorption Process to Separate Dyes from Tanning Wastewaters. Processes 2023, 11, 3006. https://doi.org/10.3390/pr11103006
Trucillo P, Lancia A, Di Natale F. Adsorption–Desorption Process to Separate Dyes from Tanning Wastewaters. Processes. 2023; 11(10):3006. https://doi.org/10.3390/pr11103006
Chicago/Turabian StyleTrucillo, Paolo, Amedeo Lancia, and Francesco Di Natale. 2023. "Adsorption–Desorption Process to Separate Dyes from Tanning Wastewaters" Processes 11, no. 10: 3006. https://doi.org/10.3390/pr11103006
APA StyleTrucillo, P., Lancia, A., & Di Natale, F. (2023). Adsorption–Desorption Process to Separate Dyes from Tanning Wastewaters. Processes, 11(10), 3006. https://doi.org/10.3390/pr11103006









