Electrostatic Spray Deposition of Al-Doped ZnO Thin Films for Acetone Gas Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
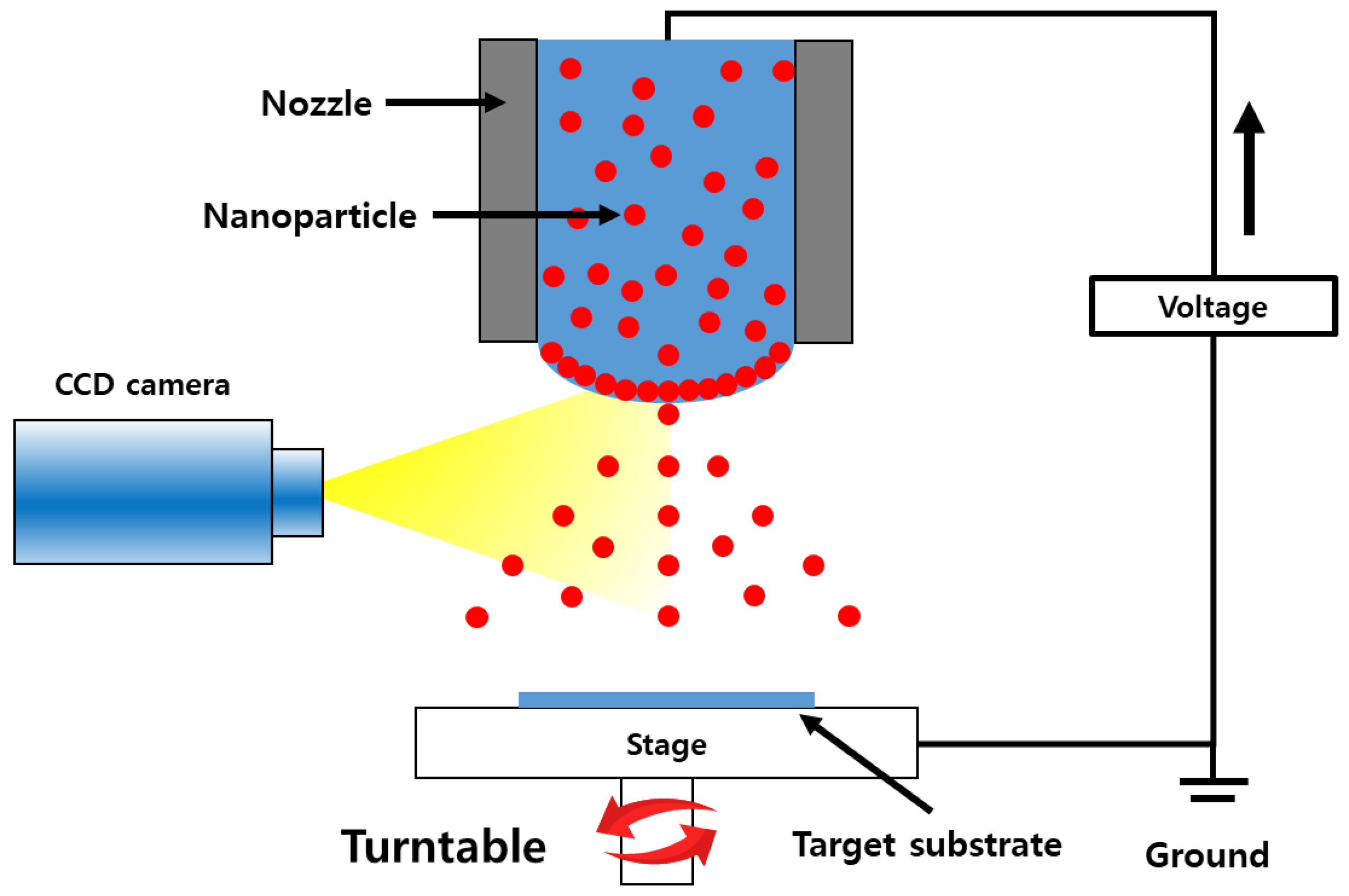
2.2. Sensing Layer Coating
2.3. Electrode Printing with Reverse Offset
2.4. Gas Sensing Measurement
3. Results and Discussion
3.1. XRD Crystal Structure Analysis
3.2. Analysis of Thin Films Formed by the ESD Process
3.3. The Effect of Coating Time on the Sensing Response
3.4. Surface Defects and the Analysis of Mechanisms
3.5. The Sensitivity and Selectivity of Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Cao, Y.; Zhang, H.; Hu, J. Acetone Sensing Properties and Mechanism of SnO2 Thick Films. Sensors 2018, 18, 3425. [Google Scholar] [CrossRef]
- Sonia, T.; Balamurugan, D.; Jeyaprakash, B.G. Investigation on sensing and stability property of spray deposited ZnO-acetone sensor at high temperature. Sens. Actuator A Phys. 2021, 331, 113041. [Google Scholar] [CrossRef]
- Šetka, M.; Bahos, F.A.; Matatagui, D.; Gràcia, I.; Figueras, E.; Drbohlavová, J.; Vallejos, S. Love Wave Sensors with Silver Modified Polypyrrole Nanoparticles for VOCs Monitoring. Sensors 2020, 20, 1432. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Luo, P.; Yu, Y.; Chen, D.; Li, X. Incorporating N Atoms into SnO2 Nanostructure as an Approach to Enhance Gas Sensing Property for Acetone. Nanomaterials 2019, 9, 445. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H.; Brown, R.J.C. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef]
- Li, J.; Tang, P.; Zhang, J.; Feng, Y.; Luo, R.; Chen, A.; Li, D. Facile Synthesis and Acetone Sensing Performance of Hierarchical SnO2 Hollow Microspheres with Controllable Size and Shell Thickness. Ind. Eng. Chem. Res. 2016, 55, 3588–3595. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B Chem. 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Li, Y.; Hua, Z.; Wu, Y.; Zeng, Y.; Qiu, Z.; Tian, X.; Wang, M. Surface Modification of Pt-loaded WO3 Nanosheets for Acetone Sensing Application. Chem. Lett. 2018, 47, 167–170. [Google Scholar] [CrossRef]
- Muthukrishnan, K.; Vanaraja, M.; Boomadevi, S.; Karn, R.K.; Singh, V.; Singh, P.K.; Pandiyan, K. Studies on ace-tone sensing characteristics of ZnO thin film prepared by sol–gel dip coating. J. Alloys Compd. 2016, 673, 138–143. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Olanrewaju Alade, I.; Qamar, M.; Akbar, S. Zinc Oxide-Based Acetone Gas Sensors for Breath Analysis: A Review. Chem. Asian J. 2021, 16, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.; Park, Y.; Jung, H.; Rim, H.J.; Cho, S.; Lee, H.-S.; Lee, W. Acetone-sensing properties of doped ZnO nano-particles for breath-analyzer applications. J. Alloys Compd. 2019, 803, 135–144. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Aziz, A.A. Sensing mechanism of hydrogen gas sensor based on RF-sputtered ZnO thin films. Int. J. Hydrogen Energy 2010, 35, 4428–4434. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N.; Bedi, R.K. Ethanol gas sensor based upon ZnO nanoparticles prepared by different techniques. Results Phys. 2017, 7, 801–806. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Noh, J.-H.; Kim, I.; Kim, D.-S.; Chun, S. Reliability of gravure offset printing under various printing con-ditions. J. Appl. Phys. 2010, 108, 102802. [Google Scholar] [CrossRef]
- Lee, T.-M.; Lee, S.-H.; Noh, J.-H.; Kim, D.-S.; Chun, S. The effect of shear force on ink transfer in gravure offset printing. J. Micromech. Microeng. 2010, 20, 125026. [Google Scholar] [CrossRef]
- Devabharathi, N.; Pradhan, J.R.; Priyadarsini, S.S.; Brezesinski, T.; Dasgupta, S. Inkjet-Printed Narrow-Channel Mesoporous Oxide-Based n-Type TFTs and All-Oxide CMOS Electronics. Adv. Mater. Interfaces 2022, 9, 2200949. [Google Scholar]
- Lee, H.; Cho, N.-J.; Kim, D. Development of a Novel Reverse Offset Printer Equipped with Double-Layer Blanket (DLB) for Micropattern Printing on 3D Curved Surfaces. Processes 2022, 10, 424. [Google Scholar] [CrossRef]
- Brooke, R.; Wijeratne, K.; Hübscher, K.; Belaineh, D.; Andersson Ersman, P. Combining Vapor Phase Polymerization and Screen Printing for Printed Electronics on Flexible Substrates. Adv. Mater. Technol. 2022, 7, 2101665. [Google Scholar] [CrossRef]
- Ogbeide, O.; Bae, G.; Yu, W.; Morrin, E.; Song, Y.; Song, W.; Li, Y.; Su, B.; An, K.; Hasan, T. Inkjet-Printed rGO/binary Metal Oxide Sensor for Predictive Gas Sensing in a Mixed Environment. Adv. Funct. Mater. 2022, 32, 2113348. [Google Scholar] [CrossRef]
- Yin, H.; Lv, P.; Gao, B.; Zhang, Y.; Zhu, Y.; Hu, M.; Tan, B.; Xu, M.; Huang, F.; Cheng, Y.-B.; et al. Slot-die coated scalable hole transporting layers for efficient perovskite solar modules. J. Mater. Chem. A 2022, 10, 25652–25660. [Google Scholar] [CrossRef]
- Henriques, A.; Rabiei Baboukani, A.; Jafarizadeh, B.; Chowdhury, A.H.; Wang, C. Nano-Confined Tin Oxide in Carbon Nanotube Electrodes via Electrostatic Spray Deposition for Lithium-Ion Batteries. Materials 2022, 15, 9086. [Google Scholar] [CrossRef] [PubMed]
- Ghimbeu, C.M.; Schoonman, J.; Lumbreras, M.; Siadat, M. Electrostatic spray deposited zinc oxide films for gas sensor applications. Appl. Surf. Sci. 2007, 253, 7483–7489. [Google Scholar] [CrossRef]
- Lee, J.; Koo, H.; Kim, S.Y.; Kim, S.J.; Lee, W. Electrostatic spray deposition of chemochromic WO3-Pd sensor for hydrogen leakage detection at room temperature. Sens. Actuators B Chem. 2021, 327, 128930. [Google Scholar] [CrossRef]
- Bharath, S.P.; Bangera, K.V. Fast detection and discriminative analysis of volatile organic compounds using Al-doped ZnO thin films. Appl. Phys. A 2021, 127, 699. [Google Scholar] [CrossRef]
- Ennaceri, H.; Taleb, A.; Boujnah, M.; Khaldoun, A.; Ebothé, J.; Ennaoui, A.; Benyoussef, A. Theoretical and experimental studies of Al-doped ZnO thin films: Optical and structural properties. J. Comput. Electron. 2021, 20, 1948–1958. [Google Scholar] [CrossRef]
- Mahmood, K.; Park, S.B. Atmospheric pressure based electrostatic spray deposition of transparent conductive ZnO and Al-doped ZnO (AZO) thin films: Effects of Al doping and annealing treatment. Electron. Mater. Lett. 2013, 9, 161–170. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Majeed Khan, M.A.; Ahamed, M. Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci. Rep. 2015, 5, 2206401. [Google Scholar] [CrossRef]
- Bie, L.-J.; Yan, X.-N.; Yin, J.; Duan, Y.-Q.; Yuan, Z.-H. Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B Chem. 2007, 126, 604–608. [Google Scholar] [CrossRef]
- Yoo, R.; Güntner, A.T.; Park, Y.; Rim, H.J.; Lee, H.-S.; Lee, W. Sensing of acetone by Al-doped ZnO. Sens. Actuators B Chem. 2019, 283, 107–115. [Google Scholar] [CrossRef]
- Kampara, R.K.; Rai, P.K.; Jeyaprakash, B.G. Highly sensitive graphene oxide functionalized ZnO nanowires for ammonia vapour detection at ambient temperature. Sens. Actuators B Chem. 2018, 255, 1064–1071. [Google Scholar]
- Sankar ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Ren, H.; Xia, A.; Liu, Y. Direct double Z-scheme O-g-C3N4/Zn2SnO4N/ZnO ternary hetero-junction photocatalyst with enhanced visible photocatalytic activity. Appl. Surf. Sci. 2019, 492, 690–702. [Google Scholar] [CrossRef]
- Han, B.; Liu, X.; Xing, X.; Chen, N.; Xiao, X.; Liu, S.; Wang, Y. A high response butanol gas sensor based on ZnO hollow spheres. Sens. Actuators B Chem. 2016, 237, 423–430. [Google Scholar] [CrossRef]
- Bai, S.; Guo, T.; Zhao, Y.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. Mechanism enhancing gas sensing and first-principle calculations of Al-doped ZnO nanostructures. J. Mater. Chem. A 2013, 1, 11335. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Sim, J.-H.; Oh, G.; Won, M.; Mantry, S.P.; Kim, D.-S. Electrostatic Spray Deposition of Al-Doped ZnO Thin Films for Acetone Gas Detection. Processes 2023, 11, 3390. https://doi.org/10.3390/pr11123390
Lee G, Sim J-H, Oh G, Won M, Mantry SP, Kim D-S. Electrostatic Spray Deposition of Al-Doped ZnO Thin Films for Acetone Gas Detection. Processes. 2023; 11(12):3390. https://doi.org/10.3390/pr11123390
Chicago/Turabian StyleLee, Geonhui, Jae-Ho Sim, Gyeongseok Oh, Mijin Won, Snigdha Paramita Mantry, and Dong-Soo Kim. 2023. "Electrostatic Spray Deposition of Al-Doped ZnO Thin Films for Acetone Gas Detection" Processes 11, no. 12: 3390. https://doi.org/10.3390/pr11123390
APA StyleLee, G., Sim, J.-H., Oh, G., Won, M., Mantry, S. P., & Kim, D.-S. (2023). Electrostatic Spray Deposition of Al-Doped ZnO Thin Films for Acetone Gas Detection. Processes, 11(12), 3390. https://doi.org/10.3390/pr11123390





