Optimization of Polyphenol Extraction with Potential Application as Natural Food Preservatives from Brazilian Amazonian Species Dalbergia monetaria and Croton cajucara
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Botanical Material and Extract Preparation
2.3. Gravimetric Analysis
2.4. Experimental Design
2.5. Total Flavonoids Content
2.6. Total Phenolic Content
2.7. DPPH Free Radical Scavenging
2.8. Total Antioxidant Capacity by Phosphomolybdenum Assay
2.9. Ferric Reduction Power Assay
2.10. Ultra High-Performance Liquid Chromatography-Diode Array Detection (UPLC-DAD)
2.11. Lipid Peroxidation Inhibition Assay
2.12. Statistical Analysis
3. Results and Discussion
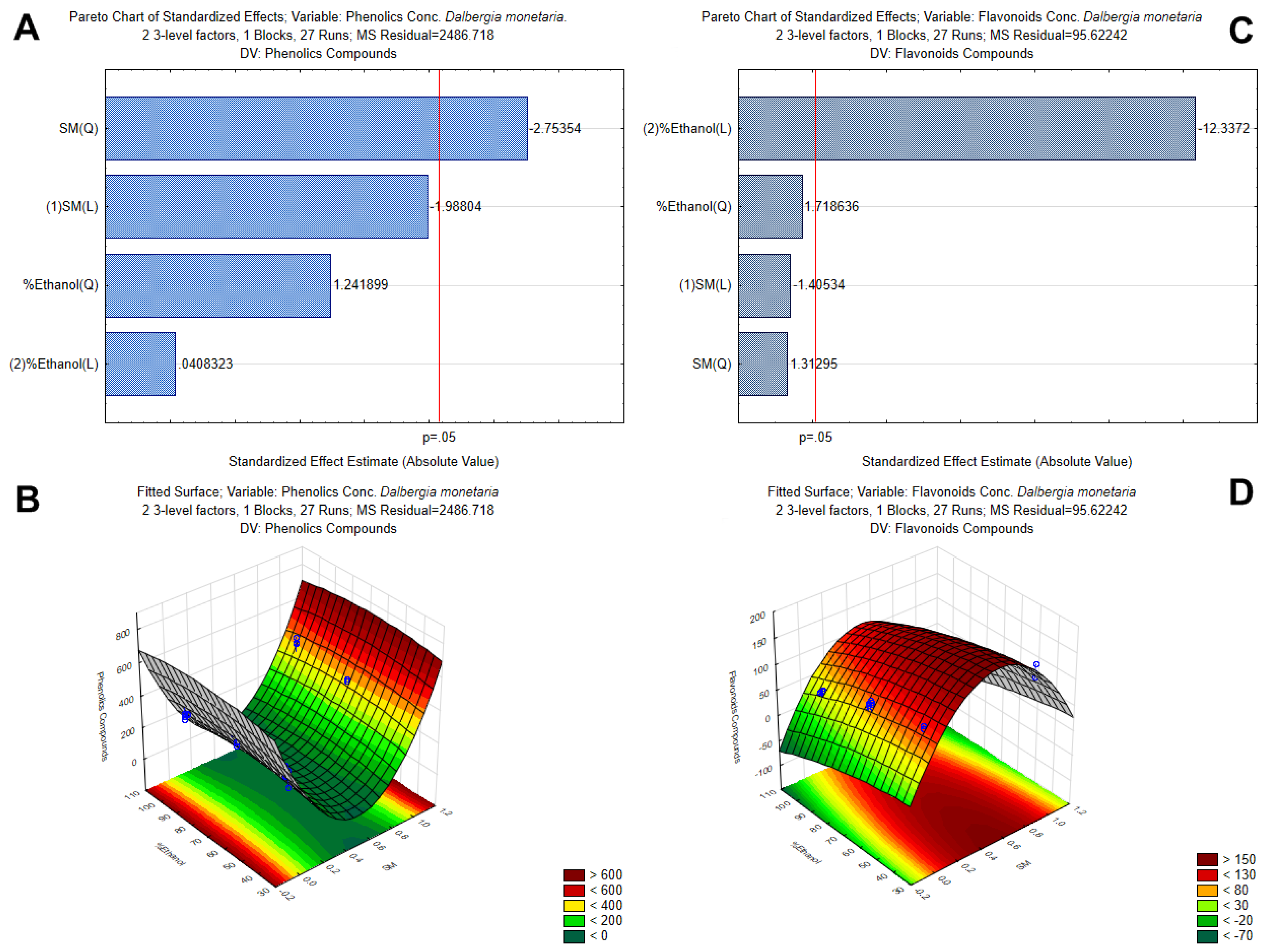
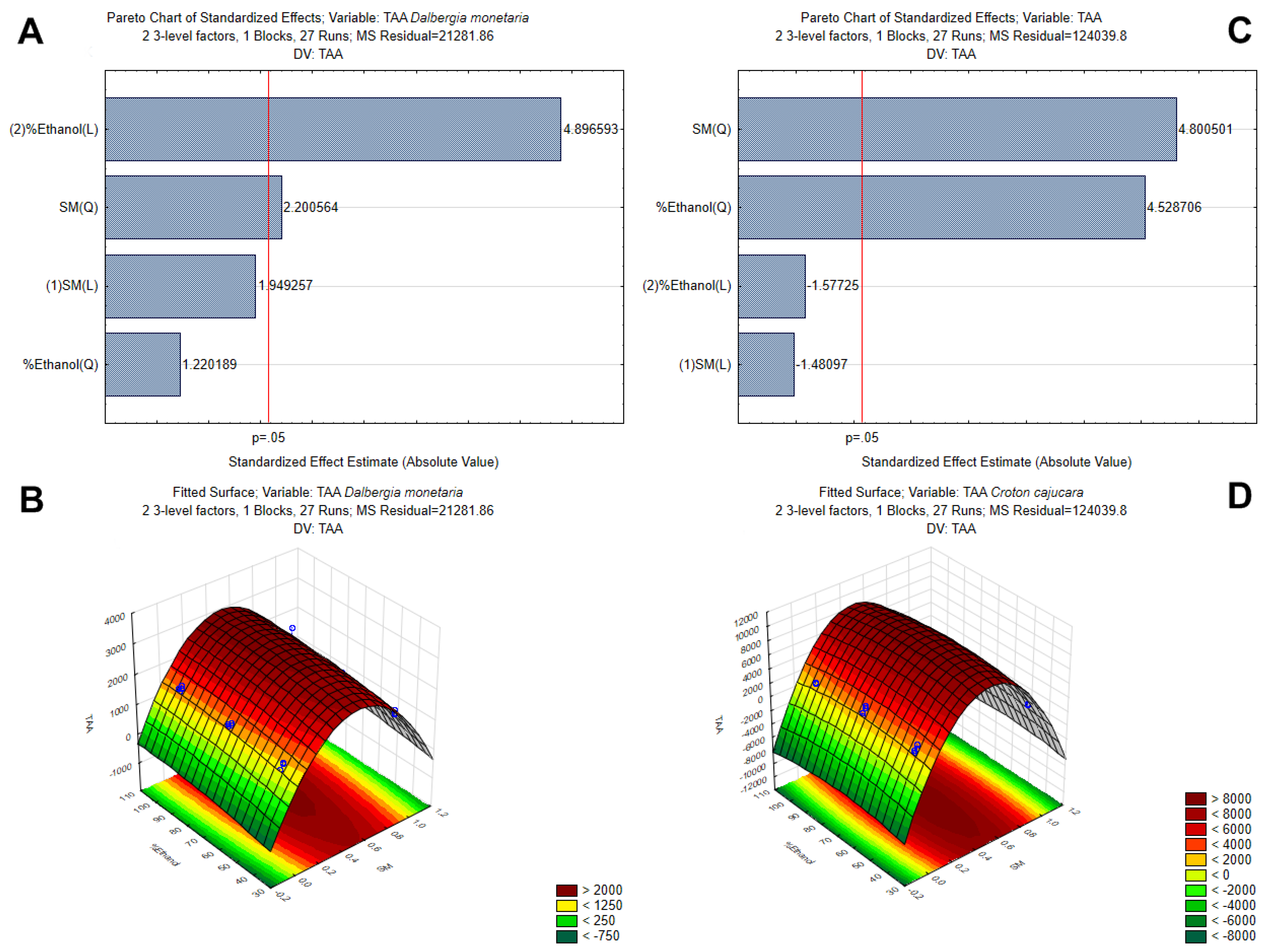
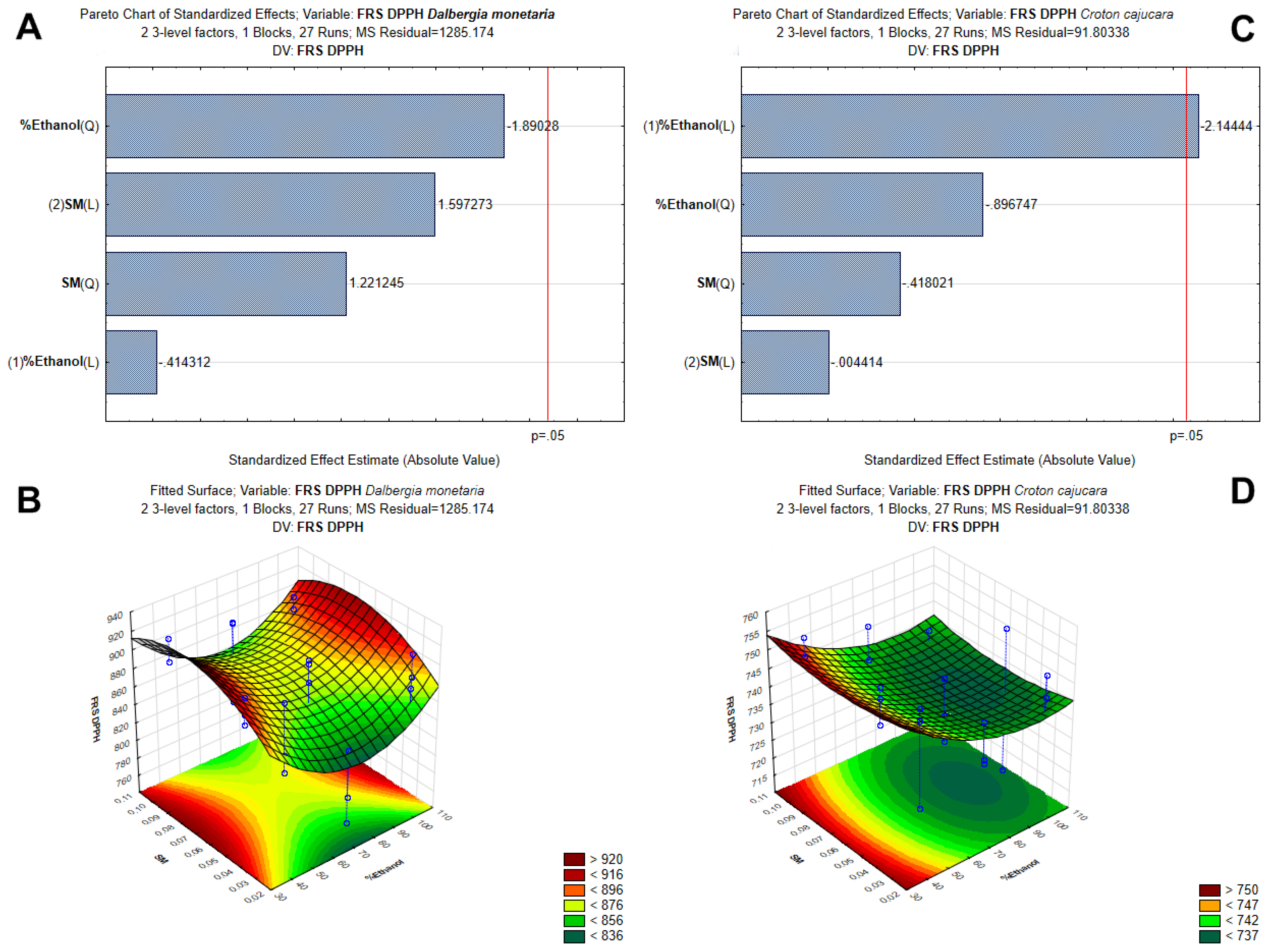
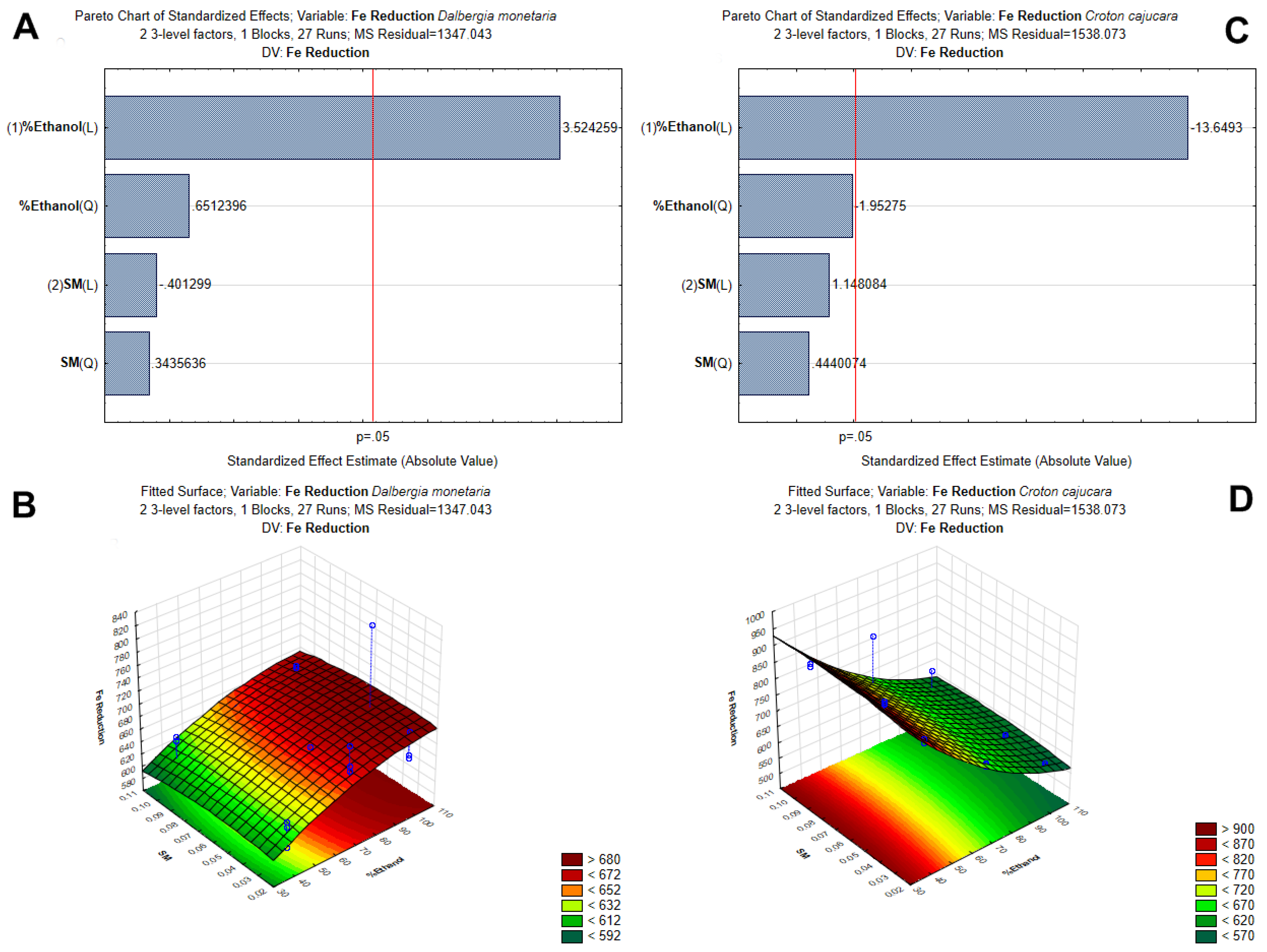
3.1. Optimization of Polyphenol Extraction by Response Surface Methodology and Characterization by Antioxidant Capacity
3.2. UPLC/DAD Analysis of Polyphenols of D. monetaria and C. cajucara Extracts
3.3. Lipid Peroxidation Inhibition of D. monetaria and C. cajucara Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Long, Y.; Chen, Q.; Zhang, W.; Liu, G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 8497–8517. [Google Scholar] [CrossRef]
- Kwon, J.; López-García, R.; Socolovsky, S.; Magnuson, B. Global regulations for the use of food additives and processing aids. In Present Knowledge in Food Safety; Knowles, M.E., Anelich, L.E., Boobis, A.R., Popping, B., Eds.; Academic Press: Cambridge, UK, 2023; pp. 170–193. [Google Scholar]
- Paula Neto, H.A.; Ausina, P.; Gomez, L.S.; Leandro, J.G.B.; Zancan, P.; Sola-Penna, M. Effects of food additives on immune cells as contributors to body weight gain and immune-mediated metabolic dysregulation. Front. Immunol. 2017, 8, 1478. [Google Scholar] [CrossRef]
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S. Food additives and child health. Pediatrics 2018, 142, e20181410. [Google Scholar] [CrossRef]
- Sambu, S.; Hemaram, U.; Murugan, R.; Alsof, A.A. Toxicological and teratogenic effect of various food additives: An updated review. Biomed Res. Int. 2022, 2022, 6829409. [Google Scholar] [CrossRef]
- Bimpizas-Pinis, M.; Santagata, R.; Kaiser, S.; Liu, Y.; Lyu, Y. Additives in the food supply chain: Environmental assessment and circular economy implications. Environ. Sustain. Ind. 2022, 14, 100172. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in natural antioxidants for food improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Vasantha Rupasinghe, H.P.; Keyata, E.O. Potentials of natural preservatives to enhance food safety and shelf life: A review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Sawabe, A.; Ohnishi, N.; Yoshioka, S.; Kusudo, K.; Kanno, K.; Watanabe, Y. Functional ingredients and food preservative in immature Persimmon “Tekka-Kaki”. Processes 2021, 9, 1989. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Nwabor, O.F. Natural bioactive compounds in food production and preservation. In Food Preservation and Safety of Natural Products; Onyeaka, H.N., Nwabor, O.F., Eds.; Academic Press: Cambridge, UK, 2022; pp. 57–73. [Google Scholar]
- Romani, A.; Suriano, R.; Levi, M. Biomass waste materials through extrusion-based additive manufacturing: A systematic literature review. J. Clean. Prod. 2023, 386, 135779. [Google Scholar] [CrossRef]
- Chisti, Y. Biotechnology for sustainable production of food. In Reference Module in Food Science; Elsevier: Philadelphia, PA, USA, 2023; in press. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Anjos, H.A.; Luna, S.; Hernández-Macedo, M.L.; López, J.A. Antimicrobial and antioxidant active food packaging: Technological and scientific prospection. Recent Pat. Biotechnol. 2020, 14, 99–111. [Google Scholar] [CrossRef]
- Haji, M.; Kerbache, L.; Al-Ansari, T. Food quality, drug safety, and increasing public health measures in supply chain management. Processes 2022, 10, 1715. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Araújo, F.F.; Farias, D.P.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Shah, M.A.; Mir, S.A. Plant extracts as food preservatives. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; Academic Press: Cambridge, UK, 2022; pp. 127–141. [Google Scholar]
- Maximo, Y.I.; Hassegawa, M.; Verkerk, P.J.; Missio, A.L. Forest bioeconomy in Brazil: Potential innovative Products from the forest sector. Land 2022, 11, 1297. [Google Scholar] [CrossRef]
- Skirycz, A.; Kierszniowska, S.; Méret, M.; Willmitzer, L.; Tzotzos, G. Medicinal Bioprospecting of the Amazon Rainforest: A Modern Eldorado? Trends Biotechnol. 2016, 34, 781–790. [Google Scholar] [CrossRef]
- Sarquis, R.S.F.R.; Sarquis, I.R.; Sarquis, I.R.; Fernandes, C.P.; da Silva, G.A.; Silva, R.B.L.; Jardim, M.A.G.; Sánchez-Ortíz, B.L.; Carvalho, J.C.T. The use of medicinal plants in the riverside community of the Mazagão River in the Brazilian Amazon, Amapá, Brazil: Ethnobotanical and ethnopharmacological studies. Evid. Based Complement. Alternat. Med. 2019, 2019, 6087509. [Google Scholar] [CrossRef]
- Braga, F.C. Brazilian traditional medicine: Historical basis, features and potentialities for pharmaceutical development. J. Tradit. Chin. Med. Sci. 2021, 8, S44–S50. [Google Scholar] [CrossRef]
- Peres, C.A.; Gardner, T.A.; Barlow, J.; Zuanon, J.; Michalski, F.; Lees, A.C.; Vieira, I.C.G.; Moreira, F.M.S.; Feeley, K.J. Biodiversity conservation in human-modified Amazonian forest landscapes. Biol. Conserv. 2010, 143, 2314–2327. [Google Scholar] [CrossRef]
- Bergamo, D.; Zerbini, O.; Pinho, P.; Moutinho, P. The Amazon bioeconomy: Beyond the use of forest products. Ecol. Econ. 2022, 199, 107448. [Google Scholar] [CrossRef]
- Guerra Junior, J.I.; Ferreira, M.R.A.; Oliveira, A.M.; Soares, L.A.L. Croton sp.: A review about popular uses, biological activities and chemical composition. Res. Soc. Dev. 2022, 11, e57311225306. [Google Scholar] [CrossRef]
- Oliveira-Melo, P.M.C.; Lima, P.G.C.; Costa, J.C.; Coelho-Ferreira, M.R. Ethnobotanical study in a rural settlement in Amazon: Contribution of local knowledge to public health policies. Res. Soc. Dev. 2022, 1, e56911125258. [Google Scholar] [CrossRef]
- de Moura, P.H.B.; Almeida, A.S.; Porzel, A.; Wessjohann, L.A.; Leal, I.C.R.; Martins, R.C.C. Characterization of antibacterial proanthocyanidins of Dalbergia monetaria, an Amazonian medicinal plant, by UHPLC-HRMS/MS. Planta Med. 2020, 86, 858–866. [Google Scholar] [CrossRef]
- Vasudeva, N.; Vats, M.; Sharma, S.K.; Sardana, S. Chemistry and biological activities of the genus Dalbergia—A review. Pharmacogn. Rev. 2009, 3, 307–319. [Google Scholar]
- Melo, E.L.; Ramos, R.S.; Almeida, S.S.M.S. Phytochemical study, chemicalphysical analysis and toxicological testing of stem bark of Dalbergia monetaria L.f. Br. J. Pharm. Res. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- de Moura, P.H.B.; Brandt, W.; Porzel, A.; Martins, R.C.C.; Leal, I.C.R.; Wessjohann, L.A. Structural Elucidation of an Atropisomeric Entcassiflavan-(4β→8)-Epicatechin Isolated from Dalbergia monetaria L.f. Based on NMR and ECD calculations in comparison to experimental data. Molecules 2022, 27, 2512. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; et al. Ethnopharmacology, phytochemistry and pharmacology: A successful combination in the study of Croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Dantas, T.N.C.; Câmara, J.K.P.; Pinto, A.C.; Veiga, V.F., Jr.; Kaiser, K.R.; Pereira, N.A.; Carneiro, C.M.T.S.; Vanderlinde, F.A.; Lapa, A.J.; et al. Pharmacological and biochemical profiling of lead compounds from traditional remedies: The case of Croton cajucara. Adv. Phytomed. 2006, 2, 225–253. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Nascimento, A.M.; Maria-Ferreira, M.D.; Lin, F.T.D.; Kimura, A.; Santana-Filho, A.P.; Werner, M.F.P.; Iacomini, M.; Sassaki, G.L.; Cipriani, T.R.; Souza, L.M. Phytochemical analysis and anti-inflammatory evaluation of compounds from an aqueous extract of Croton cajucara Benth. J. Pharm. Biomed. Anal. 2017, 145, 821–830. [Google Scholar] [CrossRef]
- Souza, A.A.M.; Barros, D.S.; Rodrigues, C.C.; Almeida, S.M.F.; Silva, N.M.M.; Dolabela, M.F.; Brandão, D.L.N.; Santos, L.S.N. Study of quantification pf phenolic compounds, evaluation of antioxidant and antimicrobial activities of the stem bark of the Croton cajucara Benth. Res. Soc. Dev. 2020, 9, e1929119742. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Rocha, T.M.; Philippini, R.R.; Martiniano, S.E.; Prado, C.A.; Mier-Alba, E.; Hernandez-Perez, A.F.; Jofre, F.M.; Abdeshahian, P.; Ribeaux, D.R.; et al. The potential of vegetal biomass for biomolecules production. In Comprehensive Renewable Energy; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–164. [Google Scholar]
- Natural Antioxidants Global Market Report 2023. Available online: https://www.thebusinessresearchcompany.com/report/natural-antioxidants-global-market-report (accessed on 12 January 2023).
- Gordon, A.; Schreurs, F. Addressing trade and market access issues based on food safety strategies. In Food Safety and Quality Systems in Developing Countries; Gordon, A., Ed.; Academic Press: Cambridge, UK, 2020; pp. 41–80. [Google Scholar]
- Isaías, R.; Frias, A.; Rocha, C.; Moura, A.P.; Cunha, L.M. Designing and development of food structure with high acceptance based on the consumer perception. In Food Structure Engineering and Design for Improved Nutrition, Health and Well-Being; Cerqueira, M.A.P.R., Castro, L.M.P., Eds.; Academic Press: Cambridge, UK, 2023; pp. 399–414. [Google Scholar]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Jemia, M.B.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdinum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Santos, M.H.; Batista, B.L.; Duarte, S.M.S.; Abreu, C.M.P.; Gouvêa, C.M.C.P. Influence of processing and roasting on the antioxidant activity of coffee. Quim. Nova. 2007, 30, 604–610. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A.; Rocha-Guzmán, N.E. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food Compos. Anal. 2017, 63, 38–46. [Google Scholar] [CrossRef]
- Melo, M.G.D.; Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; Pasquali, M.A.B.; Rabelo, T.K.; Rocha, R.F.; Quintans, L., Jr.; Araújo, A.A.S.; Silva, F.A.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. In Vitro 2011, 25, 462–468. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Segovia, J.F.O.; Sousa, V.Y.K.; Mata, E.C.G.; Gonçalves, M.C.A.; Bezerra, R.M.; Junior, P.O.M.; Kanzaki, L.I.B. Antimicrobial activity of Amazonian medicinal plants. SpringerPlus 2013, 2, 271. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trend Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods used in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Corbo, F. Optimization of a green extraction of polyphenols from sweet cherry (Prunus avium L.) pulp. Processes 2022, 10, 1657. [Google Scholar] [CrossRef]
- Picos-Salas, M.A.; Heredia, J.B.; Leyva-López, N.; Ambriz-Pérez, D.L.; Gutiérrez-Grijalva, E.P. Extraction processes affect the composition and bioavailability of flavones from Lamiaceae plants: A comprehensive review. Processes 2021, 9, 1675. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and recovery of bioactive soluble phenolic compounds from brocade orange (Citrus sinensis) peels: Effect of different extraction methods thereon. LWT-Food Sci. Technol. 2023, 173, 114337. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.-J.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Lozano-Sánchez, J.; Oliver-Simancas, R.; Alañón, M.E.; Castangia, I.; Segura-Carretero, A.; Arráez-Román, D. Application of response surface methodologies to optimize high-added value products developments: Cosmetic formulations as an example. Antioxidants 2022, 11, 1552. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Anis, N.; Ahmed, D. Modelling and optimization of polyphenol and antioxidant extraction from Rumex hastatus by green glycerol-water solvent according to response surface methodology. Heliyon 2022, 8, e11992. [Google Scholar] [CrossRef]
- Silva, L.M.P.; Inácio, M.R.C.; Silva, G.G.C.d.; Silva, J.M.d.S.e.; Luz, J.R.D.d.; Almeida, M.d.G.; Moraes, E.P.; Esposito, D.; Ferreira, L.D.S.; Zucolotto, S.M. The first optimization process from cultivation to flavonoid-rich extract from Moringa oleifera Lam. leaves in Brazil. Foods 2022, 11, 1452. [Google Scholar] [CrossRef]
- Ramos, M.; Laveriano, E.; San Sebastián, L.; Perez, M.; Jiménez, A.; Lamuela-Raventos, R.M.; Garrigós, M.C.; Vallverdú-Queralt, A. Rice straw as a valuable source of cellulose and polyphenols: Applications in the food industry. Trends Food Sci. Technol. 2023, 131, 14–27. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural food colorants and preservatives: A review, a demand, and challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Morais, L.V.F.; Luz, J.R.D.; Nascimento, T.E.S.; Cruz, M.A.S.A.; Rocha, W.P.S.; Araujo-Silva, G.; Ururahy, M.A.G.; Chaves, G.M.; Brandão Neto, J.; López, J.A.; et al. Phenolic composition, toxicity potential, and antimicrobial activi-ty of Licania rigida Benth (Chrysobalanaceae) leaf extracts. J. Med. Food. 2022, 25, 97–109. [Google Scholar] [CrossRef]
- Odabaş, H.I.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind.Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.-P.; Romdhane, M. Optimization of accelerated solvent extraction of bioactive compounds from Eucalyptus intertexta using response surface methodology and evaluation of its phenolic composition and biological activities. J. Appl. Res. Med. Aromat. Plants 2023, in press. [Google Scholar] [CrossRef]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Akanbi, T.O.; Nwachukwu, I.D.; Gunathilake, T. Perspectives on preserving lipid quality and strategies for value enhancement. Curr. Opin. Food Sci. 2022, 44, 100802. [Google Scholar] [CrossRef]
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating volatile lipid oxidation compounds with consumer sensory data in dairy based powders during storage. Antioxidants 2020, 9, 338. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Rocha, R.; Pinela, J.; Abreu, R.M.V.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Saldanha, A.L.; Alves, M.J.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L. Extraction of anthocyanins from red raspberry for natural food colorants development: Processes optimization and in vitro bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]



| Variable Coded Values | Variable Coded Values (Continuation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard Run | Replicate | Block | SB | %EtOH | Standard Run | Replicate | Block | SB | %EtOH |
| 17 | 2 | 3 | 0.025 | 100 | 1 | 1 | 1 | 0.050 | 100 |
| 4 | 1 | 2 | 0.025 | 70 | 7 | 1 | 3 | 0.100 | 40 |
| 5 | 1 | 2 | 0.100 | 100 | 20 | 3 | 1 | 0.100 | 70 |
| 10 | 2 | 1 | 0.050 | 100 | 9 | 1 | 3 | 0.050 | 70 |
| 15 | 2 | 2 | 0.050 | 40 | 12 | 2 | 1 | 0.025 | 40 |
| 16 | 2 | 3 | 0.100 | 40 | 13 | 2 | 2 | 0.025 | 70 |
| 11 | 2 | 1 | 0.100 | 70 | 8 | 1 | 3 | 0.025 | 100 |
| 24 | 3 | 2 | 0.050 | 40 | 19 | 3 | 1 | 0.050 | 100 |
| 6 | 1 | 2 | 0.050 | 40 | 3 | 1 | 1 | 0.025 | 40 |
| 18 | 2 | 3 | 0.050 | 70 | 14 | 2 | 2 | 0.100 | 100 |
| 26 | 3 | 3 | 0.0250 | 100 | 27 | 3 | 3 | 0.050 | 70 |
| 2 | 1 | 1 | 0.100 | 70 | 23 | 3 | 2 | 0.100 | 100 |
| 22 | 3 | 2 | 0.025 | 70 | 21 | 3 | 1 | 0.025 | 40 |
| 25 | 3 | 3 | 0.100 | 40 | |||||
| Compound | Retention Times (min) | Linear Range (µg/mL) | UV (nm) | R2 |
|---|---|---|---|---|
| Gallic acid | 1.71 | 1.5–50 | 271 | 0.9945 |
| Chlorogenic acid | 6.47 | 1.5–50 | 326 | 0.9973 |
| Catechin | 7.19 | 1.5–50 | 278 | 0.9993 |
| (-)-Epigallocatechin gallate | 8.18 | 1.5–50 | 275 | 0.9989 |
| Rutin | 9.20 | 1.81–100 | 275–354 | 0.9976 |
| Hyperoside | 9.24 | 1.81–100 | 256–354 | 0.9995 |
| Quercetin | 16.03 | 1.5–50 | 255–371 | 0.9989 |
| Apigenin | 18.51 | 1.5–50 | 266–337 | 0.9989 |
| Kaempferol | 18.85 | 1.5–50 | 263–367 | 0.9984 |
| Dalbergia monetaria | Croton cajucara | ||||||
|---|---|---|---|---|---|---|---|
| Peak | RT (min) | Concentration (µg/mL) | UV-λmax (min) | Peak | RT (min) | Concentration (µg/mL) | UV-λmax (min) |
| 1 | 0.807 | 51.88 | 265 A | 1 | 0.651 | 51.72 | 274 A |
| 2 | 3.094 | 468.38 | 289/477 A | 2 | 5.750 | 5.35 | 335/271 A |
| 3 | 20.756 | 119.86 | 332/286 B | 3 | 6.286 | 3.53 | 256/349 A |
| 4 | 21.960 | 138.94 | 330/662 B | 4 | 6.763 | 6.08 | 265/229/346 A |
| 5 | 23.236 | 74.76 | 331/491 B | 5 | 6.863 | 2.27 | 254/353/232 A |
| 6 | 30.737 | 6.77 | 331/491 B | 6 | 7.047 | 4.58 | 254/337 A |
| 7 | 7.696 | 2.94 | 265/240/342 A | ||||
| 8 | 7.871 | 3.63 | 254/352 A | ||||
| 9 | 8.043 | 4.98 | 259/346 A | ||||
| 10 | 15.872 | 0.39 | 246/349 B | ||||
| 11 | 19.837 | 2.59 | 267/347 B | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paranhos de Araújo, V.A.; Luz, J.R.D.d.; da Silva e Silva, N.S.; Pereira, M.P.; Barbosa, J.P.; dos Santos, D.C.; López, J.A.; da Silva Solon, L.G.; Araujo-Silva, G. Optimization of Polyphenol Extraction with Potential Application as Natural Food Preservatives from Brazilian Amazonian Species Dalbergia monetaria and Croton cajucara. Processes 2023, 11, 669. https://doi.org/10.3390/pr11030669
Paranhos de Araújo VA, Luz JRDd, da Silva e Silva NS, Pereira MP, Barbosa JP, dos Santos DC, López JA, da Silva Solon LG, Araujo-Silva G. Optimization of Polyphenol Extraction with Potential Application as Natural Food Preservatives from Brazilian Amazonian Species Dalbergia monetaria and Croton cajucara. Processes. 2023; 11(3):669. https://doi.org/10.3390/pr11030669
Chicago/Turabian StyleParanhos de Araújo, Vaneska Aimee, Jefferson Romáryo Duarte da Luz, Naikita Suellen da Silva e Silva, Matheus Pereira Pereira, Jardel Pinto Barbosa, Darlan Coutinho dos Santos, Jorge A. López, Lilian Grace da Silva Solon, and Gabriel Araujo-Silva. 2023. "Optimization of Polyphenol Extraction with Potential Application as Natural Food Preservatives from Brazilian Amazonian Species Dalbergia monetaria and Croton cajucara" Processes 11, no. 3: 669. https://doi.org/10.3390/pr11030669
APA StyleParanhos de Araújo, V. A., Luz, J. R. D. d., da Silva e Silva, N. S., Pereira, M. P., Barbosa, J. P., dos Santos, D. C., López, J. A., da Silva Solon, L. G., & Araujo-Silva, G. (2023). Optimization of Polyphenol Extraction with Potential Application as Natural Food Preservatives from Brazilian Amazonian Species Dalbergia monetaria and Croton cajucara. Processes, 11(3), 669. https://doi.org/10.3390/pr11030669










