Dynamic Parameter Simulations for a Novel Small-Scale Power-to-Ammonia Concept
Abstract
1. Introduction
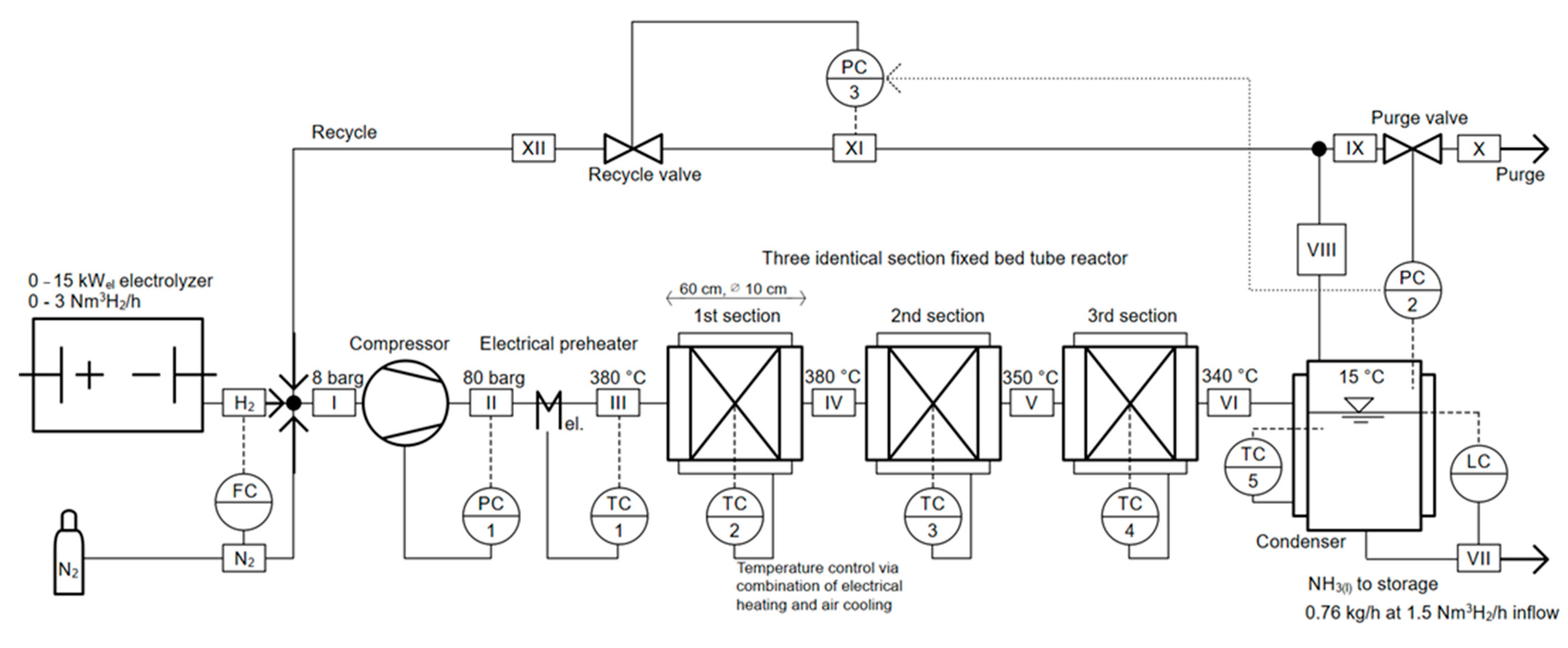
2. Materials and Method—System Description and Simulation Set-Up
- (a)
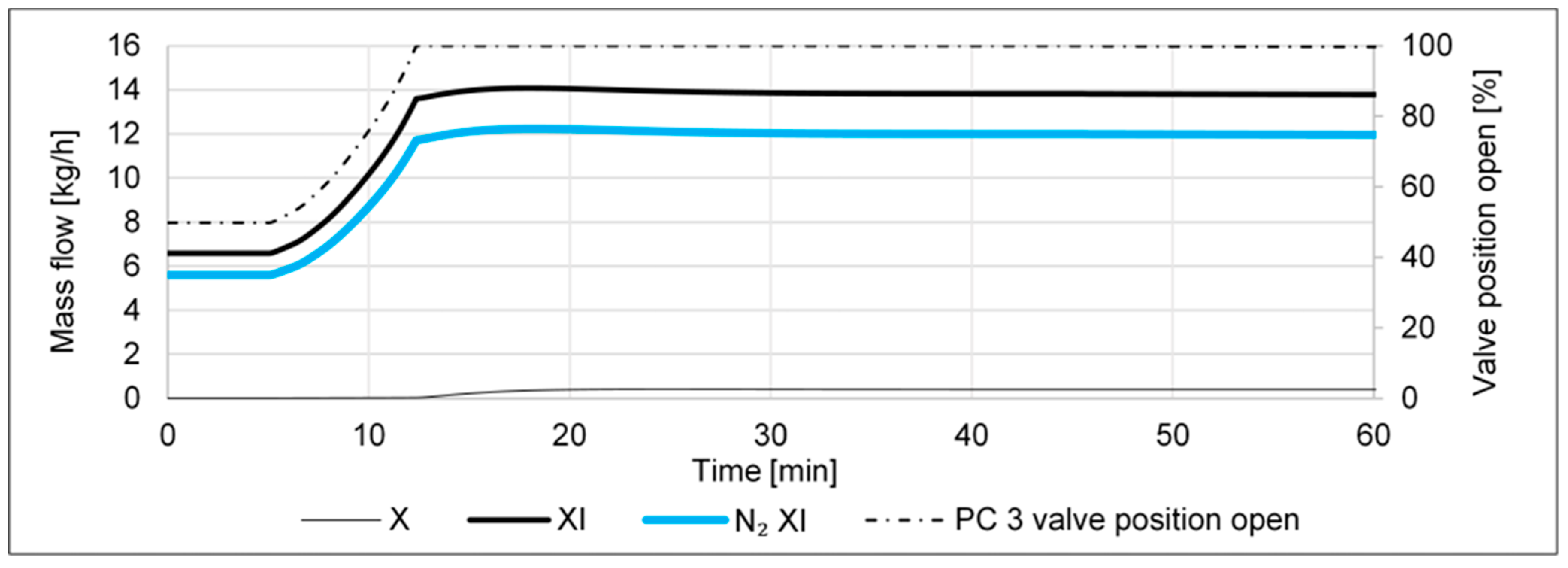
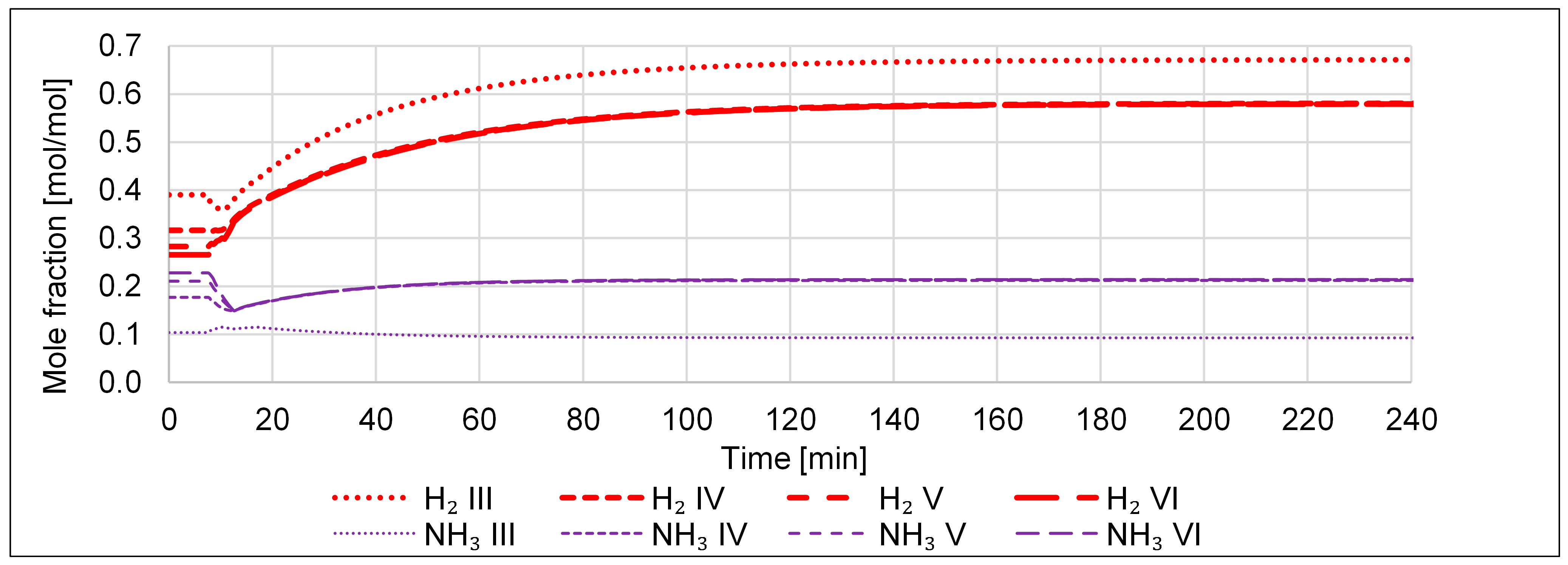
- An increase in the N2 inlet, such that the value of RH2N2 is changed from 2.96 to 2, shall emulate a case of a malfunctioning FC, i.e., what happens to the cycle if too much N2 is being fed into it.
- (b)
- A decrease in the N2 inlet, such that the value of RH2N2 is changed from 2.96 to 4, shall emulate a case of a malfunctioning FC, i.e., what happens to the cycle if too little N2 is being fed into it.
- (c)
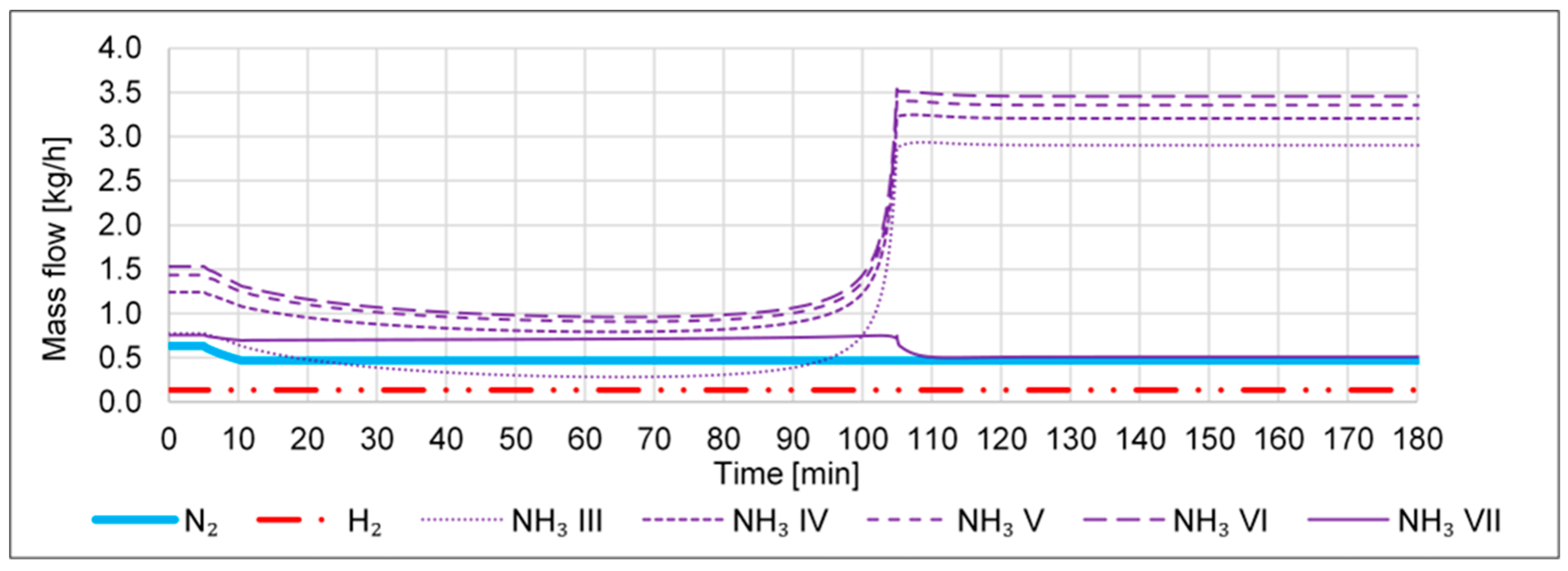
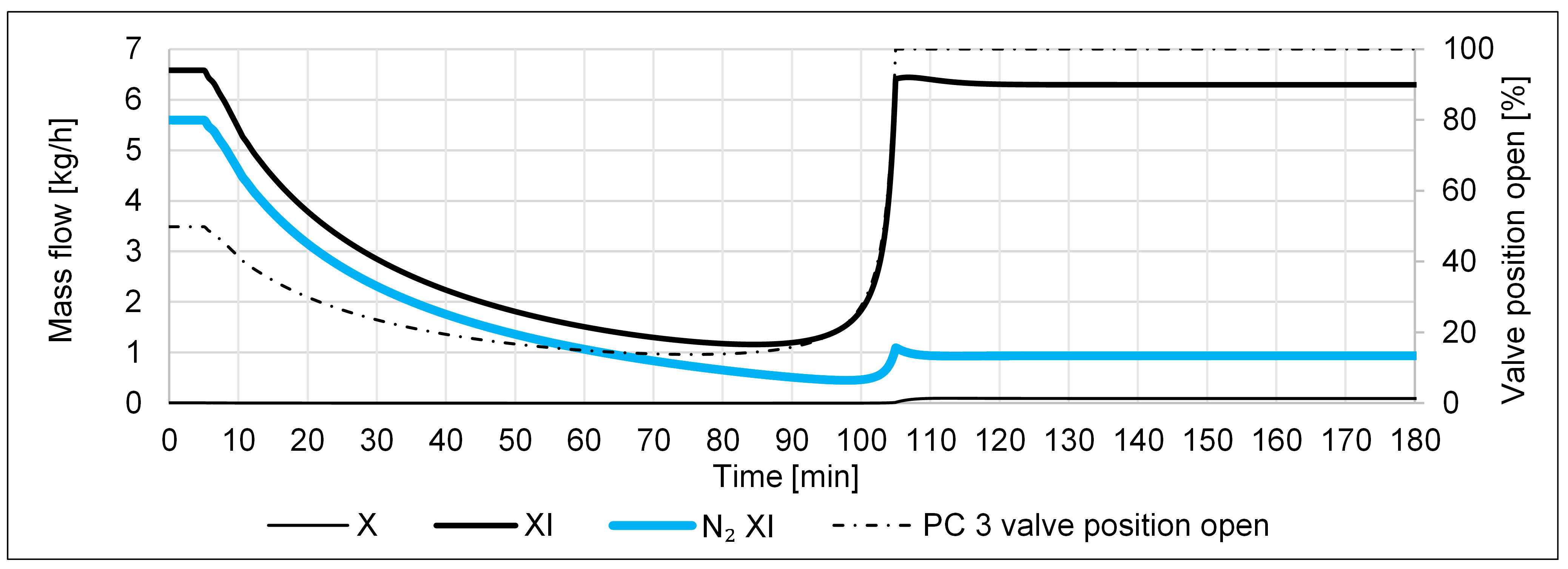
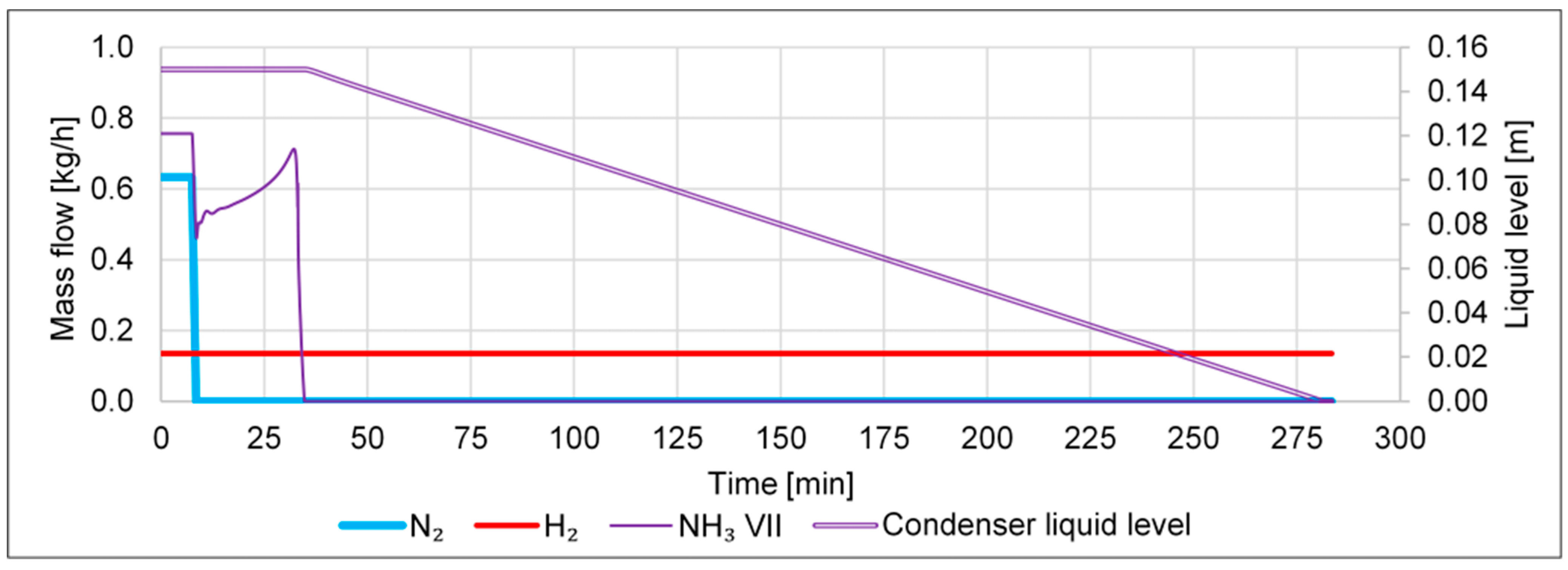
- A failure in the N2 inlet, i.e., a drop from 0.023 to 0 kmol/h within 1 min, shall emulate a sudden failure in the N2 supply or of the FC.
- (d)
- A failure in the H2 inlet, i.e., a drop from 0.067 to 0 kmol/h within 1 min, shall emulate a sudden failure in the H2 supply, e.g., due to an outage of the electrolyzer.
- (e)
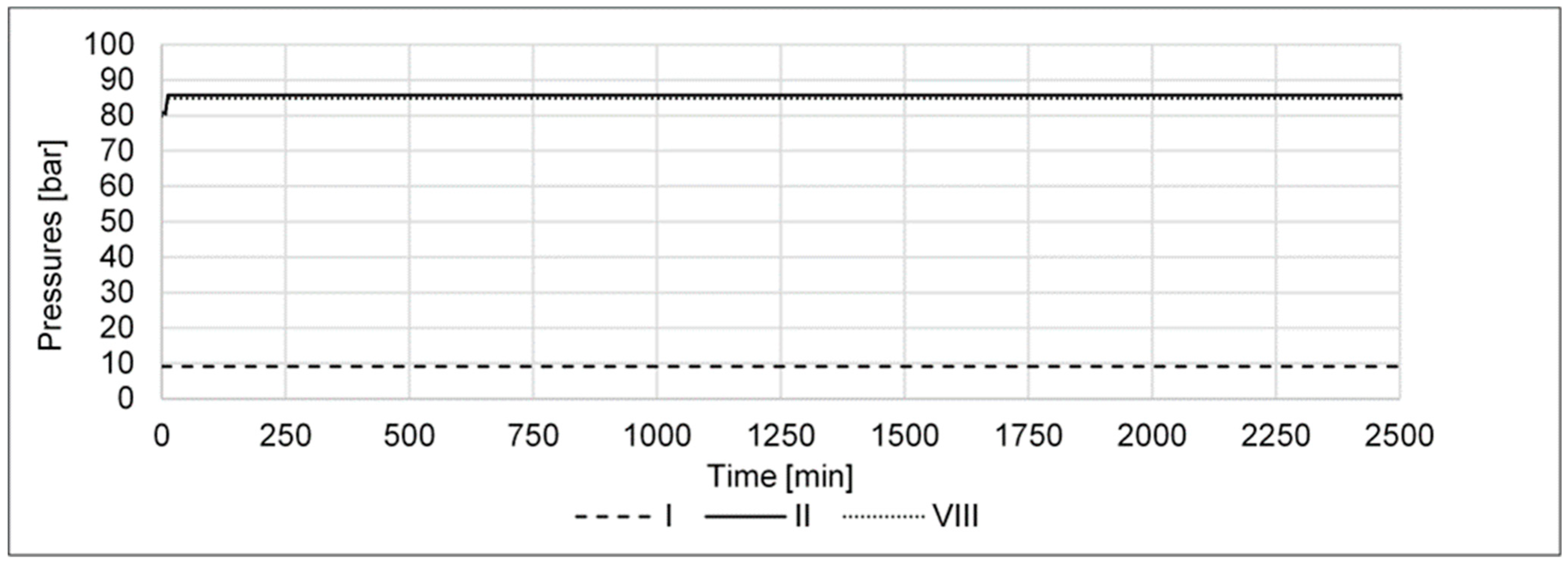
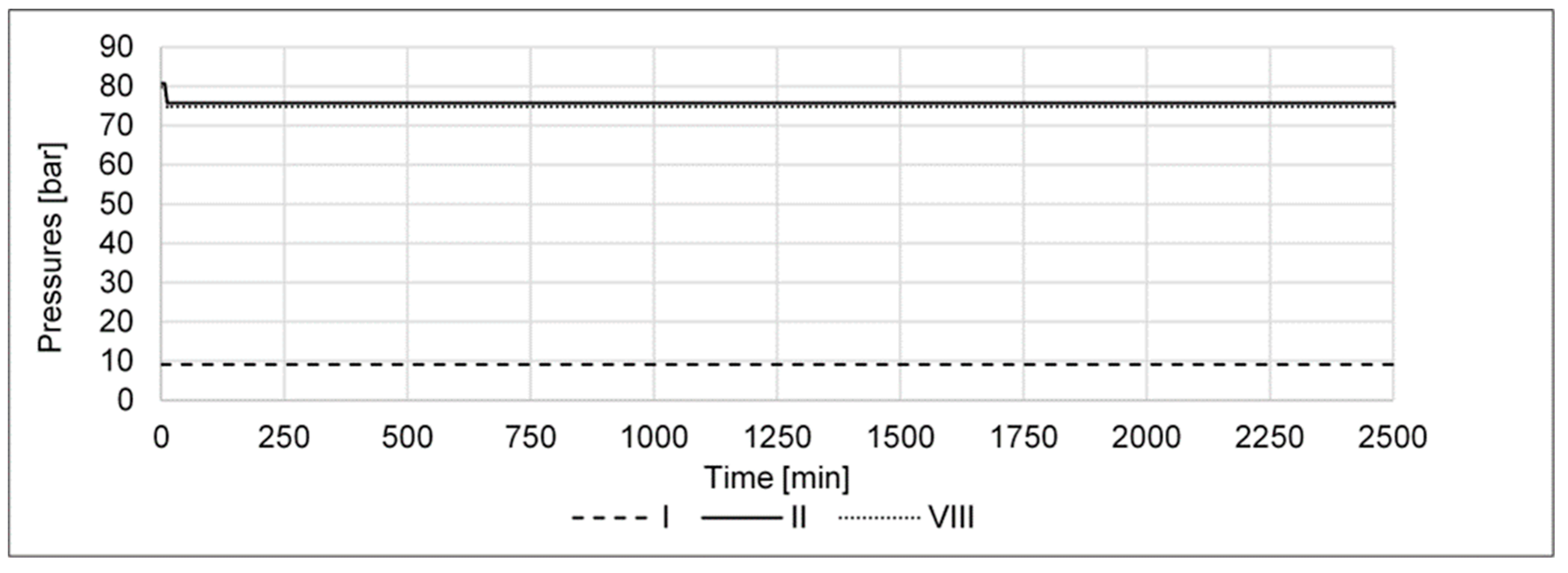
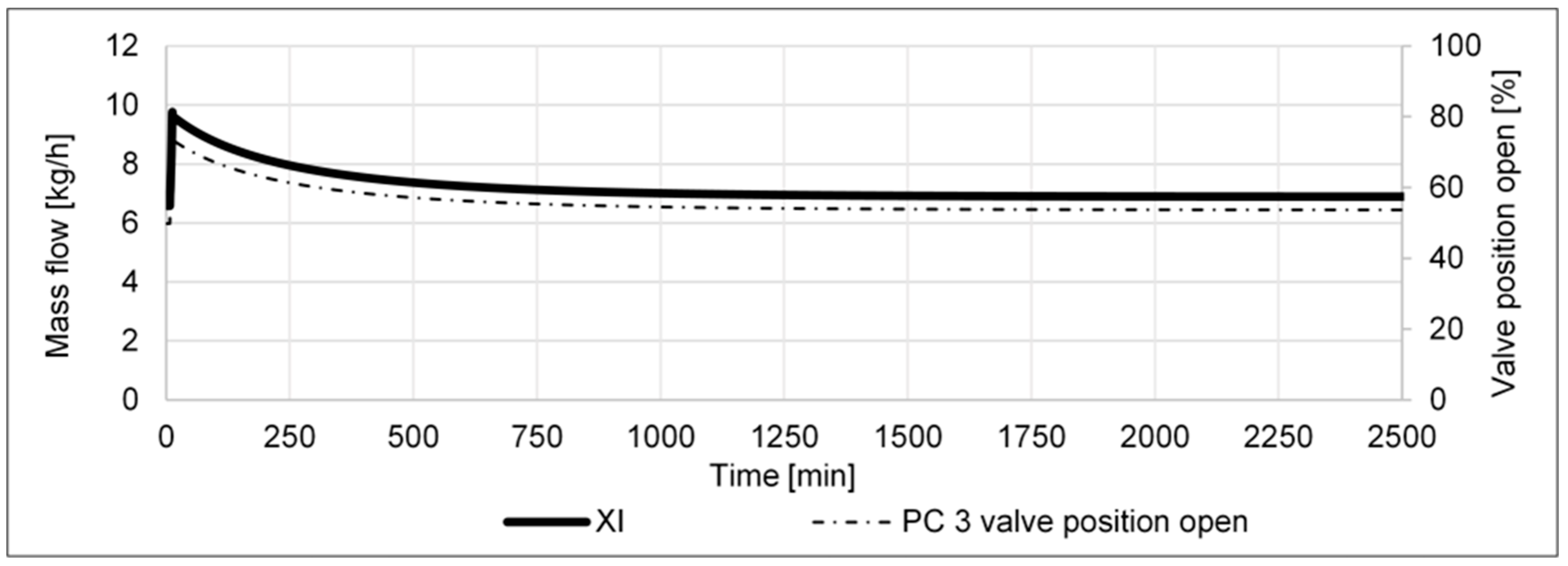
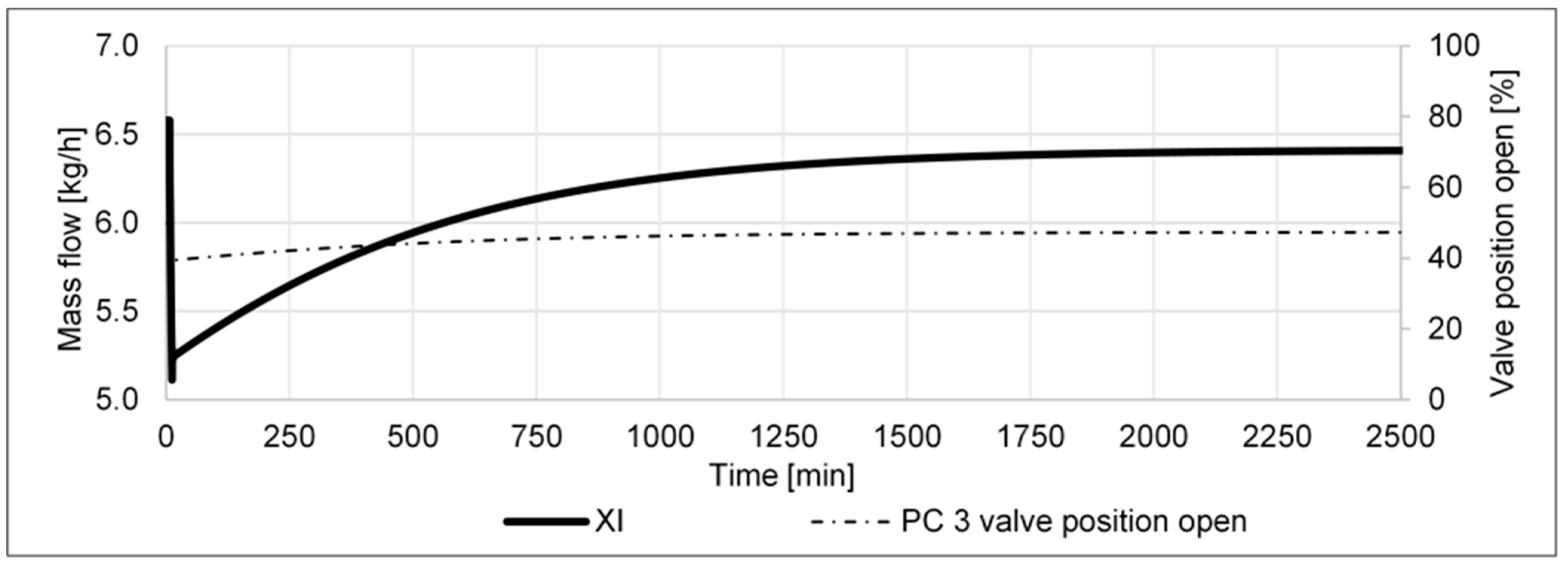
- A pressure increase from 80 to 85 barg in the high-pressure part of the cycle shall determine if the cycle’s performance can be improved by higher pressure, e.g., via a compressor retrofit.
- (f)
- A pressure decrease from 80 to 75 barg in the high-pressure part of the cycle shall determine if the cycle’s performance is reduced as a result of the lower pressure in the reactor.
- (g)
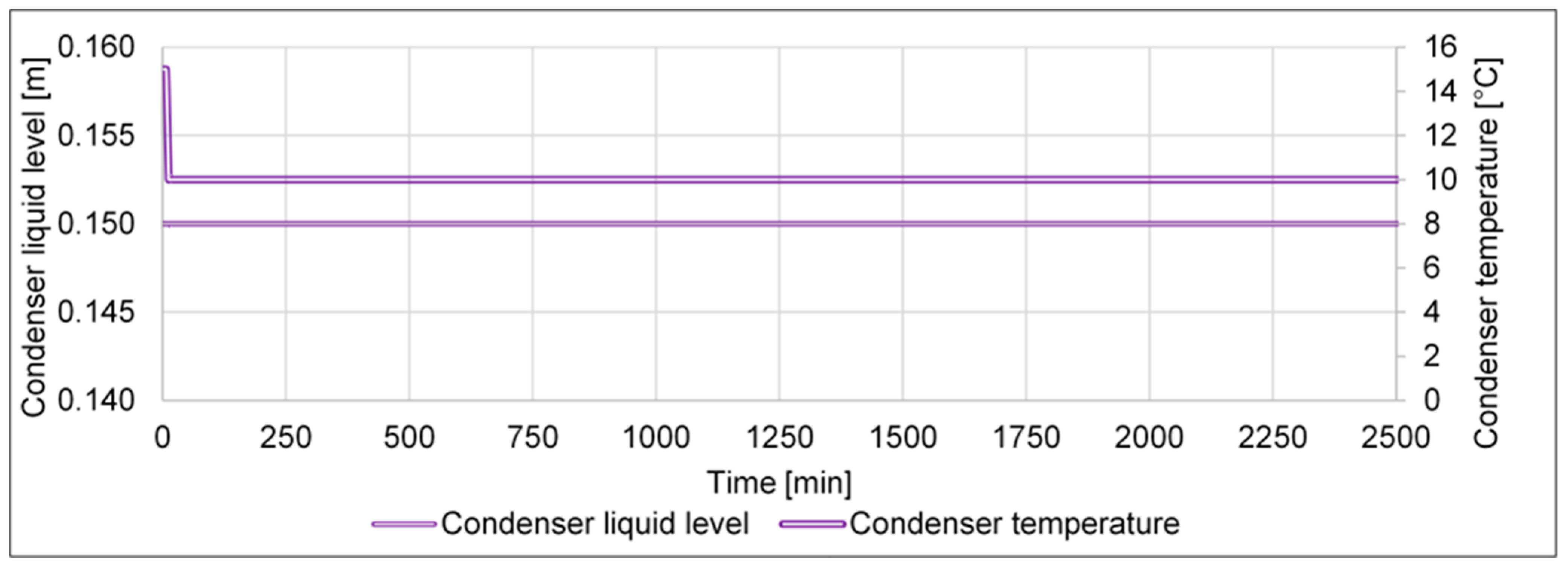
- An increase in the condenser temperature from 15 to 20 °C shall investigate how a malfunctioning cooling of the condenser affects the cycle’s performance.
- (h)
- A decrease in the condenser temperature from 15 to 10 °C shall investigate if the cycle can be improved by a cooling system that reaches lower temperatures.
- (i)
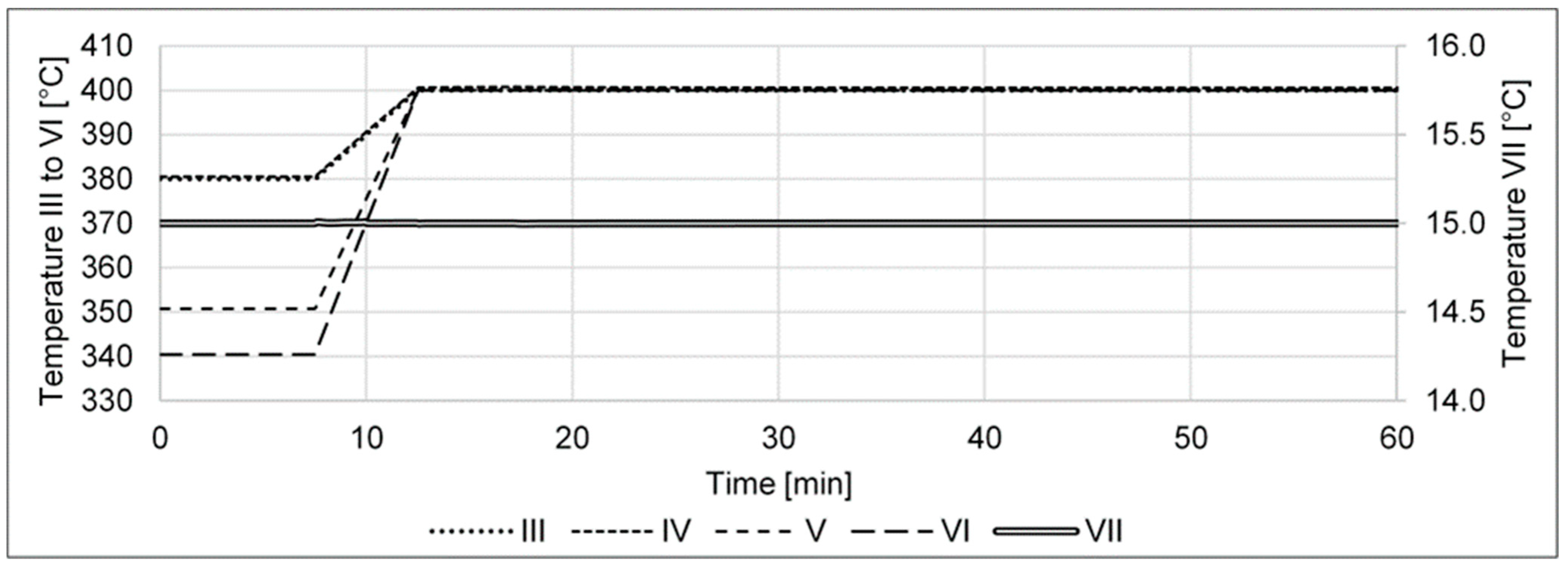
- A collective increase in the reactor temperature profile from 380, 380, 350, and 340 to 400 °C shall investigate how the cycle’s performance is affected by a malfunctioning cooling of the reactor resulting in a too-high isothermal profile of the reactor.
- (j)
- A collective decrease in the reactor temperature profile from 380, 380, 350, and 340 to 300 °C shall investigate how the cycle’s performance is affected by a malfunctioning heating of the reactor resulting in a too-low isothermal profile of the reactor.
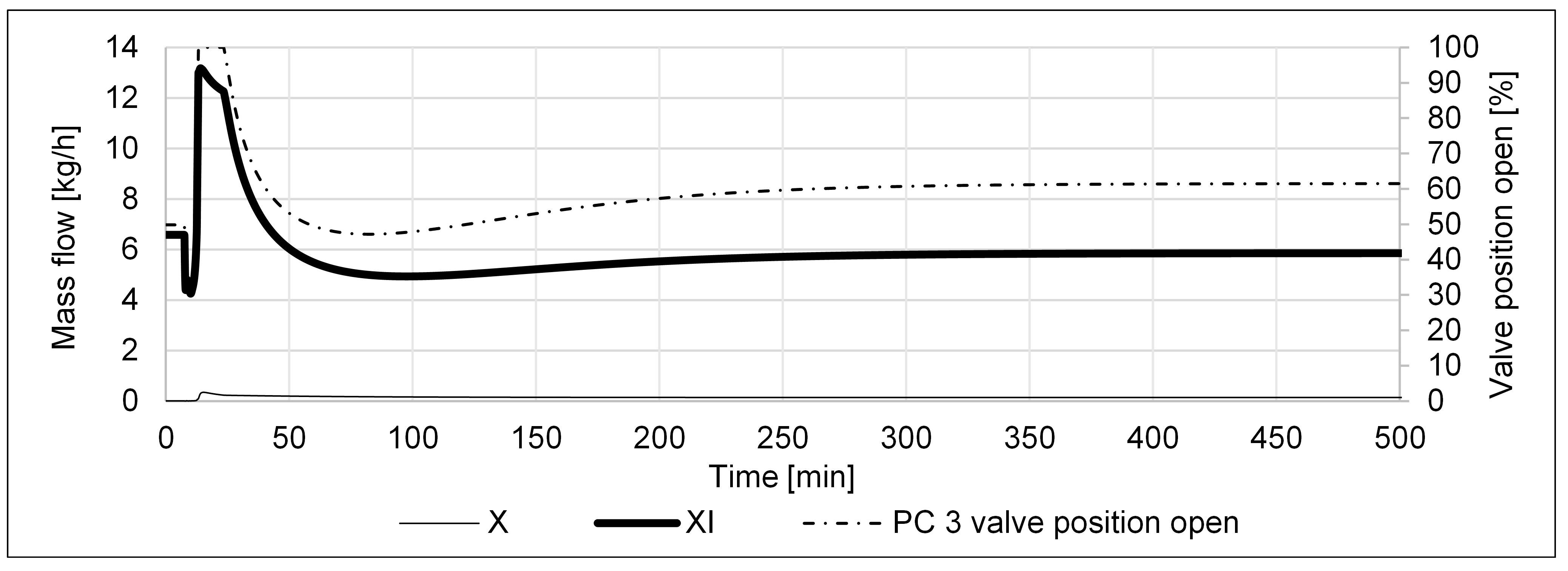
3. Results
4. Discussion & Conclusions
Author Contributions
Funding

Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| CO2 | Carbon dioxide |
| EU | European Union |
| FC | Flow control |
| FLEXnCONFU | Flexibilize combined cycle power plant through power-to-X solutions using non-conventional fuels |
| H2 | Hydrogen |
| HYSPR | Aspen HYSYS® Peng Robinson |
| LLHW | Langmuir Hinshelwood Hougen Watson |
| N2 | Nitrogen |
| NH3 | Ammonia |
| P2A | Power-to-ammonia |
| PC | Pressure control |
| PID | Proportional integration differential controller |
| RH2N2 | Cycle inlet molar ratio of H2 to N2 |
| TC | Temperature control |
| Roman letters | |
| I, II, …, XII | Stream numbering |
References
- European Commission Directorate-General for Energy. The Role and Potential of Power-to-X in 2050: METIS Studies, Study S8; European Commission: Brussels, Belgium, 2019; ISBN 978-92-76-03322-6. [Google Scholar]
- Kroch, E. Ammonia—A Fuel For Motor Buses. J. Inst. Pet. 1945, 31, 213–223. [Google Scholar]
- Atchison, J. Amogy: Ammonia-Powered Tractor. Press Release. Available online: https://www.ammoniaenergy.org/articles/amogy-ammonia-powered-tractor/ (accessed on 5 December 2022).
- MAN Energy Solutions. MAN B&W Two-Stroke Engine Operating on Ammonia. Available online: https://www.man-es.com/docs/default-source/marine/tools/man-b-w-two-stroke-engine-operating-on-ammonia.pdf?sfvrsn=544dc811_10 (accessed on 4 December 2022).
- Atchison, J. Ocean Network Express: Adding Ammonia Power to the Fleet. Press Release. Available online: https://www.ammoniaenergy.org/articles/ocean-network-express-adding-ammonia-power-to-the-fleet/?mc_cid=f6b5bf45bb&mc_eid=d2d2750d43 (accessed on 5 December 2022).
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Ito, S.; Uchida, M.; Suda, T.; Fujimori, T. Development of Ammonia Gas Turbine Co-Generation Tech. IHI Eng. Rev. 2020, 53, 1–6. [Google Scholar]
- Okafor, E.C.; Kurata, O.; Yamashita, H.; Inoue, T.; Tsujimura, T.; Iki, N.; Hayakawa, A.; Ito, S.; Uchida, M.; Kobayashi, H. Liquid ammonia spray combustion in two-stage micro gas turbine combustors at 0.25 MPa; Relevance of combustion enhancement to flame stability and NOx control. Appl. Energy Combust. Sci. 2021, 7, 100038. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Kaltschmitt, M. (Ed.) Renewable Energy: Technology, Economics and Environment; with 66 Tables; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-70947-3. [Google Scholar]
- European Commission. A European Green Deal: Striving to be the First Climate-Neutral Continent. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 5 December 2022).
- Ausfelder, F.; Herrmann, E.O.; López González, L.F. Perspective Europe 2030: Technology Options for CO2-Emission Reduction of Hydrogen Feedstock in Ammonia Production; DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V: Frankfurt am Main, Germany, 2022; ISBN 978-3-89746-237-3. [Google Scholar]
- Rouwenhorst, K.H.; van der Ham, A.G.; Mul, G.; Kersten, S.R. Islanded ammonia power systems: Technology review & conceptual process design. Renew. Sustain. Energy Rev. 2019, 114, 109339. [Google Scholar] [CrossRef]
- Reese, M.; Marquart, C.; Malmali, M.; Wagner, K.; Buchanan, E.; McCormick, A.; Cussler, E.L. Performance of a Small-Scale Haber Process. Ind. Eng. Chem. Res. 2016, 55, 3742–3750. [Google Scholar] [CrossRef]
- Duckett, A. Green Ammonia Project Set for Launch in UK Today. Press Release. Available online: https://www.thechemicalengineer.com/news/green-ammonia-project-set-for-launch-in-uk-today/ (accessed on 4 December 2022).
- Yamagami, A.; Fujiwara, H. World’s First Successful Ammonia Synthesis Using Renewable Energy-Based Hydrogen and Power Generation: Progress Toward Realization of Hydrogen Energy Carriers. Press Release. Available online: https://www.jgc.com/en/news/assets/pdf/20181019e.pdf (accessed on 4 December 2022).
- Stephens, A.D.; Richards, R.J. Steady state and dynamic analysis of an ammonia synthesis plant. Automatica 1973, 9, 65–78. [Google Scholar] [CrossRef]
- Zhang, C.; Vasudevan, S.; Rangaiah, G.P. Plantwide Control System Design and Performance Evaluation for Ammonia Synthesis Process. Ind. Eng. Chem. Res. 2010, 49, 12538–12547. [Google Scholar] [CrossRef]
- Dastjerd, F.T.; Sadeghi, J. The Simulation and Control of Ammonia Unit of Shiraz Petrochemical Complex, Iran. J. Chem. Pet. Eng. 2018, 52, 107–122. [Google Scholar] [CrossRef]
- Verleysen, K.; Parente, A.; Contino, F. How sensitive is a dynamic ammonia synthesis process? Global sensitivity analysis of a dynamic Haber-Bosch process (for flexible seasonal energy storage). Energy 2021, 232, 121016. [Google Scholar] [CrossRef]
- Bland, M.J. Optimisation of an Ammonia Synthesis Loop; Norwegian University of Science and Technology: Trondheim, Norway, 2015. [Google Scholar]
- Aspen Tech. Aspen Plus Ammonia Model. Available online: https://user.eng.umd.edu/~nsw/chbe446/Ammonia-Aspen.pdf (accessed on 5 December 2022).
- Appl, M.; Ammonia,vol. 2, 3. Production Processes. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Araújo, A.; Skogestad, S. Control structure design for the ammonia synthesis process. Comput. Chem. Eng. 2008, 32, 2920–2932. [Google Scholar] [CrossRef]
- de Tian, W.; Yu, Z.P.; Sun, S.L. Dynamic Simulation of Ammonia Synthesis Loop under Abnormal Conditions. AMR 2011, 396–398, 955–958. [Google Scholar] [CrossRef]
- Palys, M.; McCormick, A.; Cussler, E.; Daoutidis, P. Modeling and Optimal Design of Absorbent Enhanced Ammonia Synthesis. Processes 2018, 6, 91. [Google Scholar] [CrossRef]
- Verleysen, K.; Coppitters, D.; Parente, A.; de Paepe, W.; Contino, F. How can power-to-ammonia be robust? Optimization of an ammonia synthesis plant powered by a wind turbine considering operational uncertainties. Fuel 2020, 266, 117049. [Google Scholar] [CrossRef]
- Kirova-Yordanova, Z. Exergy analysis of industrial ammonia synthesis. Energy 2004, 29, 2373–2384. [Google Scholar] [CrossRef]
- Penkuhn, M.; Tsatsaronis, G. Comparison of different ammonia synthesis loop configurations with the aid of advanced exergy analysis. Energy 2017, 137, 854–864. [Google Scholar] [CrossRef]
- Morud, J.C.; Skogestad, S. Analysis of instability in an industrial ammonia reactor. AIChE J. 1998, 44, 888–895. [Google Scholar] [CrossRef]
- Kasiri, N.; Hosseini, A.R.; Moghadam, M. Dynamic simulation of an ammonia synthesis reactor. In European Symposium on Computer Aided Process Engineering-13, 36th European Symposium of the Working Party on Computer Aided Process Engineering; Elsevier: Amsterdam, The Netherlands, 2003; pp. 695–700. ISBN 9780444513687. [Google Scholar]
- Altaf, B.; Montague, G.; Martin, E.B. Dynamic Process Monitoring of An Ammonia Synthesis Fixed-Bed Reactor. Int. J. Mech. Mechatron. Eng. 2016, 10, 462–472. [Google Scholar] [CrossRef]
- Allman, A.; Daoutidis, P. Optimal scheduling for wind-powered ammonia generation: Effects of key design parameters. Chem. Eng. Res. Des. 2018, 131, 5–15. [Google Scholar] [CrossRef]
- Jinasena, A.; Bernt, L.; Bjørn, G. Dynamic model of an ammonia synthesis reactor based on open information. In Proceedings of the 9th EUROSIM Congress on Modelling and Simulation, EUROSIM 2016, The 57th SIMS Conference on Simulation and Modelling SIMS 2016. Mo, 12–16 September 2016; Linköping University Electronic Press: Linköping, Sweden, 2018; pp. 998–1004. [Google Scholar]
- Adhi, T.P.; Prasetyo, M.I. Process Stability Identification Through Dynamic Study of Single-bed Ammonia Reactor with Feed-Effluent Heat Exchanger (FEHE). MATEC Web Conf. 2018, 156, 3003. [Google Scholar] [CrossRef]
- Tripodi, A.; Compagnoni, M.; Bahadori, E.; Rossetti, I. Process simulation of ammonia synthesis over optimized Ru/C catalyst and multibed Fe + Ru configurations. J. Ind. Eng. Chem. 2018, 66, 176–186. [Google Scholar] [CrossRef]
- ICI Caldaie Company, C. Tregambe, Personal Communication in the Framework of the Collaboration in the EU H2020 FLEXnCONFU Project. Available online: www.flexnconfu.eu (accessed on 1 September 2022).
- Koschwitz, P.; Bellotti, D.; Liang, C.; Epple, B. Dynamic Simulation of a Novel Small-scale Power to Ammonia Concept. Energy Proc. 2022, 28, 1–10. [Google Scholar] [CrossRef]
- Koschwitz, P.; Bellotti, D.; Liang, C.; Epple, B. Steady state process simulations of a novel containerized power to ammonia concept. Int. J. Hydrogen Energy 2022, 47, 25322–25334. [Google Scholar] [CrossRef]
- Aspen Tech. Aspen Plus Dynamics simulation software V11 (37.0.0.395). Available online: https://www.aspentech.com/en/products/engineering/aspen-plus-dynamics (accessed on 21 February 2023).
- Bazzanella, A.M.; Ausfelder, F. DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V. In Low Carbon Energy and Feedstock for the European Chemical Industry: Technology Study; DECHEMA e.V.: Frankfurt am Main, Germany, 2017; ISBN 978-3-89746-196-2. [Google Scholar]
- Proton Ventures BV. Personal Communication in the Framework of the Collaboration in the EU H2020 FLEXnCONFU Project. Available online: www.flexnconfu.eu (accessed on 1 February 2021).
- McPhy. Electrolyser Series H 2021. Sales Brochure. Available online: https://cellar-c2.services.clever-cloud.com/com-mcphy/uploads/2020/08/20.05.McPhy_Portfolio_ELY_Piel_EN.pdf (accessed on 1 December 2022).
- Air Liquide. Alphagaz 2 Nitrogen/N2. Product Data Sheet. Available online: https://mygas.airliquide.be/files/830649f95c7d94a5d1ca3b9a428c7ede434dc08d_ALPHAGAZ____2_NITROGEN_en_BE_v1.6.pdf (accessed on 1 December 2022).













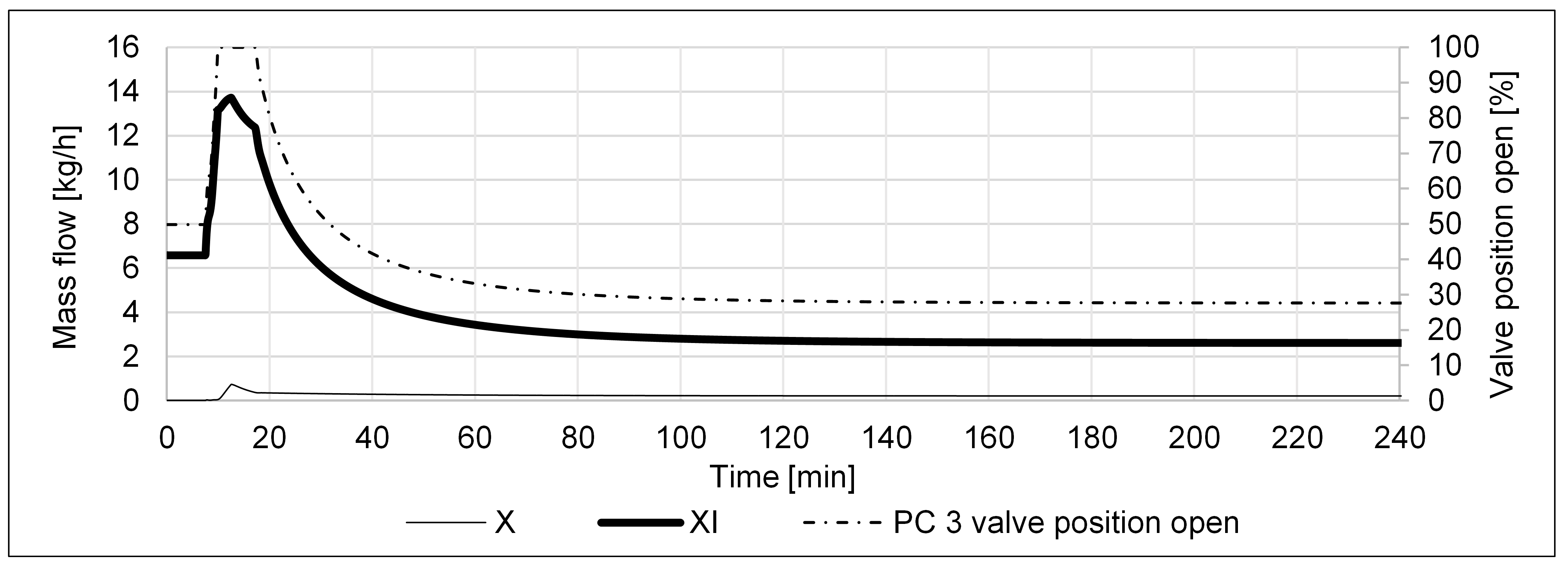
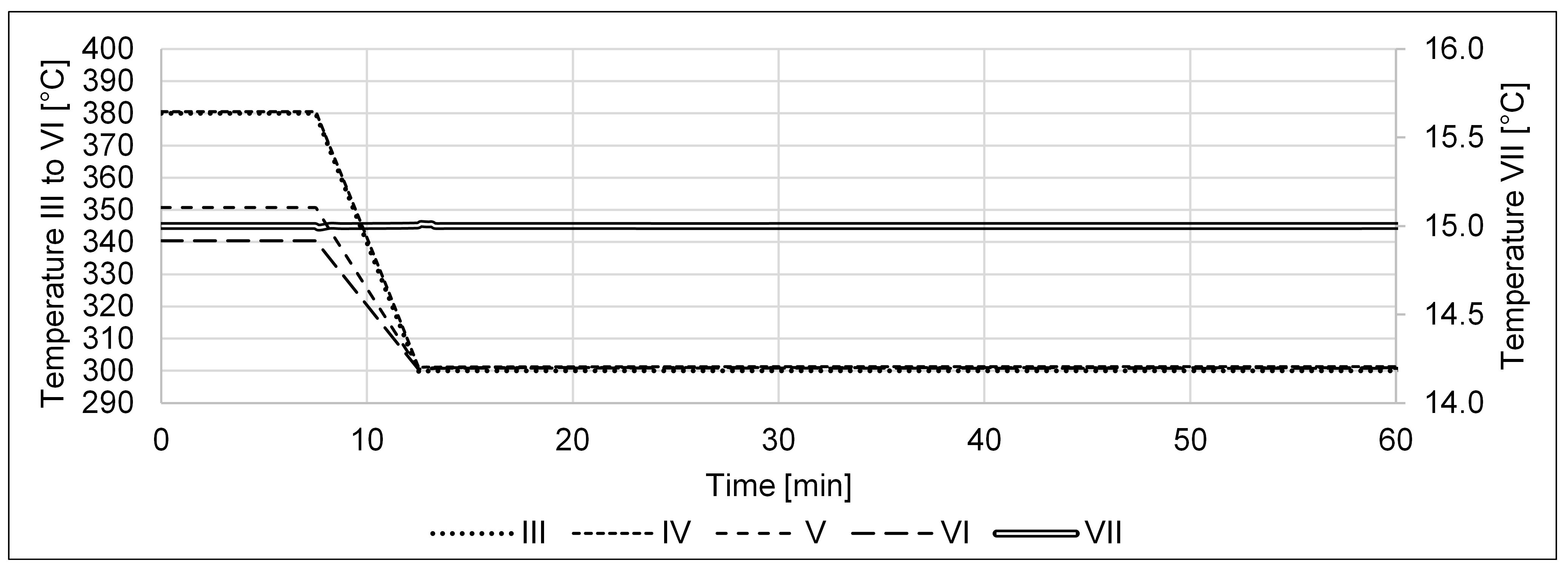
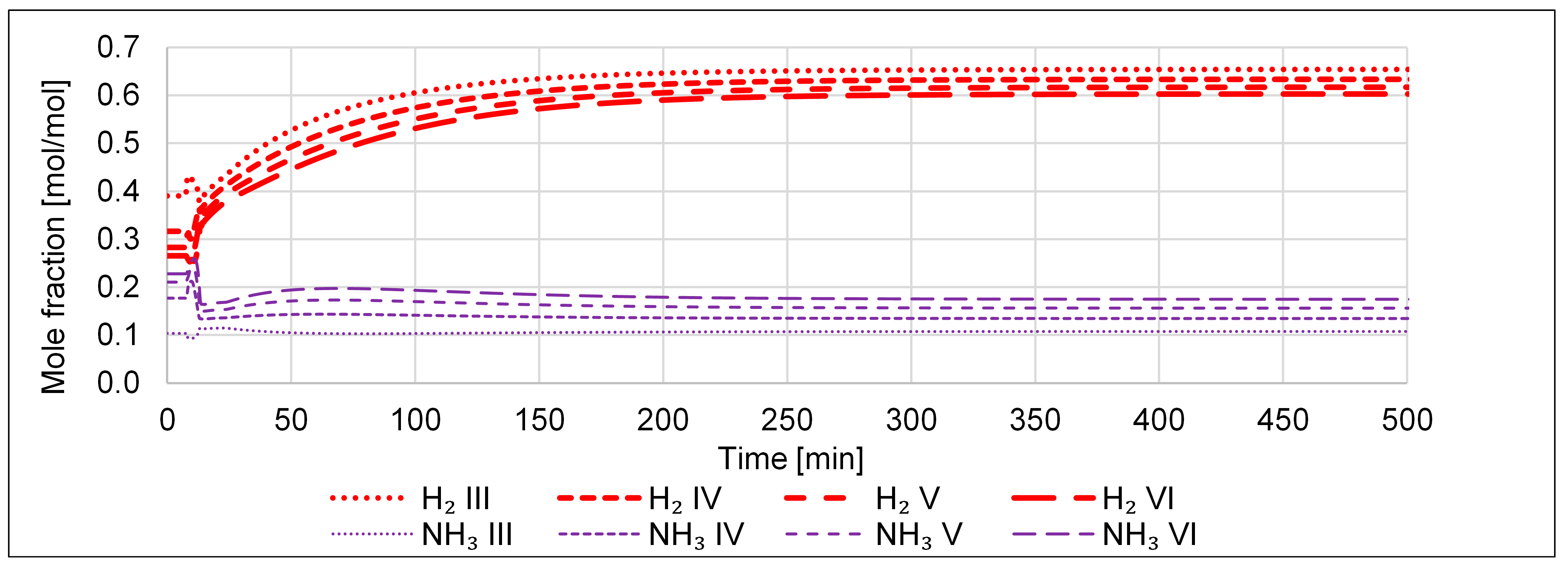
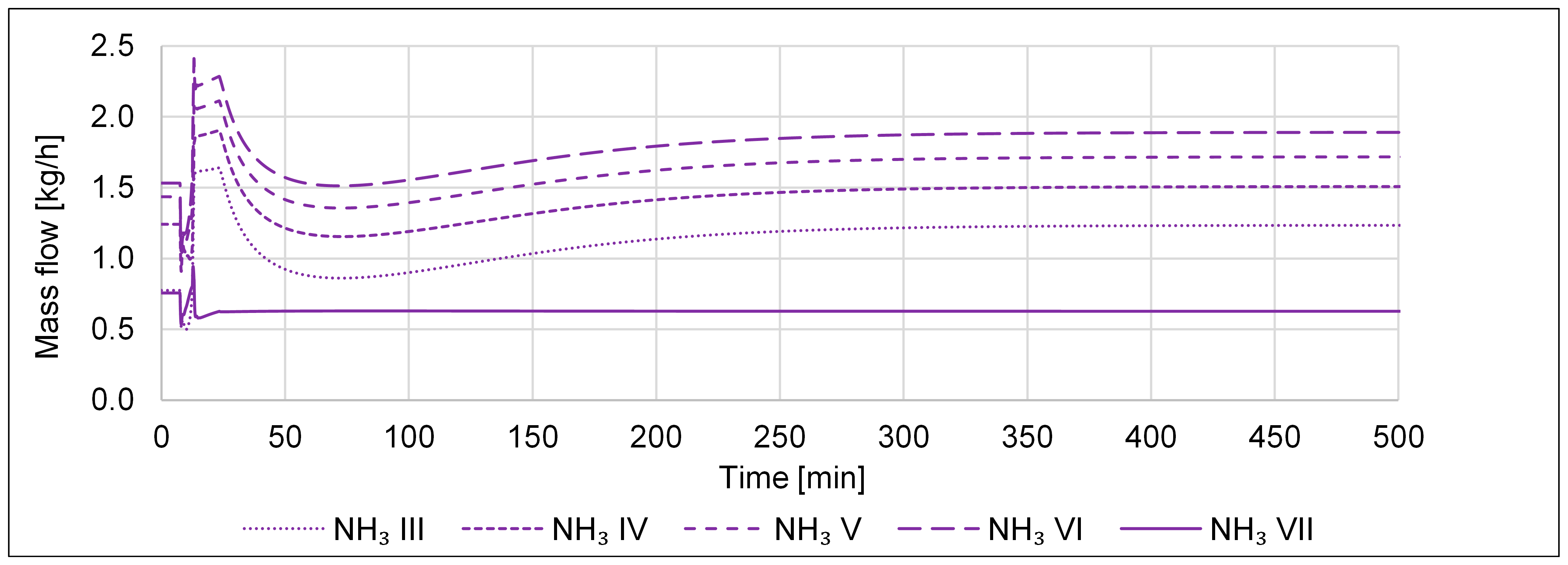
| P2A Design | Operating Flexibility | Cost of Investment |
|---|---|---|
| Conventional | Rather low | Rather high |
| The dynamic behavior of an internal gas-gas heat exchanger can be difficult due to the feedback of the internal loop brought to the system by this type of heat exchanger [35]. | The use of two compressors with about the same investment cost increases the overall investment cost by 20% [37]. | |
| Novel | Rather high | Rather low |
| The dynamic behavior of the electric preheater is fast and efficient. Further, feedback behavior is not to be expected, as there is no internal loop. | The novel layout uses a recycle valve instead of a recycle compressor. The investment cost of such a valve is negligible. |
| P2A Design | |||
| Electrolyzer | Electrical power input | 0–15 | kW |
| Hydrogen production | 0–3 | Nm3/h | |
| Reactors | Catalyst | Novel iron-based catalyst [39] | |
| Catalyst mass | 14 | kg | |
| Length | 0.6 | m | |
| Diameter | 0.1 | m | |
| Condenser | Volume | 10 | l |
| Aspen Plus Dynamics® | |||
| General | Property method | Modified HYSPR used by industry partners [39] | |
| Type of controls | PID optimized with Ziegler–Nichols | ||
| Time integration | Default Mixed Newton & Implicit Euler | ||
| Compressor | Isentropic efficiency | 80 | % |
| Inlet pressure | 8 | barg | |
| Outlet pressure | 80 | barg | |
| Reactors | Pressure drop correlation | Ergun | |
| Kinetics | Modified LLHW fitted to catalyst performance data (Equation (2), [39]) | ||
| Optimal temperature profile | 380, 350, and 340 [39] | °C | |
| Condenser | Temperature | 15 | °C |
| Initial liquid level | 50 | % | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koschwitz, P.; Bellotti, D.; Sanz, M.C.; Alcaide-Moreno, A.; Liang, C.; Epple, B. Dynamic Parameter Simulations for a Novel Small-Scale Power-to-Ammonia Concept. Processes 2023, 11, 680. https://doi.org/10.3390/pr11030680
Koschwitz P, Bellotti D, Sanz MC, Alcaide-Moreno A, Liang C, Epple B. Dynamic Parameter Simulations for a Novel Small-Scale Power-to-Ammonia Concept. Processes. 2023; 11(3):680. https://doi.org/10.3390/pr11030680
Chicago/Turabian StyleKoschwitz, Pascal, Daria Bellotti, Miguel Cámara Sanz, Antonio Alcaide-Moreno, Cheng Liang, and Bernd Epple. 2023. "Dynamic Parameter Simulations for a Novel Small-Scale Power-to-Ammonia Concept" Processes 11, no. 3: 680. https://doi.org/10.3390/pr11030680
APA StyleKoschwitz, P., Bellotti, D., Sanz, M. C., Alcaide-Moreno, A., Liang, C., & Epple, B. (2023). Dynamic Parameter Simulations for a Novel Small-Scale Power-to-Ammonia Concept. Processes, 11(3), 680. https://doi.org/10.3390/pr11030680








