Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal
Abstract
1. Introduction
2. Advanced Oxidation Processes
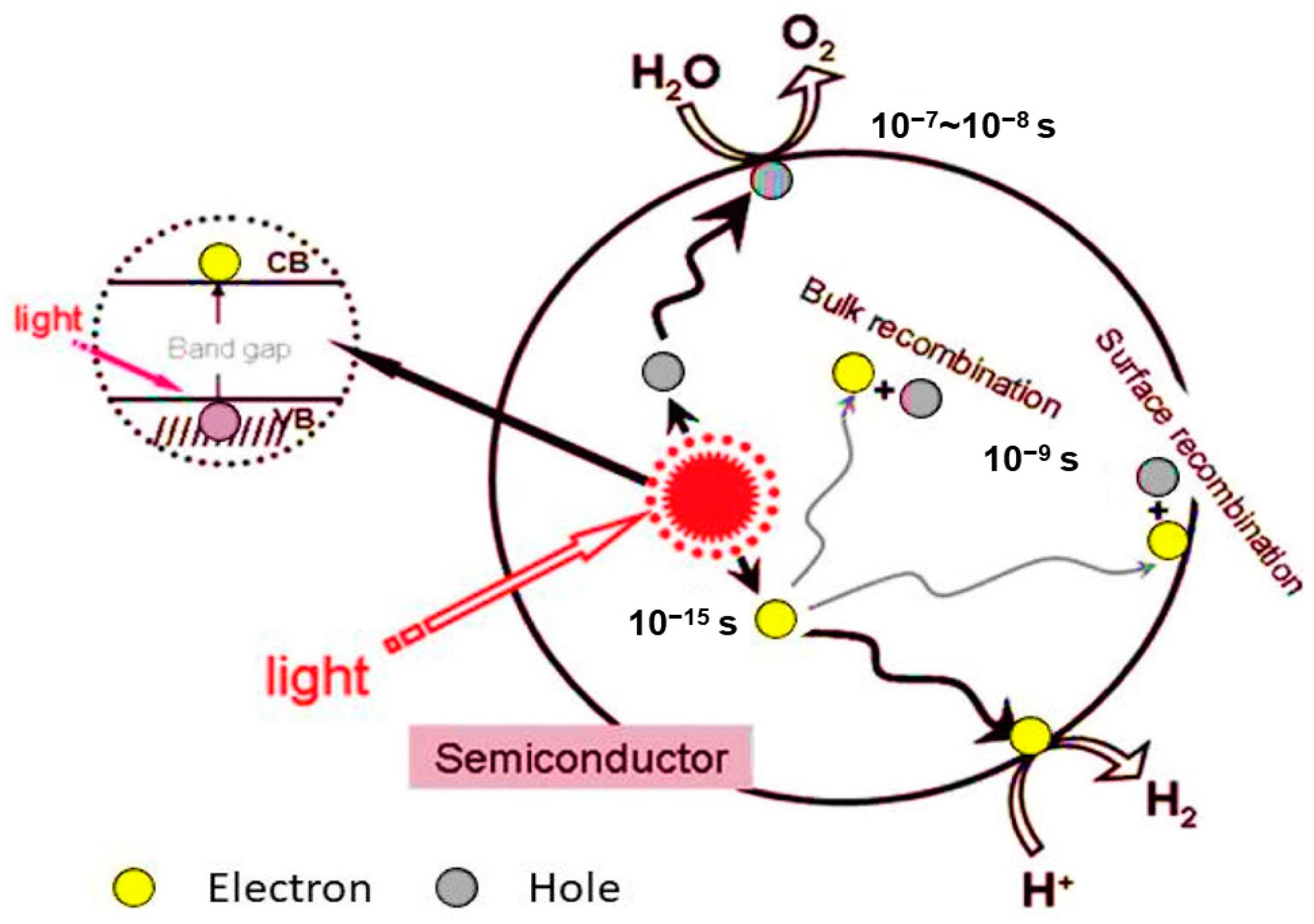
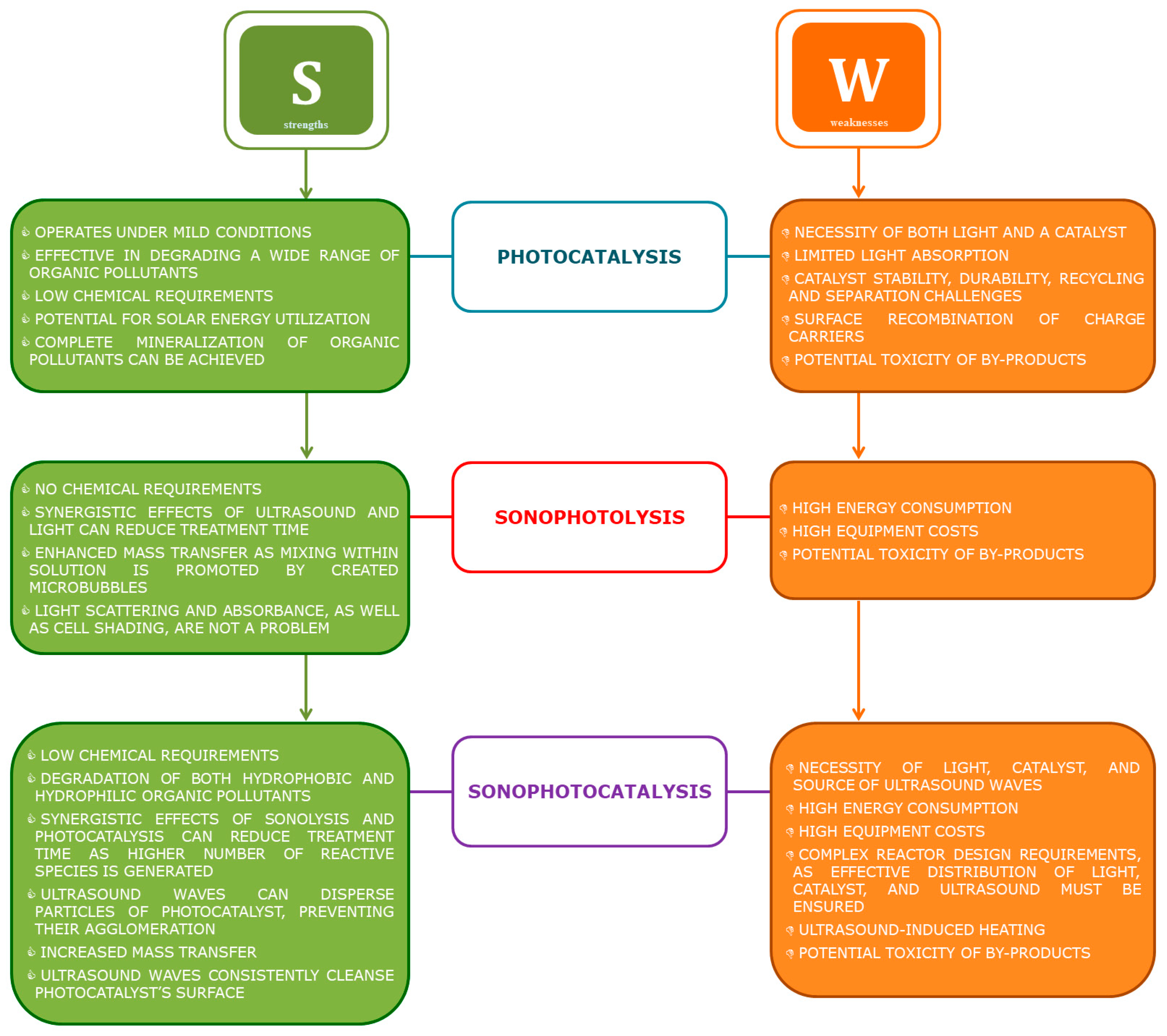
2.1. Heterogeneous Photocatalysis
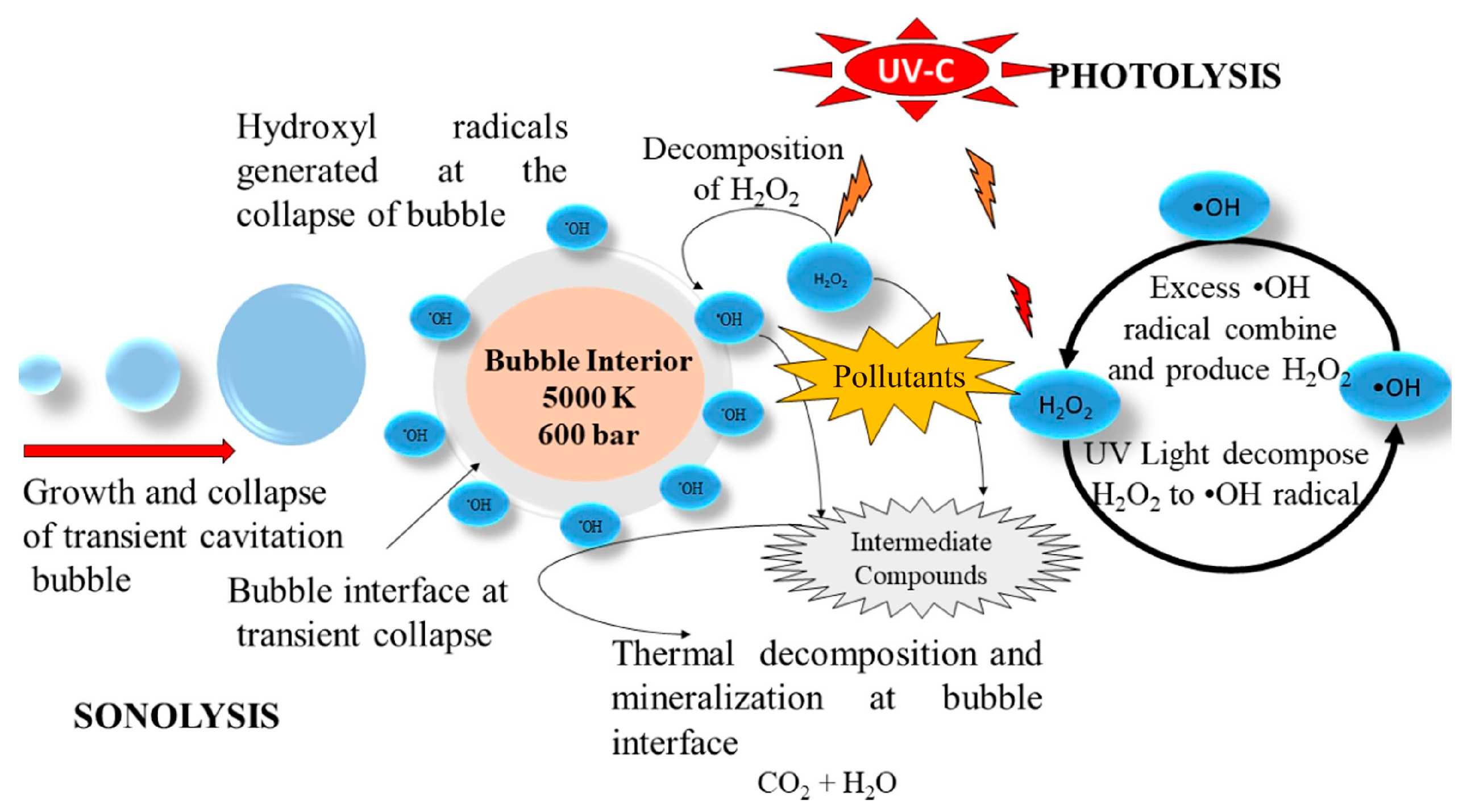
2.2. Sonophotolysis
2.3. Sonophotocatalysis
3. State-of-the-Art Application of AOPs
3.1. Photocatalysis
3.2. Sonophotolysis
3.3. Sonophotocatalysis
4. Cost Estimation and Constraints of Different AOPs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Britannica. Available online: https://www.britannica.com/explore/savingearth/pollution-overview (accessed on 20 April 2024).
- Singh, S. Water pollution in rural areas: Primary sources and associated health issues. In Water Resources Management for Rural Development; Madhav, S., Srivastav, A.L., Chibueze Izah, S., Hullebusch, E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 29–44. [Google Scholar]
- UNESCO Report. Available online: https://www.unesco.org/en/articles/imminent-risk-global-water-crisis-warns-un-world-water-development-report-2023 (accessed on 9 April 2024).
- Nelson, K.L.; Murray, A. Sanitation for Unserved Populations: Technologies, Implementation Challenges, and Opportunities. Annu. Rev. Environ. Resour. 2008, 33, 119–151. [Google Scholar] [CrossRef]
- Malaj, E.; von der Ohe, P.C.; Grote, M.; Kuhne, R.; Mondy, C.P.; Usseglio-Polatera, P.; Brack, W.; Schafer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Schoups, G.; van de Giesen, N. Organic pollution of rivers: Combined threats of urbanization, livestock farming and global climate change. Sci. Rep. 2017, 7, 43289. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Corcoles, M.T.; Rodriguez-Gomez, R.; de Alarcon-Gomez, B.; Cipa, M.; Martin-Pozo, L.; Kauffmann, J.M.; Zafra-Gomez, A. Chromatographic methods for the determination of emerging contaminants in natural water and wastewater samples: A Review. Crit. Rev. Anal. Chem. 2019, 49, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, J.; Kotowska, U. Removal of organic pollution in the water environment. Water 2019, 11, 2017. [Google Scholar] [CrossRef]
- Aravind Kumar, J.; Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent organic pollutants in water resources: Fate, occurrence, characterization and risk analysis. Sci. Total Environ. 2022, 831, 154808. [Google Scholar] [CrossRef] [PubMed]
- Shumbula, P.; Maswanganyi, C.; Shumbula, N. Type, sources, methods and treatment of organic pollutants in wastewater. In Persistent Organic Pollutants (POPs)—Monitoring, Impact and Treatment; Rashed, M., Ed.; IntechOpen: London, UK, 2022; pp. 1–20. [Google Scholar]
- Otálora, A.; Lerma, T.A.; Arrieta-Urango, Y.; Palencia, M. Emerging organic pollutants in aqueous environments: Detection, monitoring, and removal techniques. J. Sci. Technol. Appl. 2021, 10, 92–153. [Google Scholar] [CrossRef]
- Vasilachi, I.; Asiminicesei, D.; Fertu, D.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- CDC. Available online: https://www.cdc.gov/healthywater/drinking/public/water_treatment.html (accessed on 11 April 2024).
- Yeoh, J.X.; Md. Jamil, S.N.A.; Syukri, F.; Koyama, M.; Nourouzi Mobarekeh, M. Comparison between Conventional treatment processes and advanced oxidation processes in treating slaughterhouse wastewater: A Review. Water 2022, 14, 3778. [Google Scholar] [CrossRef]
- Cococeanu, A.L.; Man, T.E. Methods and characteristics of conventional water treatment technologies. In Water Safety, Security and Sustainability: Threat Detection and Mitigation; Vaseashta, A., Maftei, C., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 305–330. [Google Scholar]
- Mahy, J.G.; Wolfs, C.; Vreuls, C.; Drot, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Hermans, S.; Lambert, S.D. Advanced oxidation processes for waste water treatment: From laboratory-scale model water to on-site real waste water. Environ. Technol. 2021, 42, 3974–3986. [Google Scholar] [CrossRef]
- Jyothi, K.P.; Yesodharan, S.; Yesodharan, E.P. Ultrasound (US), Ultraviolet light (UV) and combination (US+UV) assisted semiconductor catalysed degradation of organic pollutants in water: Oscillation in the concentration of hydrogen peroxide formed in situ. Ultrason. Sonochem. 2014, 21, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Villaroel, E.; Silva-Agredo, J.; Petrier, C.; Taborda, G.; Torres-Palma, R.A. Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem. 2014, 21, 1763–1769. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef] [PubMed]
- Terki, M.; Triaa, S.; Ali, F.K.; Youcef, R.; Brahim, I.O.; Trari, M. Sono-assisted degradation of rhodamine B using the Fe modified MgO nanostructures: Characterization and catalytic activity. React. Kinet. Mech. Catal. 2023, 136, 1143–1155. [Google Scholar] [CrossRef]
- Sahani, S.; Malika Tripathi, K.; Il Lee, T.; Dubal, D.P.; Wong, C.-P.; Chandra Sharma, Y.; Young Kim, T. Recent advances in photocatalytic carbon-based materials for enhanced water splitting under visible-light irradiation. Energy Convers. Manag. 2022, 252, 115133. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Maksimović, I.; Velebit, B.; Putnik-Delić, M.; Šojić Merkulov, D. Sustainable removal of tolperisone from waters by application of photocatalysis, nanotechnology, and chemometrics: Quantification, environmental toxicity, and degradation optimization. Nanomaterials 2022, 12, 4199. [Google Scholar] [CrossRef] [PubMed]
- Finčur, N.; Šojić Merkulov, D.; Putnik, P.; Despotović, V.; Banić, N.; Bognár, S.; Jovanović, D.; Panić, S.; Ivetić, T.; Abramović, B. Sunlight-driven degradation of alprazolam and amitriptyline by application of binary zinc oxide and tin oxide powders. Separations 2023, 10, 316. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.; Vlazan, P.; Poienar, M.; Bognár, S.; Ianasi, C.; Sfirloaga, P. Sustainable removal of 17α-ethynylestradiol from aqueous environment using rare earth doped lanthanum manganite nanomaterials. Catal. Today 2023, 424, 113746. [Google Scholar] [CrossRef]
- Bognár, S.; Jovanović, D.; Putnik, P.; Despotović, V.; Ivetić, T.; Bajac, B.; Tóth, E.; Finčur, N.; Maksimović, I.; Putnik-Delić, M.; et al. Solar-driven removal of selected organics with binary ZnO based nanomaterials from aquatic environment: Chemometric and toxicological assessments on wheat. J. Environ. Chem. Eng. 2024, 12, 112016. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; Wang, Z.; Zubairu, S.M.; Ding, Y.; Zhu, G. In-situ construction of Z-scheme 2D/2D g-C3N4/BiOBr heterojunction with enhanced photocatalytic CO2 reduction. Surf. Interfaces 2024, 45, 103875. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, J.; Santos, H.A. Recent advances in Fenton and Fenton-like reaction mediated nanoparticle in cancer therapy. Biomed. Technol. 2023, 3, 40–51. [Google Scholar] [CrossRef]
- Liu, S.; Long, Z.; Liu, H.; Wang, Y.; Zhang, J.; Zhang, G.; Liang, J. Recent advances in ultrasound-Fenton/Fenton-like technology for degradation of aqueous organic pollutants. Chemosphere 2024, 352, 141286. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Zahid, M.; Bhatti, H.N.; Jamil, Y. Degradation of persistent organic pollutant using Ag-doped ZnO-ZnS–polyaniline composite as photocatalyst. Int. J. Environ. Sci. Technol. 2022, 20, 4811–4826. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Machado, F.; Teixeira, A.C.S.C.; Ruotolo, L.A.M. Critical review of Fenton and photo-Fenton wastewater treatment processes over the last two decades. Int. J. Environ. Sci. Technol. 2023, 20, 13995–14032. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J.; Naumov, S.; Sonntag, C.V. Reaction of Ozone with Hydrogen Peroxide (Peroxone Process): A Revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ. Sci. Technol. 2010, 44, 3505–3507. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Wang, Z.; Guan, C.; Jiang, J. The synergistic effect of oxidant-peroxide coupling systems for water and wastewater treatments. Water Res. 2024, 249, 120992. [Google Scholar] [CrossRef]
- Saharan, V.K.; Pinjari, D.V.; Gogate, P.R.; Pandit, A.B. Advanced oxidation technologies for wastewater treatment. In Industrial Wastewater Treatment, Recycling and Reuse; Ranade, V., Bhandari, V., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 141–191. [Google Scholar]
- Elkacmi, R.; Bennajah, M. Advanced oxidation technologies for the treatment and detoxification of olive mill wastewater: A general review. J. Water Reuse Desal. 2019, 9, 463–505. [Google Scholar] [CrossRef]
- Babu Ponnusami, A.; Sinha, S.; Ashokan, H.; Paul, M.V.; Hariharan, S.P.; Arun, J.; Gopinath, K.P.; Hoang Le, Q.; Pugazhendhi, A. Advanced oxidation process (AOP) combined biological process for wastewater treatment: A review on advancements, feasibility and practicability of combined techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chen, H.; Hu, T.; Feng, J.; Xing, W.; Tang, L.; Tang, W. Recent advances in the applications of encapsulated transition-metal nanoparticles in advanced oxidation processes for degradation of organic pollutants: A critical review. Appl. Catal. B Environ. 2024, 342, 123401. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems. Available online: https://www.iso.org/standard/62085.html (accessed on 11 June 2024).
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An overview of homogeneous and heterogeneous photocatalysis applications for the removal of pharmaceutical compounds from real or synthetic hospital wastewaters under lab or pilot scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Li, Y.; Zhao, Y.; Zhang, C.; Chen, H. Bulk phase charge transfer in focus—And in sequential along with surface steps. Catal. Today 2021, 364, 2–6. [Google Scholar] [CrossRef]
- Bognár, S.; Maksimović, I.; Putnik, P.; Orčić, D.; Putnik-Delić, M.; Šojić Merkulov, D. Integrated approach for sustainable degradation of tolperisone hydrochloride from water by photodegradation: Chemometrics, chemical kinetics, intermediates, and environmental toxicity assessment. J. Photochem. Photobiol. A Chem. 2024, 453, 115628. [Google Scholar] [CrossRef]
- Finčur, N.; Šojić Merkulov, D.; Putnik, P.; Despotović, V.; Banić, N.; Lazarević, M.; Četojević-Simin, D.; Agbaba, J.; Abramović, B. Environmental photocatalytic degradation of antidepressants with solar radiation: Kinetics, mineralization, and toxicity. Nanomaterials 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Despotović, V.; Abramović, B.; Šojić, D.; Kler, S.; Dalmacija, M.; Bjelica, L.; Orčić, D. Photocatalytic degradation of herbicide quinmerac in various types of natural water. Wat. Air Soil Pollut. 2012, 223, 3009–3020. [Google Scholar] [CrossRef]
- Abramović, B.F.; Despotović, V.N.; Šojić, D.V.; Orčić, D.Z.; Csanadi, J.J.; Četojević-Simin, D.D. Photocatalytic degradation of the herbicide clomazone in natural water using TiO2: Kinetics, mechanism, and toxicity of degradation products. Chemosphere 2013, 93, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Šojić, D.V.; Orčić, D.; Četojević-Simin, D.D.; Banić, N.D.; Abramović, B.F. Efficient removal of sulcotrione and its formulated compound Tangenta® in aqueous TiO2 suspension: Stability, photoproducts assessment and toxicity. Chemosphere 2015, 138, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Šojić Merkulov, D.; Lazarević, M.; Djordjević, A.; Nafradi, M.; Alapi, T.; Putnik, P.; Rakočević, Z.; Novaković, M.; Miljević, B.; Bognar, S.; et al. Potential of TiO2 with various Au nanoparticles for catalyzing mesotrione removal from wastewaters under sunlight. Nanomaterials 2020, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Putnik, P.; Šojić Merkulov, D. Chemometric evaluation of different parameters for removal of tembotrione (agricultural herbicide) from water by adsorption and photocatalytic degradation using sustainable nanotechnology. Food Energy Secur. 2022, 11, e368. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, B.; Cao, S.; Yu, J. Enhanced photocatalytic activity and stability of Z-scheme Ag2CrO4-GO composite photocatalysts for organic pollutant degradation. Appl. Catal. B Environ. 2015, 164, 380–388. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3 N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Castro, M.A.M.; Porto, D.L.; Aragão, C.F.S.; Souza, R.P.; Silva, U.C.; Bomio, M.R.D.; Motta, F.V. Immobilization of Bi2MoO6/ZnO heterojunctions on glass substrate: Design of drug and dye mixture degradation by solar-driven photocatalysis. J. Photochem. Photobiol. A 2024, 452, 115619. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhu, J.; Zhang, M.; Meng, X.; Chen, M.; Feng, Y.; Chen, X.; Ding, Y. Recent advances and perspectives in cobalt-based heterogeneous catalysts for photocatalytic water splitting, CO2 reduction, and N2 fixation. Chin. J. Catal. 2022, 43, 2273–2300. [Google Scholar] [CrossRef]
- Torres-Palma, R.A.; Serna-Galvis, E.A. Sonolysis. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 177–213. [Google Scholar]
- Lim, M.; Son, Y.; Khim, J. Frequency effects on the sonochemical degradation of chlorinated compounds. Ultrason. Sonochem. 2011, 18, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T. Dissolved gas and ultrasonic cavitation—A review. Ultrason. Sonochem. 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019, 166, 115085. [Google Scholar] [CrossRef] [PubMed]
- Patidar, R.; Srivastava, V.C. Evaluation of the sono-assisted photolysis method for the mineralization of toxic pollutants. Sep. Purif. Technol. 2021, 258, 117903. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent advancements in the sonophotocatalysis (SPC) and doped-sonophotocatalysis (DSPC) for the treatment of recalcitrant hazardous organic water pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Mapukata, S.; Ntsendwana, B.; Mokhena, T.; Sikhwivhilu, L. Advances on sonophotocatalysis as a water and wastewater treatment technique: Efficiency, challenges and process optimisation. Front. Chem. 2023, 11, 1252191. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yao, Y.; Ding, W.; Anandan, S. N/Ti3+ co-doping biphasic TiO2/Bi2WO6 heterojunctions: Hydrothermal fabrication and sonophotocatalytic degradation of organic pollutants. J. Alloys Compd. 2020, 820, 153172. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Sonophotocatalytic oxidation based treatment of phthalocyanine pigment containing industrial wastewater intensified using oxidising agents. Sep. Purif. Technol. 2020, 233, 115979. [Google Scholar] [CrossRef]
- Du, B.; Fan, G.; Yang, S.; Chen, Z.; Luo, J.; Yu, W.; Yu, J.; Wei, Q.; Lu, Y. Sonophotocatalytic degradation of 17β-estradiol by Er3+-CdS/MoS2: The role and transformation of reactive oxygen species. J. Clean. Prod. 2022, 333, 130203. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Dimitrievska, M.R.; Finčur, N.L.; Đačanin, L.R.; Gúth, I.O.; Abramović, B.F.; Lukić-Petrović, S.R. Effect of annealing temperature on structural and optical properties of Mg-doped ZnO nanoparticles and their photocatalytic efficiency in alprazolam degradation. Ceram. Int. 2014, 40, 1545–1552. [Google Scholar] [CrossRef]
- Šojić, D.V.; Orčić, D.Z.; Četojević-Simin, D.D.; Despotović, V.N.; Abramović, B.F. Kinetics and the mechanism of the photocatalytic degradation of mesotrione in aqueous suspension and toxicity of its degradation mixtures. J. Mol. Catal. A Chem. 2014, 392, 67–75. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Đačanin, L.R.; Abramović, B.F.; Lukić-Petrović, S.R. Ternary and coupled binary zinc tin oxide nanopowders: Synthesis, characterization, and potential application in photocatalytic processes. Mater. Res. Bull. 2015, 62, 114–121. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Abramović, B.F.; Dimitrievska, M.; Štrbac, G.R.; Čajko, K.O.; Miljević, B.B.; Đačanin, L.R.; Lukić-Petrović, S.R. Environmentally friendly photoactive heterojunction zinc tin oxide nanoparticles. Ceram. Int. 2016, 42, 3575–3583. [Google Scholar] [CrossRef]
- Thennarasu, G.; Sivasamy, A. Enhanced visible photocatalytic activity of cotton ball like nano structured Cu doped ZnO for the degradation of organic pollutant. Ecotoxicol. Environ. Saf. 2016, 134, 412–420. [Google Scholar] [CrossRef]
- Finčur, N.L.; Krstić, J.B.; Šibul, F.S.; Šojić, D.V.; Despotović, V.N.; Banić, N.D.; Agbaba, J.R.; Abramović, B.F. Removal of alprazolam from aqueous solutions by heterogeneous photocatalysis: Influencing factors, intermediates, and products. Chem. Eng. J. 2017, 307, 1105–1115. [Google Scholar] [CrossRef]
- León, D.E.; Zúñiga-Benítez, H.; Peñuela, G.A.; Mansilla, H.D. Photocatalytic removal of the antibiotic cefotaxime on TiO2 and ZnO suspensions under simulated sunlight radiation. Water Air Soil Pollut. 2017, 228, 361. [Google Scholar] [CrossRef]
- Silva, G.; De Souza, G.M.; Neto, A.A.; De Matos Jorge, L.M.; Santos, O.A. Influence of ZnO content in mixed oxides catalysts applied in the photocatalytic degradation of atrazine. Chem. Eng. Trans. 2017, 57, 637–642. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.; Lazarević, M.; Despotović, V.; Banić, N.; Finčur, N.; Maletić, S.; Abramović, B. The effect of inorganic anions and organic matter on mesotrione (Callisto®) removal from environmental waters. J. Serbian Chem. Soc. 2017, 82, 343–355. [Google Scholar] [CrossRef]
- Azadi, M.; Hasani, A.H.; Olya, M.E.; Borghei, S.M. Application of ZnO-Ag-Nd nanocomposite as a new synthesized nanophotocatalyst for the degradation of organic compounds: Kinetic, thermodynamic and economic study. Toxicol. Ind. Health 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Guyer, G.T.; Abbas, G. Photocatalytic degradation of ibuprofen in water using TiO2 and ZnO under artificial UV and solar irradiation. Water Environ. Res. 2019, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; el Aatik, A.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Removal of Pesticides with Endocrine Disruptor Activity in Wastewater Effluent by Solar Heterogeneous Photocatalysis Using ZnO/Na2S2O8. Water Air Soil Pollut. 2019, 230, 134. [Google Scholar] [CrossRef]
- Suresh, M.; Sivasamy, A. Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq. 2020, 317, 114112. [Google Scholar] [CrossRef]
- Ángel-Hernández, B.; Hernández-Aldana, F.; Pérez-Osorio, G.; Gutiérrez-Arias, G.E.M. Municipal wastewater treatment by photocatalysis: Comparison between UV lamp and solar radiation using TiO2 and ZnO/TiO2 synthesized catalysts. Rev. Mex. Ing. Quim. 2021, 20, Cat2438. [Google Scholar] [CrossRef]
- Caracciolo, D.; Vaiano, V.; Gambicorti, T.; Sacco, O. Photocatalytic treatment of industrial wastewaters using structured photocatalysts. Chem. Eng. Trans. 2021, 84, 151–156. [Google Scholar]
- Finčur, N.; Sfirloaga, P.; Putnik, P.; Despotović, V.; Lazarević, M.; Uzelac, M.; Abramović, B.; Vlazan, P.; Ianasi, C.; Alapi, T.; et al. Removal of emerging pollutants from water using environmentally friendly processes: Photocatalysts preparation, characterization, intermediates identification and toxicity assessment. Nanomaterials 2021, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushik, R.D.; Upadhyay, G.K.; Purohit, L.P. rGO-ZnO nanocomposites as efficient photocatalyst for degradation of 4-BP and DEP using high temperature refluxing method in in-situ condition. J. Hazard. Mater. 2021, 406, 124300. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Jiang, W. Research on the sustainable heterogeneous catalyst development for photocatalytic treatment of phenol. Sustainability 2021, 13, 4670. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lejeune, L.; Haynes, T.; Body, N.; De Kreijger, S.; Elias, B.; Marcilli, R.H.M.; Fustin, C.-A.; Hermans, S. Crystalline ZnO photocatalysts prepared at ambient temperature: Influence of morphology on p-nitrophenol degradation in water. Catalysts 2021, 11, 1182. [Google Scholar] [CrossRef]
- Baladi, E.; Davar, F.; Hojjati-Najafabadi, A. Synthesis and characterization of g-C3N4-CoFe2O4-ZnO magnetic nanocomposites for enhancing photocatalytic activity with visible light for degradation of penicillin G antibiotic. Environ. Res. 2022, 215, 114270. [Google Scholar] [CrossRef]
- Tamashiro, J.R.; Lima, I.S.; Paiva, F.F.G.d.; Silva, L.H.P.; Oliveira, D.V.M.d.; Baffa, O.; Kinoshita, A. Treatment of Sugarcane Vinasse Using Heterogeneous Photocatalysis with Zinc Oxide Nanoparticles. Sustainability 2022, 14, 16052. [Google Scholar] [CrossRef]
- Despotović, V.; Finčur, N.; Bognar, S.; Šojić Merkulov, D.; Putnik, P.; Abramović, B.; Panić, S. Characterization and Photocatalytic performance of newly synthesized ZnO nanoparticles for environmental organic pollutants removal from water system. Separations 2023, 10, 258. [Google Scholar] [CrossRef]
- El Golli, A.; Contreras, S.; Dridi, C. Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment. Sci. Rep. 2023, 13, 20809. [Google Scholar] [CrossRef]
- Pavithra, M.; M B, J.R. Reusable porous chromium- zinc oxide nano-sheets for efficient detoxification of xenobiotics through integrated advanced oxidation water clean-up process. J. Hazard. Mater. Adv. 2024, 13, 100403. [Google Scholar] [CrossRef]
- Sarvothaman, V.P.; Velisoju, V.K.; Subburaj, J.; Panithasan, M.S.; Kulkarni, S.R.; Castano, P.; Turner, J.; Guida, P.; Roberts, W.L.; Nagarajan, S. Is cavitation a truly sensible choice for intensifying photocatalytic oxidation processes?—Implications on phenol degradation using ZnO photocatalysts. Ultrason. Sonochem. 2023, 99, 106548. [Google Scholar] [CrossRef]
- Thongam, D.D.; Chaturvedi, H. Induced defect and ZnO nano-flower formation by N, N, dimethylformamide solvent for natural sunlight responsive floating photocatalytic advanced oxidation process. Chemosphere 2023, 313, 137600. [Google Scholar] [CrossRef] [PubMed]
- Alsharyani, A.K.; Muruganandam, L. Fabrication of zinc oxide nanorods for photocatalytic degradation of docosane, a petroleum pollutant, under solar light simulator. RSC Adv. 2024, 14, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, A.S.; Popoola, L.T.; Gbadamosi, A.O.; Igbafe, A.I. Coal fly ash-supported ZnO-promoted TiO2 towards UV photocatalytic degradation of anthraquinone dye: Parametric optimization, kinetics and mechanism studies. Mater. Today Commun. 2024, 38, 107999. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Jung, C.; Im, J.-K.; Boateng, L.K.; Flora, J.R.V.; Jang, M.; Heo, J.; Park, C.M.; Yoon, Y. Sonocatalytic degradation coupled with single-walled carbon nanotubes for removal of ibuprofen and sulfamethoxazole. Chem. Eng. Sci. 2017, 162, 300–308. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Cazzato, G.; Saleemi, H.A.; Macadangdang, R.R., Jr.; Aftab, M.N.; Ismail, M.; Khalid, H.; Ali, S.; Bakhtiar, S.U.H.; Ismail, A.; et al. Sonophotocatalytic degradation of organic pollutant under visible light over Pt decorated CeO2: Role of ultrasonic waves for unprecedented degradation. J. Mol. Struct. 2022, 1247, 131397. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Sonochemical, photocatalytic and sonophotocatalytic oxidation of flonicamid pesticide solution using different catalysts. Chem. Eng. Process. 2020, 154, 108040. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Rezaei Kalantary, R.; Dehghani Fard, E. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Lewis, G.D.; Ashokkumar, M. Inactivation of Enterobacter aerogenes in reconstituted skim milk by high- and low-frequency ultrasound. Ultrason. Sonochem. 2014, 21, 2099–2106. [Google Scholar] [CrossRef]
- Gemici, B.T.; Karel, F.B.; Karaer, F.; Koparal, A.S. Water disinfection with advanced methods: Successive and hybrid application of antibacterial column with silver, ultrasound and UV radiation. Appl. Ecol. Environ. Res. 2018, 16, 4667–4680. [Google Scholar] [CrossRef]
- Giannakis, S.; Papoutsakis, S.; Darakas, E.; Escalas-Canellas, A.; Petrier, C.; Pulgarin, C. Ultrasound enhancement of near-neutral photo-Fenton for effective E. coli inactivation in wastewater. Ultrason. Sonochem. 2015, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Huddersman, K.; Ekpruke, A.; Asuelimen, L. Application of AOPs in the treatment of OSPAR chemicals and a comparative cost analysis. Crit. Rev. Environ. Sci. Technol. 2019, 49, 277–317. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of advanced oxidation processes (AOPs) in industrial applications: A review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. Chem. Eng. J. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef]
- Moradi, S.; Rodriguez-Seco, C.; Hayati, F.; Ma, D. Sonophotocatalysis with photoactive nanomaterials for wastewater treatment and bacteria disinfection. ACS Nanosci. Au 2023, 3, 103–129. [Google Scholar] [CrossRef]



| Organic Pollutant | Type of AOPs | Removal Efficiency (%) | Reference |
| Photocatalysis | |||
| Alprazolam | Heterogeneous photocatalysis with ZnO and TiO2 P25, under UV irradiation | 100 | [75] |
| Phenol | Heterogeneous photocatalysis with ZnO under UV irradiation | 55 | [17] |
| Mesotrione | Heterogeneous photocatalysis with ZnO and TiO2 P25 under UV | 100 | [76] |
| Alprazolam | Heterogeneous photocatalysis zinc–tin oxide nanocrystalline powders (both ternary and coupled binary) under UV irradiation | 90–100 depending on catalyst type | [77] |
| Amitriptyline | Heterogeneous photocatalysis with ZnO/SnO2, ZnO, and TiO2 P25 under UV irradiation | 80 | [78] |
| Direct blue 71 | Heterogeneous photocatalysis with Cu-doped ZnO under visible light | 96 | [79] |
| Alprazolam | Heterogeneous photocatalysis with ZnO and TiO2 Degussa P25 under UVA, visible, and solar irradiation | 100 (UV/ZnO) | [80] |
| Cefotaxime | Heterogeneous photocatalysis with ZnO under SSI | 90.6 | [81] |
| Atrazine | Heterogeneous photocatalysis with mixed oxide catalysts (ZnO/TiO2) using various ZnO concentrations, under UV irradiation | 97.5 (5% ZnO/TiO2) 94 (10% ZnO/TiO2) | [82] |
| Mesotrione | Heterogeneous photocatalysis with ZnO and TiO2 under UV irradiation | 40 (ground water and ZnO) 50 (river water and ZnO) | [83] |
| Acid red 18 | Heterogeneous photocatalysis with ZnO-Ag-Nd under UV irradiation | 100 | [84] |
| Ibuprofen | Heterogeneous photocatalysis with TiO2 and ZnO under UV irradiation | 99 | [85] |
| Two fungicides (vinclozoline and fenarimol) and four insecticides (malathion, fenotrothion, quinalphos, and dimethoate) | Heterogeneous photocatalysis with ZnO in pilot plant under artificial light | 92 (DOC) | [86] |
| Methylene blue | Heterogeneous photocatalysis with reduced graphene oxide-N-ZnO, under visible irradiation | 98.5 | [87] |
| Selected organic compounds (tetrachlorethylene; chlorodifluoroacetamide; amphetamine; dibutyl phthalate; (2,3-dihydroxypropyl Z)-9-octadecenoate; fluoxetine; furazan; phenylpropanolamine; phthalic acid; 1,2-benzisothiazol-3-amine; 2,3-dihydroxypropyl elaidate) | Heterogeneous photocatalysis with ZnO/TiO2 and TiO2 catalysts, under SSI | 85.5 | [88] |
| Wastewater with various organics | Heterogeneous photocatalysis with ZnO immobilized on polystyrene pellets under UV irradiation | 61.7 (TOC) | [89] |
| Two antibiotics (ciprofloxacin and ceftriaxone) and two herbicides (tembotrione and fluroxypyr). | Heterogeneous photocatalysis with TiO2, ZnO, and MgO under UV and SSI | 90–100 (depending on pollutant, under UV) 70–100 (depending on pollutant, under SSI) | [90] |
| Amitriptyline hydrochloride | Heterogeneous photocatalysis under UV and SSI using ZnO, TiO2 P25, and TiO2 Hombikat | 30 (TOC) | [50] |
| 4-bromophenol and diethyl phthalate | Heterogeneous photocatalysis with nanocomposites composed of reduced graphene oxide and ZnO under UV irradiation | 99 (4-bromophenol) 98.6 (diethyl phthalate) | [91] |
| Phenol | Heterogeneous photocatalysis with CdO/ZnO/Yb2O3 under SSI | 71.5 97.8 (with H2O2) | [92] |
| p-nitrophenol | Heterogeneous photocatalysis under UV irradiation using ZnO | 80 | [93] |
| Penicillin G | Heterogeneous photocatalysis with graphitic carbon nitride–calcium or magnesium co-doped cobalt ferrite–zinc oxide nanocomposite under natural sunlight | 74 | [94] |
| Various organics | Heterogeneous photocatalysis with ZnO nanoparticles under natural sunlight | 17.1 (COD) 71.7 (BOD) | [95] |
| Clomazone, amitriptyline, and sulcotrione | Heterogeneous photocatalysis with ZnO under SSI | 20–70% (depending on the pollutant) | [96] |
| Phenol, o-cresol, toluene, and xylene | Heterogeneous photocatalysis with green ZnO under SSI | 51 52 88 93 | [97] |
| Methylene blue dye | Heterogeneous photocatalysis with Ag/ZnO-ZnS/PANI under UV irradiation | 95 | [31] |
| Methylene orange and nitrophenol | Heterogeneous photocatalysis with chromium-doped ZnO under UV irradiation | 99 98.2 | [98] |
| Phenol | Heterogeneous photocatalysis with ZnO under UV and solar irradiation | >25 | [99] |
| Rhodamine B | Heterogeneous photocatalysis with ZnO under natural sunlight and UV irradiation | 91 (sunlight) 99 (UV) | [100] |
| Docosane | Heterogeneous photocatalysis with ZnO nanorods under natural sunlight | 68.5 | [101] |
| Clomazone, ciprofloxacin, and 17α-ethynilestradiol | Heterogeneous photocatalysis with ZnO/MeOx nanopowders (ZnO/MgO, ZnO/CeO2, and ZnO/ZrO2) under SSI | 77 86 71 | [25] |
| Acid blue 25 | Heterogeneous photocatalysis with TiO2-ZnO/coal fly ash under UV irradiation | 98 | [102] |
| Sonophotolysis/sonocatalysis | |||
| Ibuprofen Sulfamethoxazole | Sonolysis | 97 92 | [103] |
| Ciprofloxacin | Sonocatalysis | 61 | [104] |
| Sonophotocatalysis | |||
| Acid red 17 | Sonophotocatalysis with Pt/CeO2 | 90 | [105] |
| Amoxicilin | Sonophotocatalysis with N-doped TiO2 | 37 | [19] |
| Flonicamid | Sonophotocatalysis with CuO, ZnO, and TiO2 | 98.36 (COD, for TiO2) | [106] |
| Tetracycline | Sonophotocatalysis with TiO2 decorated on magnetic activated carbon | 93 | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bognár, S.; Jovanović, D.; Despotović, V.; Finčur, N.; Putnik, P.; Šojić Merkulov, D. Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal. Processes 2024, 12, 1256. https://doi.org/10.3390/pr12061256
Bognár S, Jovanović D, Despotović V, Finčur N, Putnik P, Šojić Merkulov D. Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal. Processes. 2024; 12(6):1256. https://doi.org/10.3390/pr12061256
Chicago/Turabian StyleBognár, Szabolcs, Dušica Jovanović, Vesna Despotović, Nina Finčur, Predrag Putnik, and Daniela Šojić Merkulov. 2024. "Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal" Processes 12, no. 6: 1256. https://doi.org/10.3390/pr12061256
APA StyleBognár, S., Jovanović, D., Despotović, V., Finčur, N., Putnik, P., & Šojić Merkulov, D. (2024). Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal. Processes, 12(6), 1256. https://doi.org/10.3390/pr12061256











