Abstract
Wound healing is the process of skin and soft tissue repair following an injury. Angiogenesis is an essential process in wound healing and plays an important role in tissue regeneration. Ginseng is mainly composed of saponins and protopanaxadiol-based ginsenosides, namely Rb1, Rb2, Rc, Re, Rg1, and Rf. 20(S)-protopanaxadiol (PPD) and 20(S)-protopanaxatriol (PPT) are aglycones of ginsenosides produced by metabolic processes and heat treatment. This study aimed to investigate the wound healing effects of active ingredients of ginseng, namely ginsenosides and aglycones, in various cellular and animal skin wound models. The angiogenic effects of ginsenosides were investigated in human umbilical vein endothelial cells (HUVECs). All experiments were conducted at increased intracellular glucose concentrations and the induction of angiogenesis through tube formation was evaluated. Among the ginsenosides and aglycones used in this study, PPD showed the strongest wound-healing activity. Cell scratch experiments confirmed that PPD increased intracellular proliferation and cell migration at high glucose concentrations, and western blotting of HUVECs showed that phosphorylated ERK, Akt, and p38 were regulated. We observed accelerated wound healing with PPD treatment in STZ-treated mice. Overall, the findings suggested that PPD could possibly help improve skin wound healing in patients with diabetes, although further research is recommended.
1. Introduction
Diabetes mellitus (DM) is a complex metabolic disease characterized by chronic hyperglycemia with impaired endovascular metabolism due to the defective secretion and action of insulin [1]. Diabetes does not allow the normal wound healing process, often resulting in patients having chronic wounds that are prone to complications, such as infection. Several complex factors are involved in the chronicity of diabetes. Many studies have been conducted to date on the defects of angiogenesis, which is important for normal wound healing [2,3,4]. In patients with diabetes, inflammatory processes that promote wound healing are impaired, the immune system is weakened, and wound healing is delayed [5,6].
Current treatments for diabetic wounds include platelet-derived growth factors (PDGFs) and epidermal growth factors (EGFs), which are domestic therapeutics. EGF has many side effects, including malignancy, hypersensitivity reactions, seizures, high blood pressure, weight gain, increased risk of death from endothelial cell damage, and decreased expression of growth factor receptors at ulcer sites [7]. Owing to such challenges, research is currently focused on finding a safe and effective treatment for diabetic wounds [8,9,10,11].
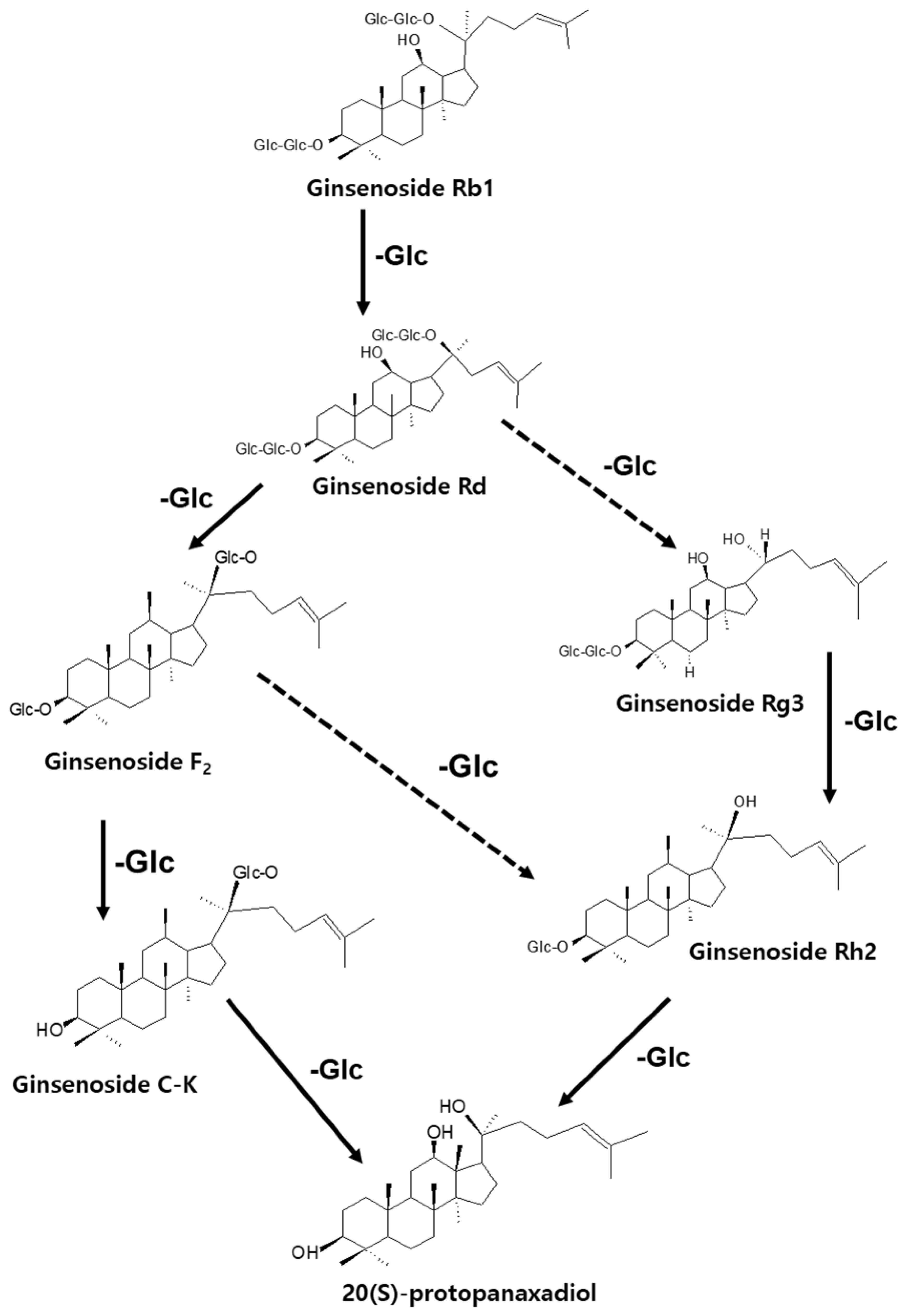
Medicinal plants, especially those with active compounds, have been used for a long time to promote wound healing without side effects, or with fewer side effects, than other drugs [12]. In the biological activity evaluation project of various herbal medicines, based on several screening results, we found that protective effect of ginseng (root of Panax ginseng C. A. Meyer) against the death of pancreatic β-cells can be helpful for patients with type 2 diabetes (T2D). Ginseng is a major herbal medicine used in oriental prescriptions, such as Samsoeum, Soshihotang, and Hyeongbangpaedoksan [13]. In vitro and in vivo experimental evidence has shown that the beneficial effects of ginseng in T2D are attributable to ginsenosides, the main active components found in ginseng [13,14]. The saponin component of ginseng contains approximately 180 types of ginsenosides and exhibits pharmacological action in the cardiovascular system [14], along with anti-inflammatory action [15], anti-fatigue action [16], antioxidant action [17], liver damage prevention action [18], antidiabetic effects [19], and anti-cancer effects [20]. Ginseng is used to treat various diseases in both Eastern and Western medicine [21]. The representative pharmacological functions of these ingredients include anticancer, anti-inflammatory, and antidiabetic effects. As shown in Figure 1, the saponin components of ginseng can be converted in various ways; for example, Rb1, Rb2, and Rc are converted to Rg3, Rg5, and Rk1 during the steaming and drying of fresh ginseng to produce red ginseng or boiling white ginseng [22,23]. Ginsenosides can be metabolized to Rh2, Rh3, and Rk2 by the gut flora and further converted to protopanaxadiol [24,25]. Re, Rg1, and Rf are also metabolized to Rh1, F1, and protopanaxatriol [26,27]. An earlier study on diabetic rats had shown that the ginsenoside Rg1 effectively promotes angiogenesis in human umbilical vein endothelial cells (HUVECs) and tubular angiogenesis in vivo [28]. Ginseng has been studied for its preventive effects against stress and aging, anticancer and anti-inflammatory effects, protection against hepatotoxicity, ischemic stroke, hormone action and production, and metabolic regulation; however, its curative effect on diabetic wounds remains unknown [29].
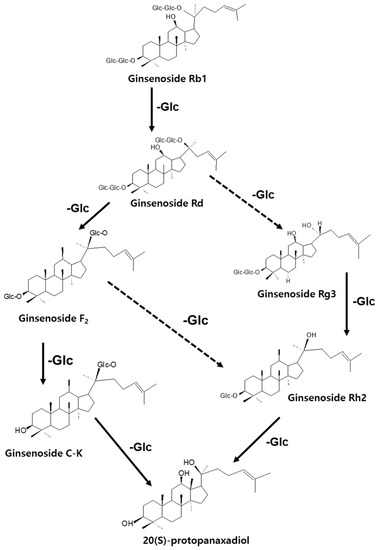
Figure 1.
Production of less-polar ginsenosides by the elimination of sugar moieties.
In this study, the pharmacological activity of ginsenosides (Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, Rg3) and aglycones (20(S)-protopanaxadiol) on wound healing in normal condition and high glucose condition was evaluated using HUVECs. Additionally, we analyzed the potential usefulness of 20(S)-protopanaxadiol (PPD), an active ginseng ingredient, using a diabetic mice model.
2. Materials and Methods
2.1. Reagents
The EZ-Cytox Cell Viability Assay Kit was obtained from DoGENBio (Seoul, Republic of Korea). The primary antibodies for extracellular signal-regulated kinase (ERK), phosphorylated-ERK (p-ERK), p38, phosphorylated-p38 (p-p38), Akt and phosphorylated-Akt (p-Akt) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Fetal bovine serum (FBS) was obtained from Invitrogen (Grand Island, NY, USA). The ginsenosides (Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, Rg3) and PPD were purchased from Biopurify (Sichuan, China).
2.2. Cell Culture
The HUVECs were obtained from ATCC (Manassas, VA, USA) and cultured in a humidified atmosphere (5% CO2, 95% air). They were maintained using the Clonetics EGM-2 MV BulletKit (Lonza, Walkersville, MD, USA).
2.3. Assessment of Cell Viability
Cytotoxicity of the eight types of ginsenosides (Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, and Rg3) and PPD in HUVECs was tested using the EZ-Cytox cell viability assay kit. Briefly, cells were seeded in 96-well plates at a density of 2.0 × 104 cells/mL, treated with various concentrations of ginsenoside or PPD, and then incubated for 24 h at 37 °C. After treatment, cell viability was assessed according to the manufacturer’s instructions.
2.4. Assessment of Tube Formation
Cells were seeded (2.5 × 105 cells/mL) in Matrigel-coated plates. Media with or without the samples were added to the plates. Cells were fixed with 4% paraformaldehyde and stained with Mayer’s hematoxylin (Muto Pure Chemicals, Tokyo, Japan). The extent of tube formation was quantified by measuring the lengths of the tubes in the images captured using ImageJ software. Changes in cell morphology and tubular structure were observed under a microscope (IX51, Olympus, Tokyo, Japan).
2.5. Cell Scratch Wound Healing Assay of HUVECs
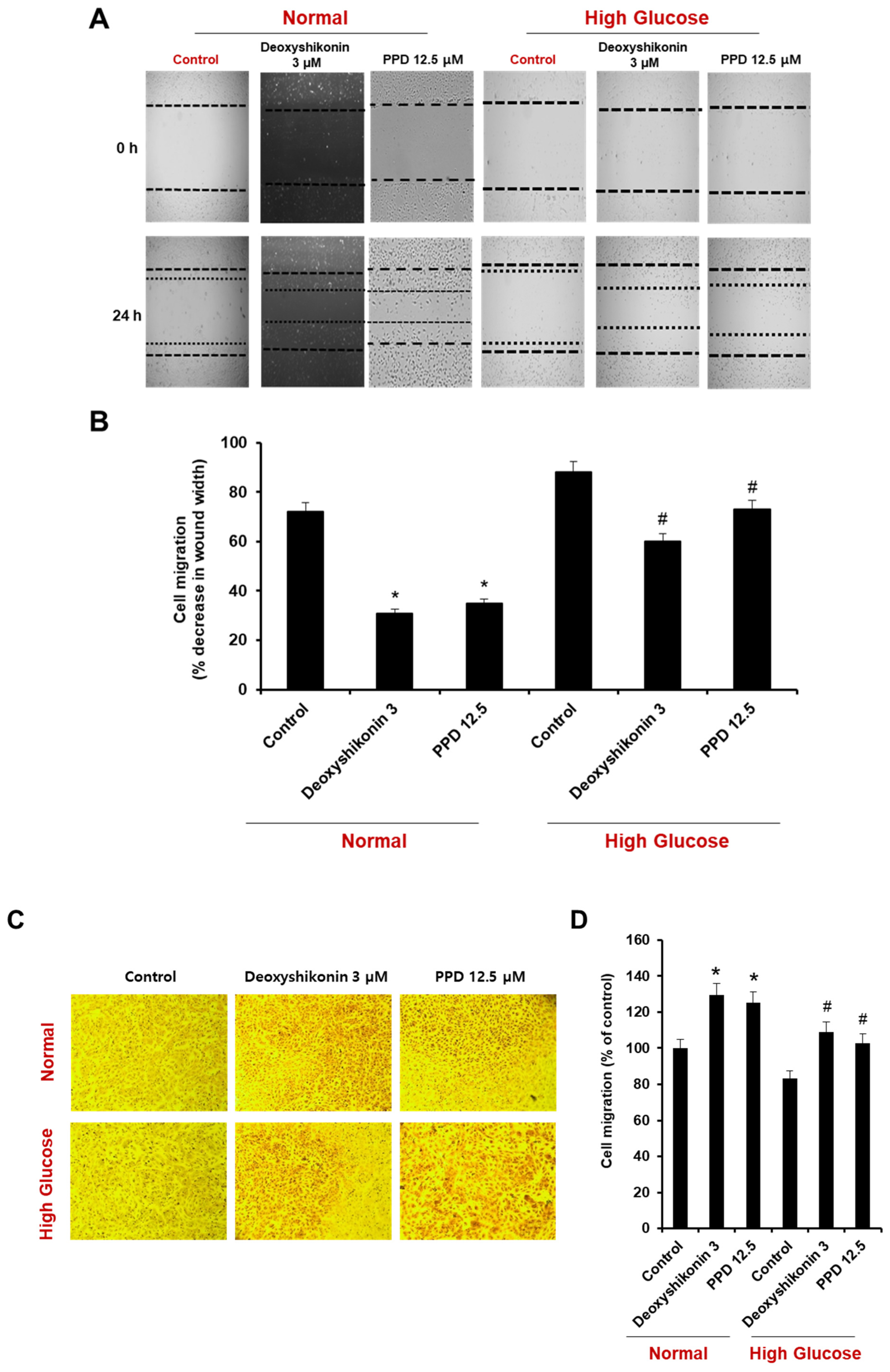
A cell scratch wound-healing assay was performed by measuring cell migration after scratch formation in a cell monolayer. Briefly, HUVECs were plated in a 35-mm dish at a density of 8 × 105 cells/mL. A cell scratch wound-healing assay was performed on the HUVEC monolayer using a sterile pipette tip. PPD (12.5 μM) and deoxyshikonin (3 μM) were added after washing away the detached cells. Wound width was measured at randomly chosen points under a light microscope equipped with a digital camera after incubating the deoxyshikonin or PPD for 24 h. The extent of wound closure was assessed in terms of the percentage of the original scratch width.
2.6. Western Blot Analysis
The HUVECs (8 × 105 cells/dish) grown in 6-cm dishes for western blotting were treated with two concentrations of PPD (6.25 μM and 12.5 μM) for 24 h under normal or high-glucose conditions. Cell lysates were prepared using radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA, USA) supplemented with 1 mM phenyl methyl sulfonyl fluoride and 1× protease inhibitor cocktail. The proteins (30 μg/lane) were electrophoresed, transferred onto polyvinylidene fluoride membranes, and visualized by ECL reagents (GE Healthcare, Buckinghamshire, UK) using a Fusion Solo Chemiluminescence ECL detection system (Vilber Lourmat, Paris, France).
2.7. Gene Expression Analysis by Real-Time PCR (qPCR)
HUVECs (8 × 105 cells/dish, 6-cm dish) were cultured with the deoxyshikonin or PPD under normal or high-glucose conditions. After 12 h, the cells were homogenized and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Total RNA was reverse-transcribed into cDNA using the AccuPower CycleScript RT premix (dT18) (Bioneer, Daejeon, Republic of Korea). Specific primers were used to amplify the cDNAs encoding vascular endothelial growth factor A (VEGF-A) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). Analysis was performed using QuantStudio 3 real time PCR System (Applied Biosystems, Waltham, MA, USA).

Table 1.
Primer sequences used for semi-quantitative reverse transcription PCR.
2.8. Measurement of Intracellular Reactive Oxygen Species (ROS) in HUVECs
The degree of intracellular ROS accumulation was assessed using a fluorescence plate reader after staining the cells with 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA), a fluorescent indicator capable of detecting ROS. HUVECs (2.0 × 104 cells/well) were seeded in each well of a 96-well black plate and incubated with the PPD (12.5 μM) and deoxyshikonin (3 μM) under normal or high-glucose conditions for 48 h. After that, 10 μM H2DCFDA was added to each well, and the cells were further incubated for 30 min. After removing the residual H2DCFDA that was not bound to ROS, using phosphate-buffered saline (PBS), the fluorescence intensity of DCF-DA was measured at 485 nm/535 nm (excitation/emission) using a fluorescence plate reader (Spark M10, Tecan, Switzerland), and Fluorescence by DCF-DA inside cells was observed under a fluorescence microscope (IX51, Olympus, Tokyo, Japan).
2.9. Cell Migration Assay in HUVECs
Cells migration assay was performed in an invasion chamber containing cell culture insert (Transparent PET membrane, 8.0 μm pore size, Corning, NY, USA) in 24-well plates according to our previous study [30]. Briefly, HUVECs were seeded on the inside of cell culture inserts (1.0 × 105 cells/insert) then incubated with normal media or high glucose media for overnight. The next day, deoxyshikoni or PPD treated the inside of each insert for 24 h. We then gently washed out the media and removed non-migrating cells using a cotton-tipped swab in culture inserts. After that, we dried the culture inserts by air, then fixed the cells with methanol. Migrated cells were then stained with hematoxylin and eosin. Cells on the membrane of insert were observed under a light microscope (IX51, Olympus, Tokyo, Japan).
2.10. VEGF Cytokine Analysis in HUVECs
Cells were seeded onto a 96-well microplate, at 2.0 × 104 cells/well, and cultured at 37 °C in a 5% incubator overnight. The following day, deoxyshikoni or PPD was incubated at an indicated concentration for 24 h. The culture supernatant was harvested, and human VEGF-A secretion levels in the supernatants were determined using ELISA kits (R&D, Minneapolis, MN, USA) according to the manufacturer’s instructions.
2.11. Assessment of Wound Healing in a Cutaneous Wound Model in Diabetic Mice
The animal testing protocols were approved by the Animal Research Ethics Committee of Gachon University (GAICUC-R2015009). Six-week-old male BALB/c mice were purchased from Orient Bio Co. Ltd. (Seongnam, Republic of Korea). The animals were randomly divided into two groups, with ten mice in each. Diabetes was induced by administering 60 mg/kg streptozotocin (STZ) after a fasting period of 16 h. Citrate buffer (0.1 M, pH 4.5) and STZ (S0130, Sigma-Aldrich, St. Louis, MO, USA) in citrate buffer (0.1 M, pH 4.5) were used in this study. After 24 h, blood glucose levels were measured in the tail vein using an Accu-Chek blood glucose meter (Roche Diagnostics, Germany). Mice with blood glucose levels >250 mg/dL were considered to have diabetes and were used in the subsequent experiment. The mice were anesthetized with ethyl ether (0.5 mg/g body weight), and a 5-mm-thick full-thickness skin incision was made on the back of each mouse. Each wound was treated with 25 μM PPD or PBS (control) for 10 days. Digital images of the wound were captured on days 0, 2, 4, 6, and 8 using a 10-megapixel digital camera. The wound size was calculated using ImageJ software. Using the ImageJ software, we measured the “pixel area” of the wound and the standard circular hole as a “polygon” and “5-point ellipse”, respectively. The wound closure rate was calculated as ((area of original wound—area of actual wound)/area of original wound) × 100.
2.12. Statistical Analysis
Statistical significance was determined using one-way analysis of variance (ANOVA), and multiple comparisons were made with Bonferroni correction. Statistical analyses were performed using SPSS Statistics ver. 19.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). Assays were performed in triplicate and repeated at least thrice.
3. Results and Discussion
3.1. Effects of Ginsenosides and PPD on HUVEC
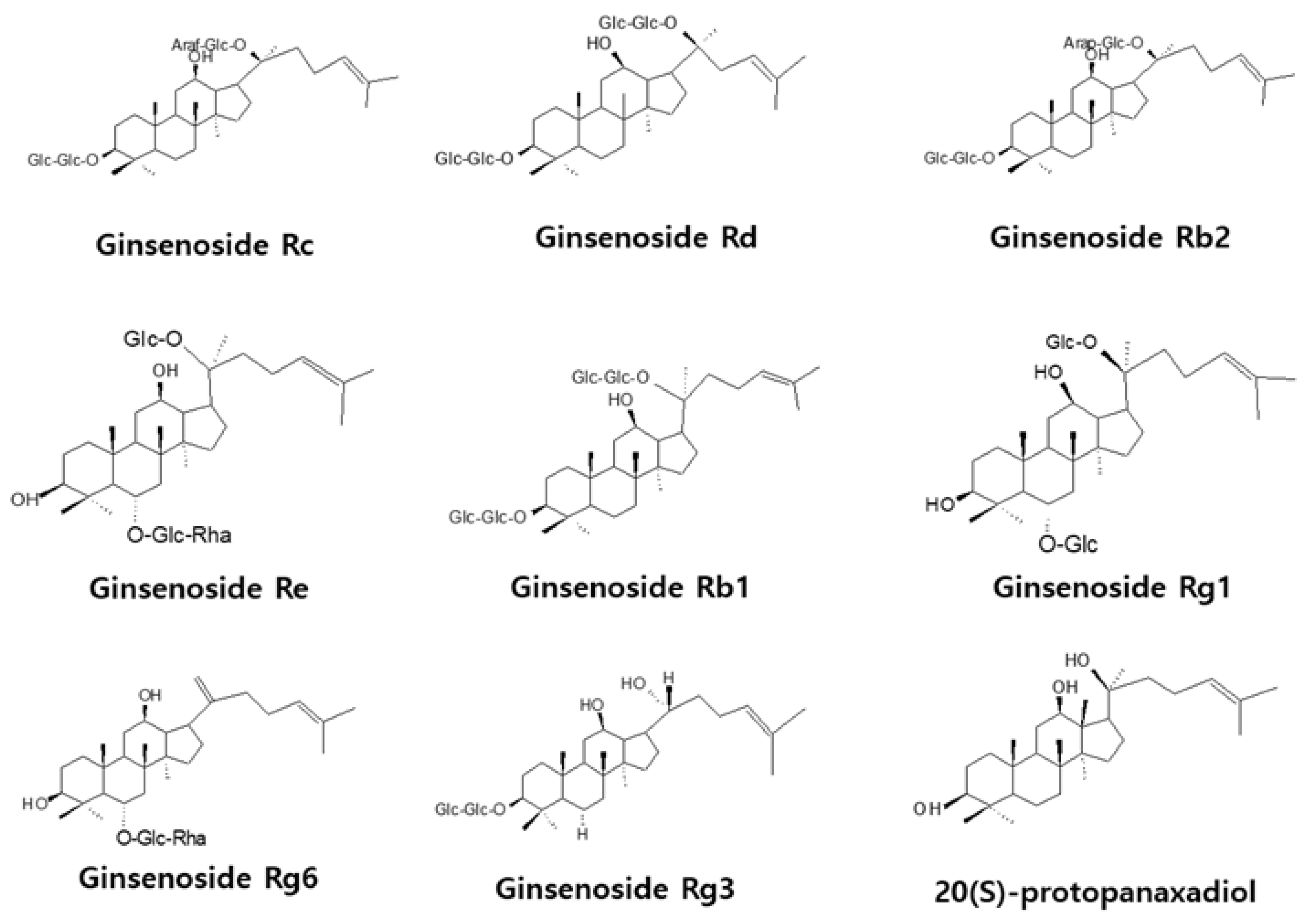
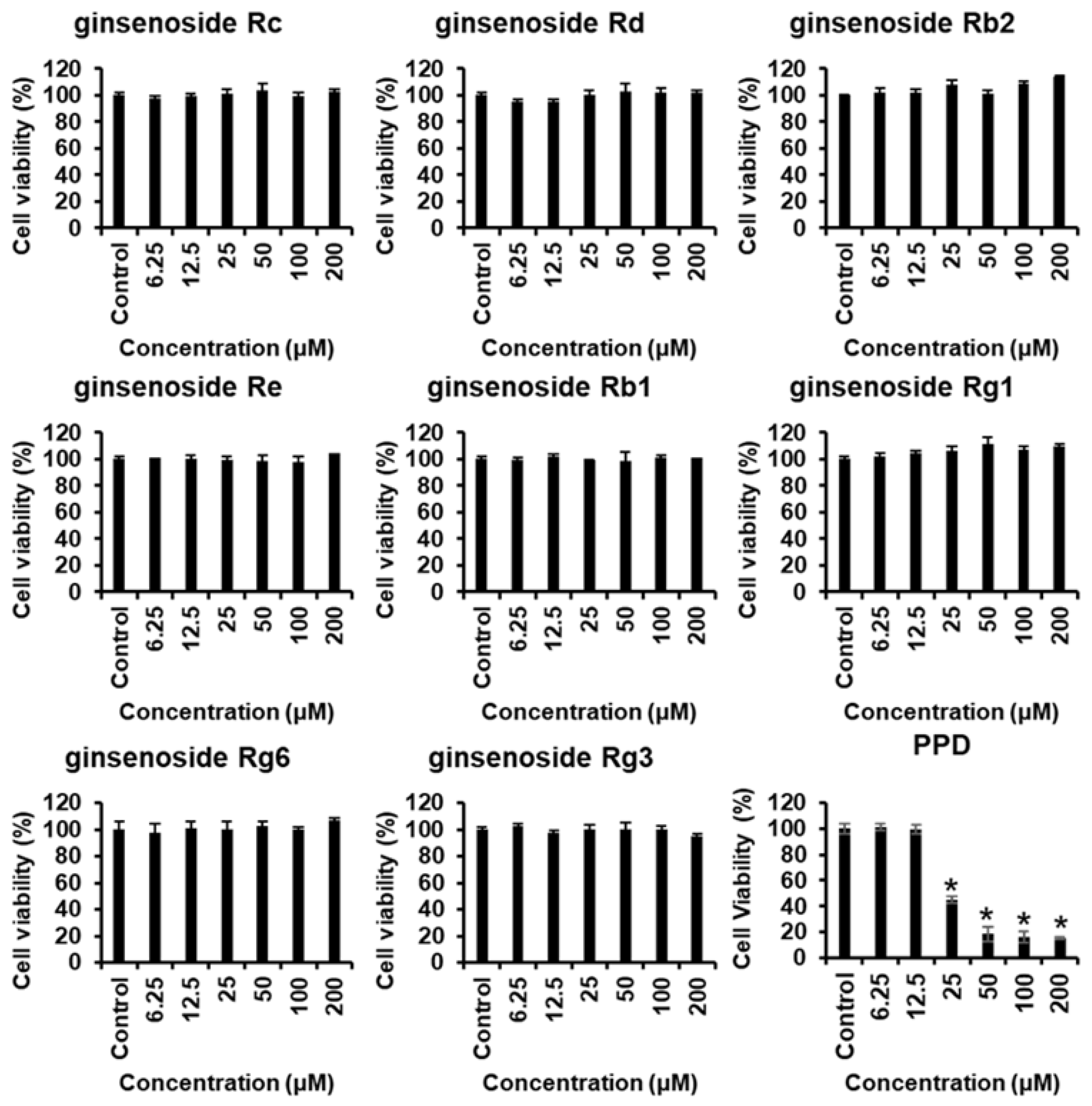
Proliferation of cells, such as endothelial cells and fibroblasts, is essential for wound healing, and cytotoxicity influences angiogenesis and cell migration. Therefore, the effects of various concentrations of ginsenosides and PPD on the survival of HUVECs were investigated using the MTT assay. Figure 2 shows the chemical structures of ginsenosides and PPD used in this experiment. Figure 3 shows the cell viability results with the use of ginsenosides. Ginsenosides Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, and Rg3 showed no change in cell viability in HUVECs compared to that in the control group, even at high concentrations, whereas PPD showed concentration-dependent toxicity in HUVECs; 12.5 μM PPD had no effect on the HUVECs. Therefore, the concentration of 12.5 μM was selected as the highest concentration of PPD that did not cause cytotoxicity.
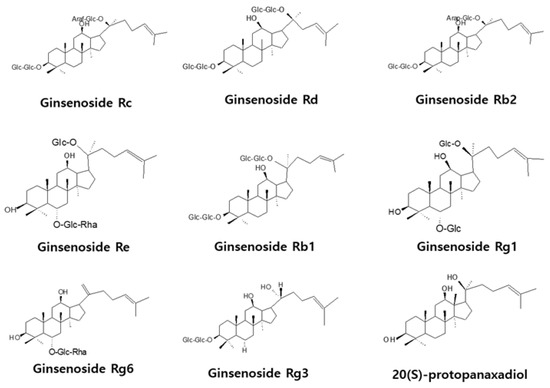
Figure 2.
Chemical structures of ginsenosides and aglycone.
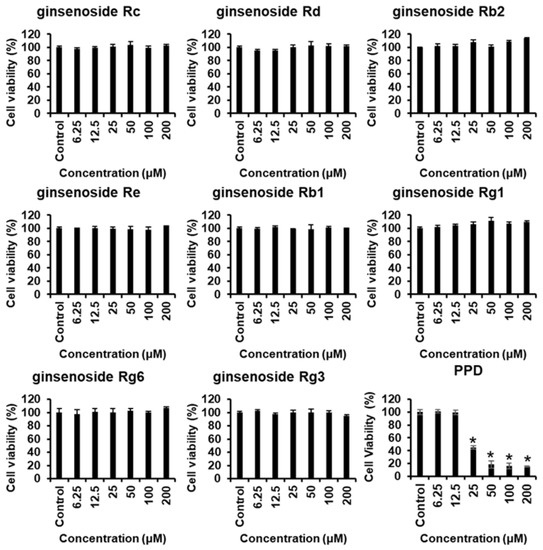
Figure 3.
Cytotoxicity of ginsenosides and PPD on HUVECs. The HUVECs were treated with ginsenosides or PPD at various concentrations (6.25–200 μM) or with 0.5% dimethyl sulfoxide only (control) for 24 h, followed by the evaluation of cell viability by the EZ-Cytox assay. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the control group.
3.2. Effects of Ginsenosides and PPD on Tube Formation in HUVECs
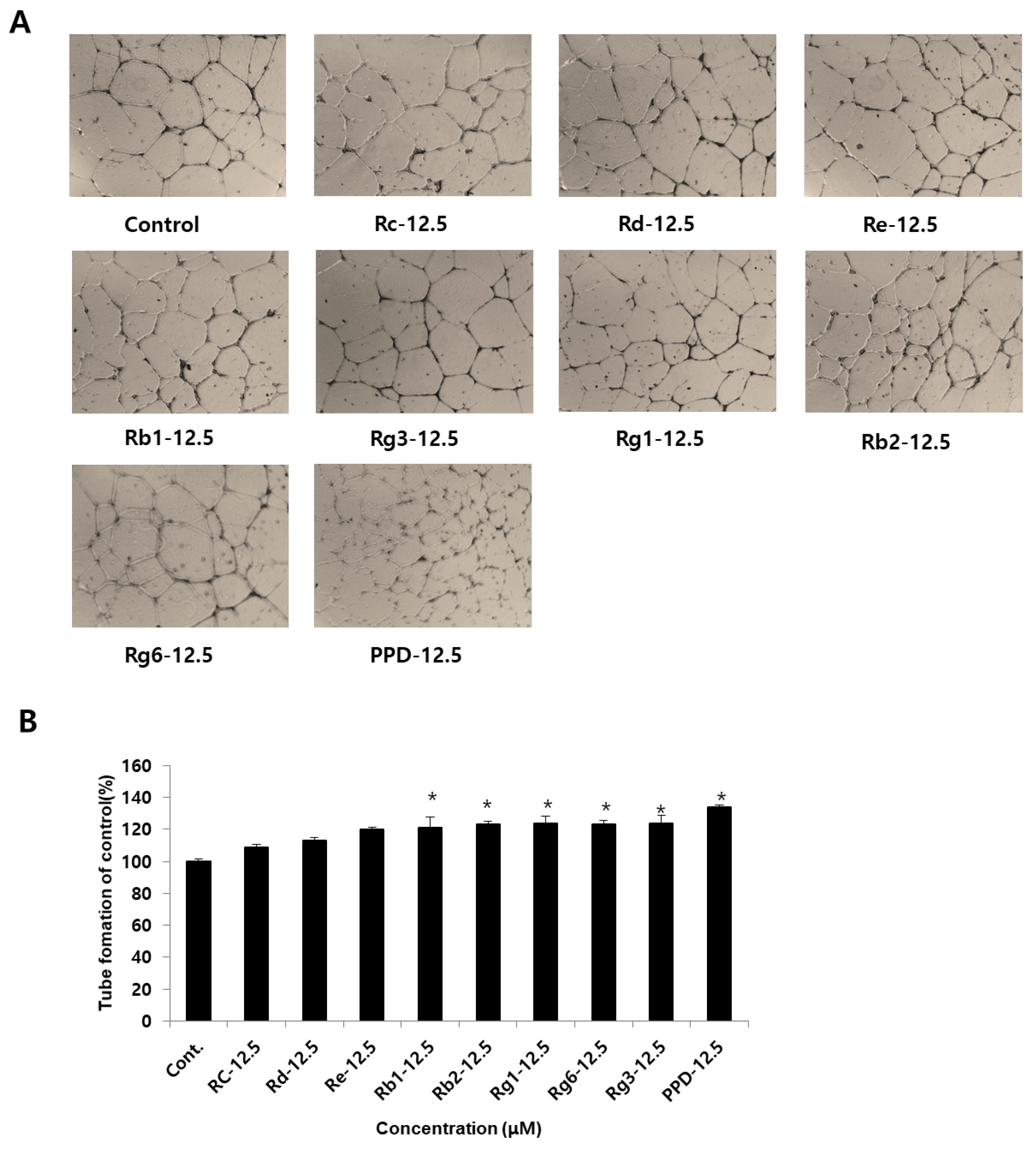
In the cytotoxicity test, angiogenic effects of ginsenosides and PPD at non-toxic concentrations were investigated in HUVECs (Figure 4A). Examination of the angiogenic effect of ginsenosides at a concentration of 12.5 μM in HUVECs revealed no significant difference across the ginsenosides Rc, Rd, and Re compared to that in the control group (Figure 4B). When the ginsenosides and PPD were treated at a concentration of 12.5 μM, angiogenesis increased by 21.7 ± 6.9% for Rb1 and by 23.3% for Rb2 compared to that in the control group. Angiogenesis was increased by 24.3 ± 4.1% for Rg1, 23.2 ± 2.8% for Rg6, 24.3 ± 5.1% for Rg3, and 34 ± 1.4% for PPD, indicating PPD to be 10% more effective than the other eight types of ginsenosides: Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, and Rg3.
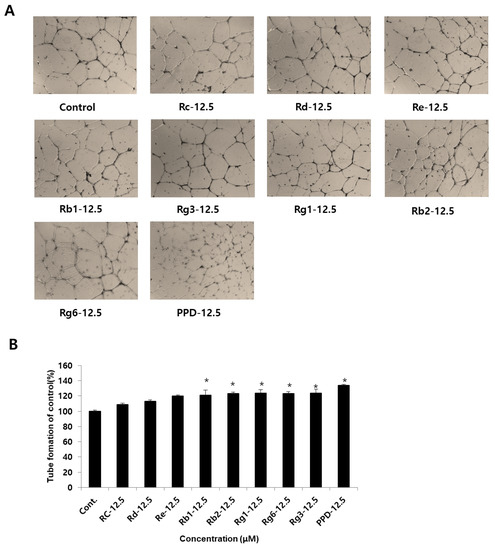
Figure 4.
Effects of ginsenosides and PPD on HUVEC tube formation. (A) Photographs of tube formation of HUVECs with or without ginsenosides and PPD (12.5 μM) after 24 h. (B) Tube formation effects were quantified using ImageJ software. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the control group.
3.3. Effect of PPD on Scratch-Induced Wound Healing under High-Glucose Conditions in HUVECs
Wound healing is the process by which damaged tissues regenerate through a continuous and complex process involving cell differentiation and proliferation, angiogenesis, synthesis of matrix proteins, and promotion of wound restoration through various complex reactions to skin tissue damage [31,32]. Wounds in patients with DM do not heal as well as in normal individuals for a combination of several reasons, including a decrease in collagen organization, blood flow, and angiogenesis, and an increase in blood viscosity in patients with DM.
To investigate the effect of PPD on cell scratch wound healing, we evaluated the extent of reduction of wound area in a high-glucose state and in a model created by inducing wounds in normal condition cells (Figure 5A). The treatment of deoxyshikonin (3 μM) was used for a positive control for the reduction of scratch wound width in HUVECs. Under normal conditions, the scratch wounds width naturally reduced by 28%, and reduced 69% by deoxyshikonin and 65% by PPD treatment, respectively. Under the high glucose condition, naturally occurring wounds reduction (12%) was found to be slower than that of the control. However, the treatment of deoxyshikonin enhanced the reduction of scratch wounds width to 40%, and PPD treatment also enhanced the reduction of scratch wounds width to 27% (Figure 5A,B). Similar results were obtained using cell migration assay. In normal conditions, deoxyshikonin and PPD treatment significantly increased cell mobility (Figure 5C,D) and, as expected, high-glucose slightly decreased cell mobility compared to control. However, deoxyshikonin and PPD treatment promoted cell mobility in high-glucose condition. This result suggested that PPD treatment is effective for in vitro wound healing in high glucose-exposed HUVECs.
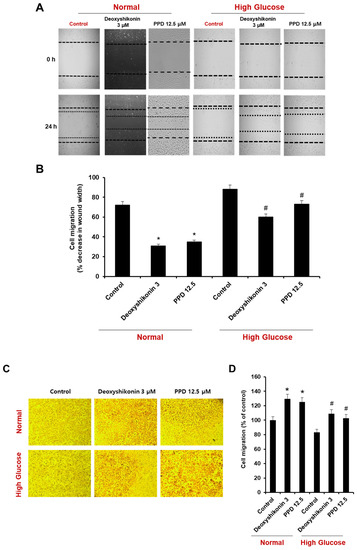
Figure 5.
Effects of deoxyshikonin and PPD on scratch wound healing in HUVECs. (A) Scratch wound healing in HUVECs was evaluated using a cell-scratch wound healing assay. Images of the same location were captured at 0 and 24 h after wounding. (B) Scratch wound healing of the deoxyshikonin- or PPD-treated HUVECs was detected using the scratch wound healing assay. (C) Cell migration of deoxyshikonin or PPD-treated HUVECs for 24 h in normal media or high-glucose media. (D) Quantification of migration was calculated as a percentage of the cell density by ImageJ software. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the control in normal, # p < 0.1 compared to the control in high glucose.
3.4. Effects of PPD on Angiogenic Protein Expression in HUVECs
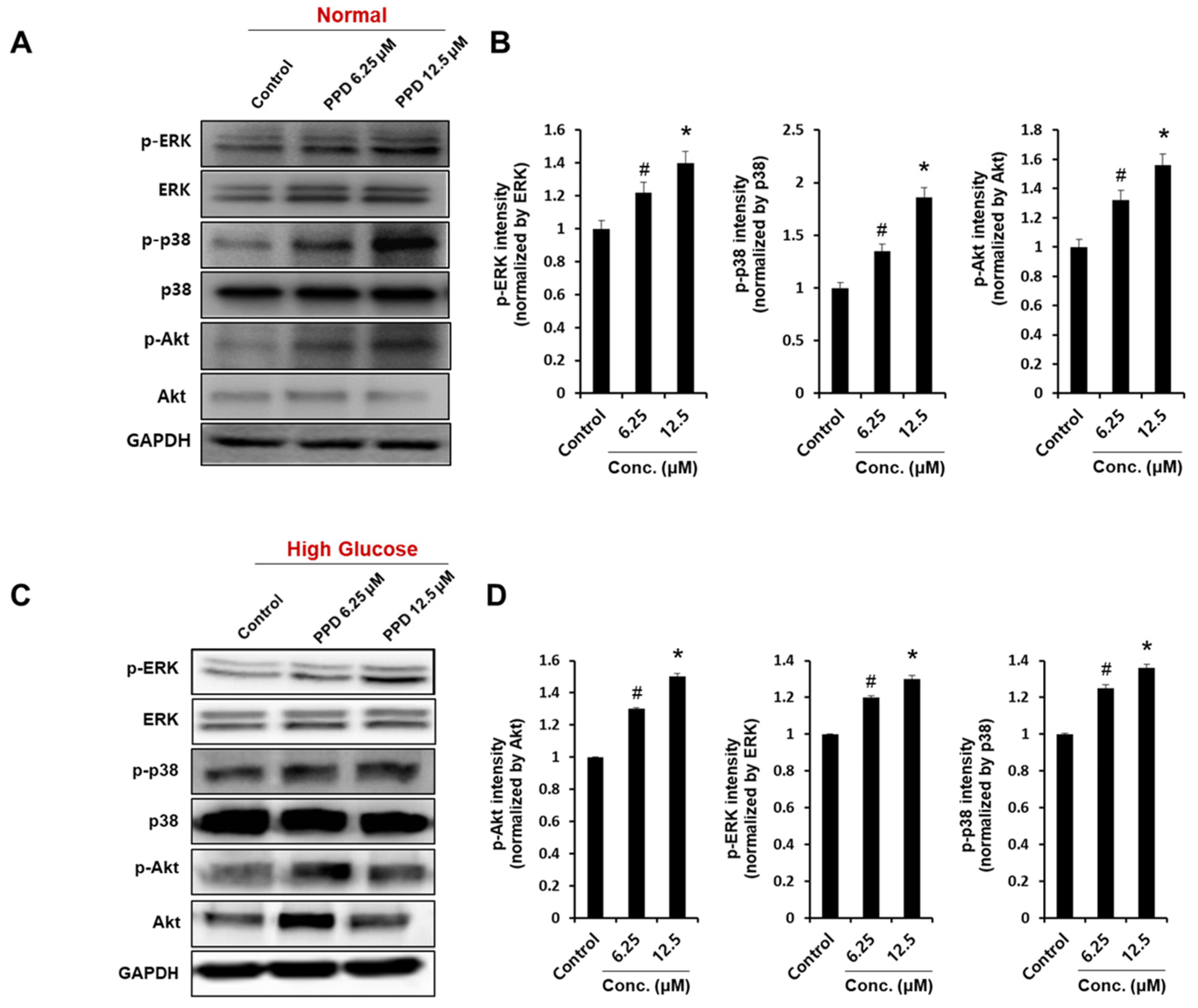
The phosphorylation of Akt, extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK), and p38 signaling proteins are known to be involved in the main signaling pathways for tube formation in HUVECs [30]. Therefore, we investigated the effect of PPD on Akt and MAPK phosphorylation in normal condition and high glucose condition. First, we confirmed that PPD treatment strongly increased phosphorylation of ERK, p38 and Akt at indicated concentrations in normal condition (Figure 6A). Additionally, PPD treatment significantly increased the phosphorylation of ERK, p38 and Akt in high glucose-exposed HUVEC (Figure 6C). These results suggested that PPD treatment might promote the intracellular signaling cascades in HUVECs in high glucose conditions as well as normal conditions.
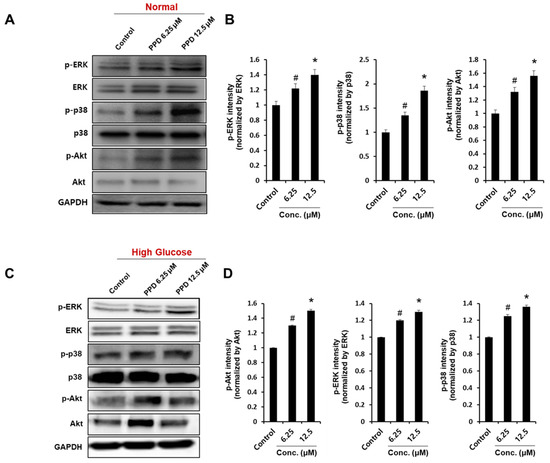
Figure 6.
Effects of PPD on angiogenic protein expression in HUVECs. Cells (8 × 105 cells/6-cm dish) were cultured in normal media or high-glucose media. (A,C) Cells were treated with PPD at indicated concentration under normal condition or high glucose condition for 24 h. Whole-cell lysates were immunoblotted with the specific antibodies indicated on the left side of each panel. The level of GAPDH was measured as internal loading control. (B,D) p-ERK, p-p38, and p-Akt protein levels were quantified by total protein using the ImageJ software. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 and # p < 0.1 compared to the control.
3.5. Regulation of VEGF Expression by PPD Treatment under High-Glucose Conditions in HUVECs
In diabetic ulcers, abnormalities in various functions, such as growth factor production and response [7,30,31,33], angiogenesis [7,34], and macrophage function [35], have been reported. VEGF, an endothelial cell mitogen and vascular permeability inducer, specifically stimulates wound healing via several mechanisms, including the promotion of collagen angiogenesis, deposition, epithelialization, and upregulation of several components of the wound-healing cascade to increase angiogenesis [36,37,38,39].
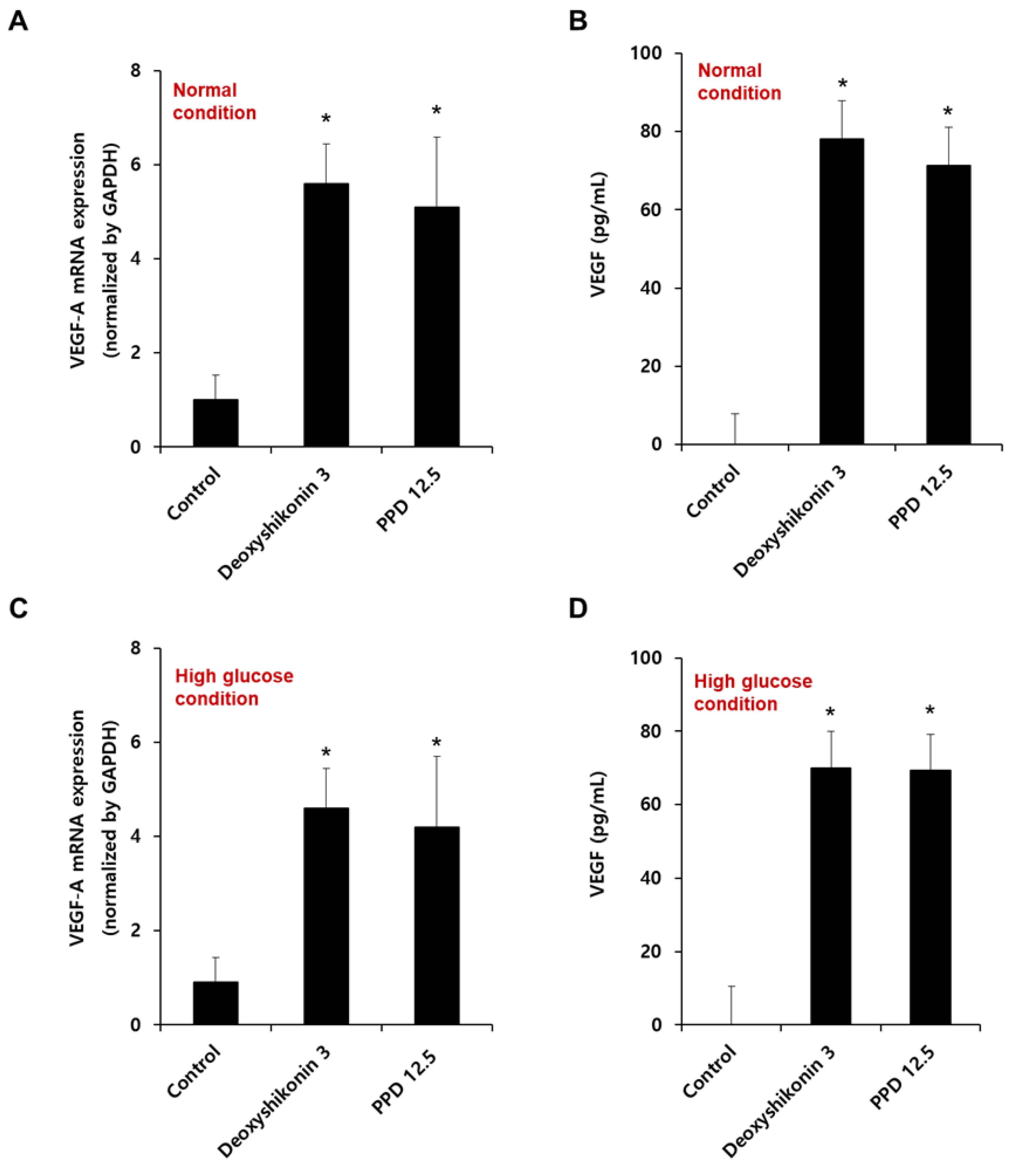
We confirmed the VEGF-A mRNA expression by PPD in HUVECs cultured at normal (Figure 7A) or high glucose conditions (Figure 7C). As shown in Figure 7A,C, PPD treatment for 12 h significantly increased VEGF-A mRNA levels, as well as normal and high-glucose conditions. In addition, we also confirmed VEGF protein secretion in HUVEC in normal (Figure 7B) and high-glucose condition (Figure 7D). PPD treatment for 24 h in normal and high glucose-exposed HUVECs produced VEGF cytokines effectively. Collectively, the up-regulation of VEGF was attributed to the phosphorylation of ERK, p38, and Akt upon PPD treatment in HUVECs.
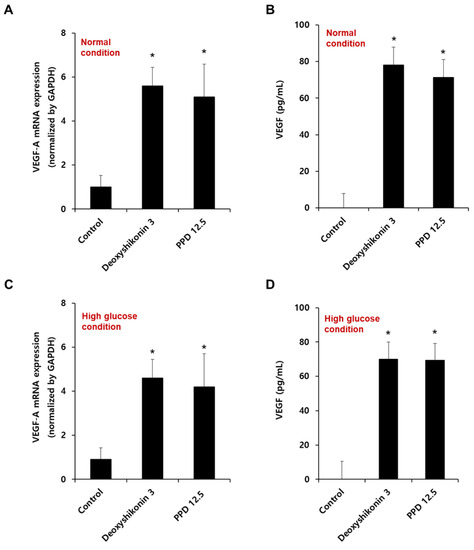
Figure 7.
Effect of PPD on the mRNA expression and cytokine production of VEGF in HUVECs. (A,C) Cells (8 × 105 cells/6-cm dish) were stimulated with deoxyshikonin (3 μM) or PPD (12.5 μM) for 12 h under normal or high glucose conditions. The mRNA expression of VEGF-A was checked using qRT-PCR with specific VEGF-A primers. Deoxyshikonin (3 μM) was used as a positive control for VEGF-A gene expression. GAPDH gene was used as a housekeeping gene for normalization. (B,D) Cells were stimulated with deoxyshikonin (3 μM) or PPD (12.5 μM) for 24 h in normal or high glucose conditions. The cell supernatant was collected, and VEGF secretion was measured by a commercial ELISA kit. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the control.
3.6. Inhibitory Effect of PPD on ROS Production under High-Glucose Conditions in HUVECs
The general wound healing process involves complex interactions among hemostasis, inflammation, proliferation, epithelialization, and scar maturation. This process is affected by the pathology of DM, which includes the generation of high levels of ROS due to hyperglycemia and continuous inflammatory responses to balance redox reactions within cells. Thus, DM interferes with normal metabolism, making treatment difficult [40,41,42,43].
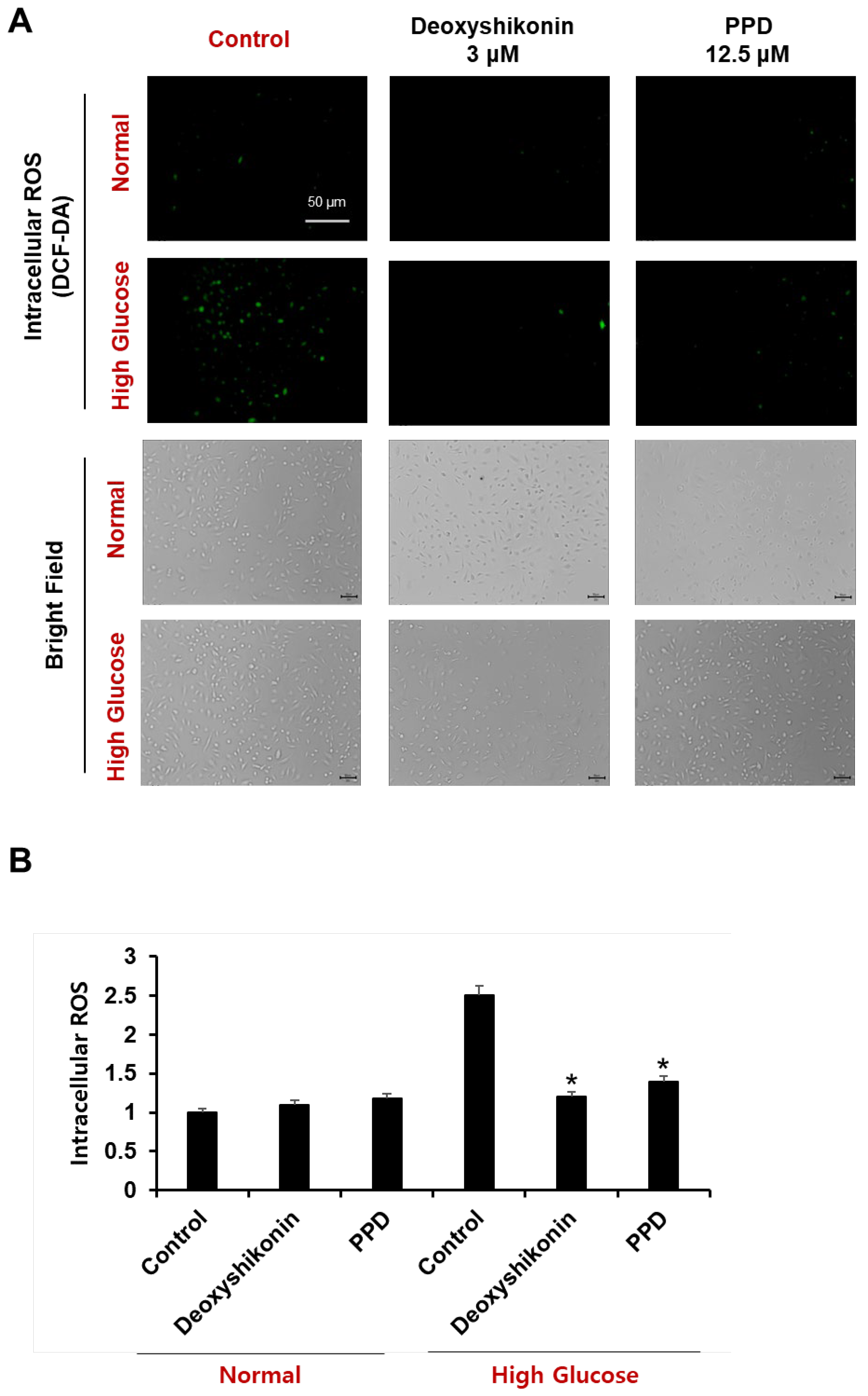
To determine the effect of PPD on ROS formation in HUVECs cultured in a high glucose concentration, the cells were stained with H2DCFDA, a fluorescent indicator of ROS. Accumulation of ROS in cells was evaluated by measuring the fluorescence intensity of H2DCFDA using a fluorescence plate reader and observed using a fluorescence microscope (Figure 8A). The measurement of H2DCFDA fluorescence intensity showed that the amount of ROS in cells cultured with high glucose was increased by 2.5 times compared to that in cells cultured under normal conditions and cells cultured with high glucose, and PPD 12.5 μM is high. We confirmed that the levels of intracellular ROS, which were increased by glucose, were significantly reduced by PPD treatment (Figure 8B). Additionally, no experimental group showed any toxicity (bright-field).
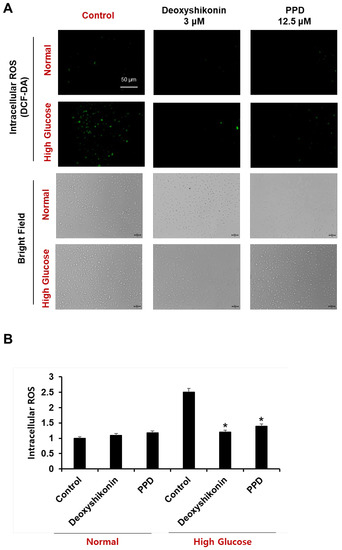
Figure 8.
HUVECs were treated with deoxyshikonin (3 μM) or PPD (12.5 μM) for 48 h under normal or high glucose conditions, then cells were stained with dichlorofluorescein diacetate (DCF-DA). Deoxyshikonin was used as a positive control. (A) Fluorescence images of DCF-DA were captured using fluorescence microscopy. Scale bar, 50 μm. (B) Bars represent a fold increase in fluorescence intensity of DCF-DA using microplate reader. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the control in high glucose.
3.7. Wound Healing Effect of PPD Treatment in STZ-Induced Diabetic Mouse Model
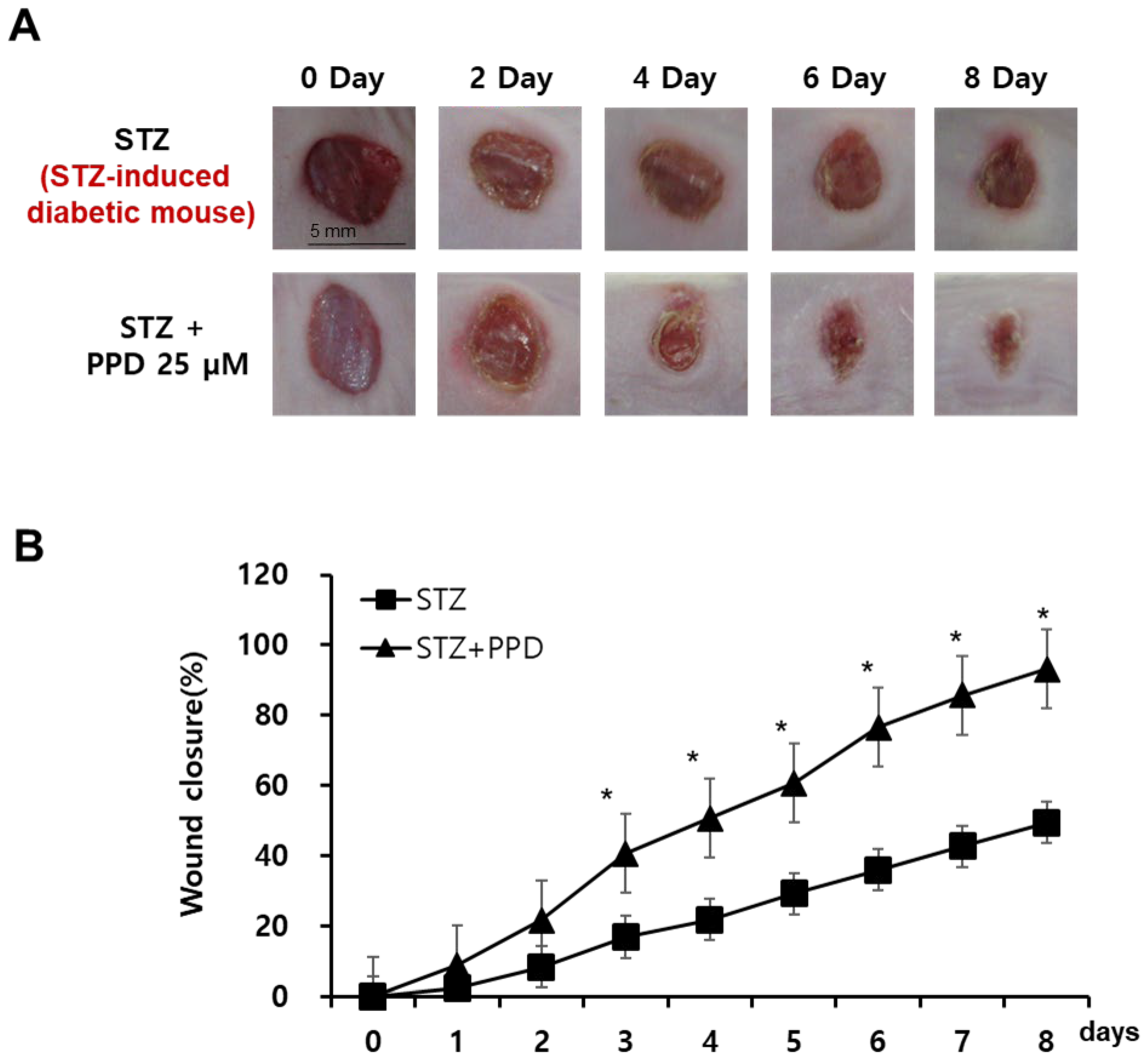
The therapies currently used to treat diabetic wounds have side effects and are sometimes insufficient as single treatments. Therefore, we aimed to evaluate wound healing in diabetic mice through multiple mechanisms, including the regulation of VEGF expression and ERK- and Akt-related mechanisms. STZ-induced diabetic mice were treated with PPD daily for 10 d after the wound induction. From the day of wound induction to 10 d after, the wound site of each subject was photographed at 1-day intervals to observe changes in the wound area (Figure 9A). On the day of wound induction, inflammatory exudates were secreted by all mice in the wound induction group, and the acute inflammatory change persisted until 5 days thereafter. In particular, five days after the induction of wounds, the STZ group, for which only the solvent was applied, had a higher amount of secretion of inflammatory exudates than the others, and foreign substances were attached to the wound. In contrast, in the PPD-treated group, a scab that was judged to inhibit the inflammatory reaction was formed. The wound radius was confirmed to be significantly lesser in the PPD-treated group than in the STZ group, and the wounds had recovered in the PPD-treated group. When 25 μM PPD was administered daily, the wound area decreased by 21% on day 2, 40% on day 3, 50% on day 4, 60% on day 5, 76% on day 6, 85% on day 7, and 93% on day 8. The wound area showed a significant reduction, approximately twice that in the STZ group, in the PPD-treated group starting on day 4 (Figure 9B). Therefore, wound closure in the PPD-treated group was confirmed to have improved compared to that in the STZ group. Ginsenosides have been reported to reduce oxidative stress-induced damage and improve peripheral nerve function and blood flow in diabetic rats [40] and are structurally divided into PPD and PPT groups [44]. The sugar moiety of the PPD group is bound to a β-OH at C-3 and another -OH at C-20 of an aglycone (ginsenosides Rb1, Rb2, Rc, Rd, Rg3, and Rh2) [45]. The pharmacological activity of ginseng saponins is known to be related to the number of sugar molecules, the number and location of hydroxyl groups, and stereo-selectivity [45]. Since the sugar-free aglycone showed the best effect, the PPD aglycone structure itself was considered to contribute to wound healing in diabetic mice model.
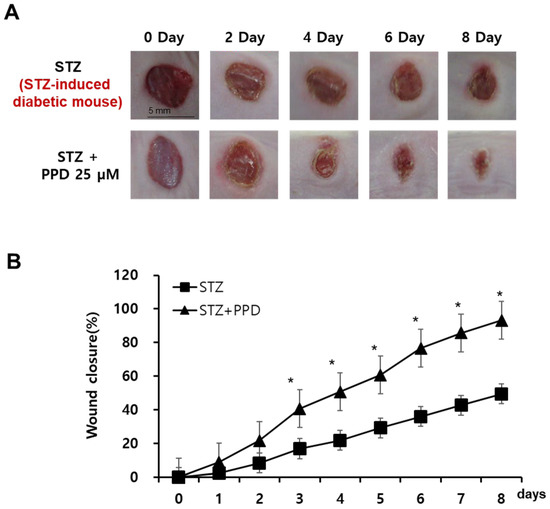
Figure 9.
Effect of PPD on wound healing in an experimental mouse model with skin wound. (A) Comparison of wound closure between the two groups (mice treated with 25 μM PPD and those in the control group). Representative pictures of wounds at 0, 2, 4, 6, and 8 days after treatment. Scale bar, 5 mm. (B) Quantitative analysis of the wound size in the PPD treatment groups compared to that in the control group. Data are presented as the mean ± standard deviation (SD) of three independent experiments. * p < 0.05 compared to the STZ group.
4. Conclusions
In this study, nine types of ginsenosides (Rc, Rd, Re, Rb1, Rb2, Rg1, Rg6, Rg3, and PPD) were used in in vitro and in vivo experimental models for diabetic wound healing. Among the 9 types of ginsenosides, 6 types, namely Rb1, Rb2, Rg1, Rg6, Rg3, and PPD, showed significantly increased tube formation activity in HUVECs. In addition, PPD treatment in high glucose-exposed HUVECs resulted in effective scratch wound healing compared to deoxyshikonin treatment. Analysis of the phosphorylation of signaling proteins, such as ERK, p38, and Akt, by PPD at high glucose levels in HUVECs revealed that PPD significantly increased the phosphorylation of ERK, p38, and Akt at 12.5 mg/mL concentration, whereas at 6.25 mg/mL it phosphorylated ERK and Akt only slightly. Similarly, PPD treatment upregulated VEGF-A mRNA and cytokine secretion in HG condition in HUVECs. PPD treatment for 8 days effectively improved wound closure in STZ-induced diabetic mice compared to that in STZ-induced control mice. Taken together, ginsenosides and PPDs, the active components of ginseng extract, can serve as useful therapeutic agents for the prevention of abnormal wounds in patients with DM. However, although the wound healing efficacy of the ginseng ingredient, PPD, in STZ-induced diabetic mice was confirmed in this study, detailed mechanisms using animal tissues have not been studied.
Author Contributions
Conceptualization, M.-S.S. and G.S.H.; methodology, D.H.P.; formal analysis, J.Y.P.; investigation, D.H.P. and J.Y.P.; writing—original draft preparation, D.H.P. and J.Y.P.; writing—review and editing, M.-S.S.; project administration, G.S.H.; funding acquisition, J.Y.P. and M.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Research Foundation of Korea (NRF-2021R1F1A1063787 and NRF-2021R1F1A1046521).
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gudehithlu, K.P.; Patri, S.; Litbarg, N.O.; Sethupathi, P.; Arruda, J.A.; Dunea, G. Impaired integration of endothelial progenitor cells in capillaries of diabetic wounds is reversible with vascular endothelial growth factor infusion. Transl. Res. 2007, 149, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Velazquez, O.C. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid. Redox Signal. 2008, 10, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Liu, Z.-J.; Xiao, M.; Chen, H.; Goldstein, L.J.; Buerk, D.G.; Nedeau, A.; Thom, S.R.; Velazquez, O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J. Clin. Investig. 2007, 117, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and emerging technology for continuous glucose monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef]
- Ng, M.G.; Ng, C.H.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Anticancer Properties of Strobilanthes crispus: A Review. Processes 2021, 9, 1370. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of allogeneic adipose-derived stem cell–hydrogel complex for treating diabetic foot ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature Review on the Management of Diabetic Foot Ulcer. World J. Diabetes 2015, 6, 37. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Seo, S.H.; Park, G.K.; Park, J.D. Korean Ginseng and Diabetes: An Insight into Antidiabetic Effects of Korean Ginseng (Panax Ginseng Ca Meyer) in Cultured Cells, Animal Models and Human Studies. Korean J. Pharmacogn. 2020, 51, 1–29. [Google Scholar]
- Peng, L.; Sun, S.; Xie, L.H.; Wicks, S.M.; Xie, J.T. Ginsenoside Re: Pharmacological effects on cardiovascular system. Cardiovasc. Ther. 2012, 30, e183–e188. [Google Scholar] [CrossRef]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Gao, J.; Ding, X.; Gao, S. The anti-fatigue effect of 20 (R)-ginsenoside Rg3 in mice by intranasally administration. Biol. Pharm. Bull. 2008, 31, 2024–2027. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Wang, P.; Fan, C.; Zhang, P.; Shen, J.; Yu, S.Y. Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats. Oxidative Med. Cell. Longev. 2020, 2020, 2325391. [Google Scholar] [CrossRef]
- Lee, H.U.; Bae, E.A.; Han, M.J.; Kim, N.J.; Kim, D.H. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005, 25, 1069–1073. [Google Scholar] [CrossRef]
- Park, Y.K.; Min, J.Y.; Hee, J.H. The Effect of Methyl Gallate Isolated from Paeonia Suffruticosa on Inflammatory Response in LPS-stimulated RAW264.7 Cells. Kor. J. Herbol. 2009, 24, 181–188. [Google Scholar]
- Jo, H.; Jang, D.; Park, S.K.; Lee, M.-G.; Cha, B.; Park, C.; Shin, Y.S.; Park, H.; Baek, J.-M.; Heo, H. Ginsenoside 20 (S)-protopanaxadiol induces cell death in human endometrial cancer cells via apoptosis. J. Ginseng Res. 2021, 45, 126–133. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, X.; Li, F.; Xu, C.; Zheng, F.; Li, J.; Zhao, H.; Dai, Y.; Liu, S.; Feng, Y. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci. Rep. 2017, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- So, S.-H.; Lee, J.W.; Kim, Y.-S.; Hyun, S.H.; Han, C.-K. Red Ginseng Monograph. J. Ginseng Res. 2018, 42, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, S.; Zhao, L.; Yang, X.; Yao, Y.; Hou, Z.; Xue, P. Stem-Leaves of Panax as a Rich and Sustainable Source of Less-Polar Ginsenosides: Comparison of Ginsenosides from Panax Ginseng, American Ginseng and Panax Notoginseng Prepared by Heating and Acid Treatment. J. Ginseng Res. 2021, 45, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-A.; Han, M.J.; Choo, M.-K.; Park, S.-Y.; Kim, D.-H. Metabolism of 20 (S)-and 20 (R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-K.; Choo, M.-K.; Kim, E.-J.; Han, M.J.; Kim, D.-H. Antiallergic activity of ginsenoside Rh2. Biol. Pharm. Bull. 2003, 26, 1581–1584. [Google Scholar] [CrossRef]
- Park, E.-K.; Choo, M.-K.; Han, M.J.; Kim, D.-H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef]
- Kim, D.-H. Gut Microbiota-Mediated Pharmacokinetics of Ginseng Saponins. J. Ginseng Res. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical role of transforming growth factor beta in different phases of wound healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Yue, P.Y.K.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.P.; Yeung, H.W.; Wong, R.N.S. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007, 2, 6–21. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.-S.; Yoo, J.-E.; Shin, M.-S.; Yamabe, N.; Kim, S.-N.; Lee, S.; Kim, K.H.; Lee, H.-J. Abietic Acid Isolated from Pine Resin (Resina Pini) Enhances Angiogenesis in Huvecs and Accelerates Cutaneous Wound Healing in Mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef]
- Steed, D.L. Modifying the wound healing response with exogenous growth factors. Clin. Plast. Surg. 1998, 25, 397–405. [Google Scholar] [CrossRef]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Goren, I.; Müller, E.; Pfeilschifter, J.; Frank, S. Severely impaired insulin signaling in chronic wounds of diabetic ob/ob mice: A potential role of tumor necrosis factor-α. Am. J. Pathol. 2006, 168, 765–777. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.-M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (Vegf)-Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Zhang, F.; Oswald, T.; Lin, S.; Cai, Z.; Lei, M.; Jones, M.; Angel, M.; Lineaweaver, W. Vascular endothelial growth factor (VEGF) expression and the effect of exogenous VEGF on survival of a random flap in the rat. Br. J. Plast. Surg. 2003, 56, 653–659. [Google Scholar] [CrossRef]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Calvente, M.I.A.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Harding, K.G. Diabetic foot ulcers. Lancet 2003, 361, 1545–1551. [Google Scholar] [CrossRef]
- Babaei, S.; Bayat, M.; Nouruzian, M.; Bayat, M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2013, 700, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kesici, U.; Kesici, S.; Ulusoy, H.; Yucesan, F.; Turkmen, A.U.; Besir, A.; Tuna, V. Effects of glutamine on wound healing. Int. Wound J. 2015, 12, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Han, G.; Owens, P.; Siddiqui, Y.; Li, A.G. Role of TGFβ-mediated inflammation in cutaneous wound healing. J. Investig. Dermatol. Symp. Proc. 2006, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Bai, Y.; Du, Z.; Chen, S.; Deligema, D.; Pang, Z. The effect of chinese medicine pu-ren-dan on pancreatic angiogenesis in high fat diet/streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013, 45, 556. [Google Scholar]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. American Ginseng: Potential Structure–Function Relationship in Cancer Chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).